Keywords
contrast agents for ultrasound, Microbubbles, echocardiographic, M-mode
Efforts have been made over the last five decades to create effective ultrasonic contrast media (UCM) for cardiac and noncardiac applications. The initial UCM was established in the 1980s, following publications from the 1960s that detailed the discovery of ultrasonic contrast enhancement using small gaseous bubbles in echocardiographic examinations. An optimal contrast agent for echography should possess the following characteristics: non-toxicity, suitability for intravenous injection, ability to traverse pulmonary, cardiac, and capillary circulations, and stability for recirculation. Definity, Optison, Sonazoid, and SonoVue are examples of current commercial contrast media. These contrast media have shown potential for various clinical reasons, both on-label and off-label. Several possible UCMs have been developed or are in progress. Advancements in comprehending the physical, chemical, and biological characteristics of microbubbles have significantly improved the visualization of tumor blood vessels, the identification of areas with reduced blood supply, and the enhanced detection of narrowed blood vessels. Innovative advances are expected to enhance future applications such as ultrasonic molecular imaging and therapeutic utilization of microbubbles.
contrast agents for ultrasound, Microbubbles, echocardiographic, M-mode
1. The grammatical and typo errors in all manuscript sections are corrected.
2. The conclusion is rephrased in a good Scientific way.
See the author's detailed response to the review by Hamad Yahia Abu Mhanna
See the author's detailed response to the review by Haytham Alewaidat
In obstetrics, cardiology, and radiology, ultrasound imaging is a common clinical tool for the morphological examination of soft tissues.1–7 As an ultrasonic wave—a longitudinal wave—travels through the body, tissue surfaces with various acoustic characteristics, such as speed of sound and density, produce reflections. The same transmitting transducer captures these scattered impulses and uses them to create an image. However, because to the size and characteristics of red blood cells, the intrinsic scattering from the blood pool is often several orders of magnitude lower than tissue at standard diagnostic frequencies (1–9 MHz). As a result, blood appears black on typical ultrasound images, making it difficult to determine the properties of blood flow. Doppler techniques can be used to measure blood velocity in bigger veins by comparing the relative motion of red blood cells to the surrounding tissue.8,9 This technique is frequently used in clinical settings (e.g., obstetrics,10 assessment of peripheral artery disease,11 cardiology12). However, this method has drawbacks when applied to areas with poor blood flow, significant tissue motion, and/or low hematocrit percentage.13–15
Ultrasound imaging’s diagnostic applications have significantly expanded during the past few decades. The advancement of UCM has resulted in the presentation of valuable physiological and pathological information, as well as the accessibility of perfusion imaging for cardiac or tumor tissue in routine clinical decision-making.16,17 The early 1960s saw the first reports of the ultrasonic contrast effect was studies by Joyner. Further research revealed the existence of UCM made of saline, indocyanine green, hydrogen peroxide, dextrose, and renografin.16,18 UCM comprise of a suspension of small spheres of gas with a poor solubility in blood (e.g., perfluorocarbon), often ranging in size from below 10 μm in diameter. The relatively large size of UCMs guarantees that they remain strictly intravascular and function as red blood cell tracers, in contrast to contrast media employed in other modalities like (magnetic resonance imaging) MRI and computer tomography (CT).19
Around 1980, achieving stability long enough for the UCM to reach the correct heart was one of the primary objectives in creating efficient UCMs. Left heart contrast was not possible until the 1990s because lung capillaries are effective filters. In 1995, contrast-enhancing substances with enhanced blood pool enhancement capabilities first surfaced. The next goal was to create bubbles that would allow for real-time imaging. In order to achieve this, air was substituted with weakly soluble gases, such as perfluorocarbons, which increased bubble endurance and allowed the development of software algorithms that could effectively distinguish UCM from tissue signals.20–23
Microbubbles vibrate about their equilibrium radius in an ultrasonic field due to the compressibility of their gas cores, and they have scattering cross-sections that are many orders of magnitude higher than a solid particle of the same size.16,24,25 A thin biocompatible encapsulation layer, often a phospholipid monolayer, stabilizes the bubbles by striking a balance between their ability to vibrate freely and their resistance to dissolving in-vivo during timeframes important for imaging, like half-lives of minutes.26,27
Contrast echocardiography has a virtually limitless potential. Contrast echocardiography is currently the subject of extensive interest and research, as this review demonstrates. The creation of novel contrast-producing chemicals is arguably the most intriguing component of this research. It will be fascinating to watch how these different agents grow. Ideally, one or more of these novel agents will be able to cross the capillaries, allowing for peripheral venous injection-based visualization of the left side of the heart.
Over the world, contrast-enhanced ultrasound imaging is used in numerous medical and off-label applications. On multiple fronts, including the creation of novel pulse sequences and image processing techniques, the development of devices, and the creation of remote monitoring for ultrasonic therapies, this field is seeing cutting-edge breakthroughs at the same time.
The only UCM that has received clinical approval is microbubbles. These bubbles have the advantage of remaining intravascular because of their size, making it possible to perform diagnostic tests that would be challenging with diffusible tracers. The use of these “conventional” UCM is being expanded, though, to include molecular-based imaging, imaging of the extravascular space, and as a platform for both imaging and therapeutic administration.28–32
Extensive study was done beginning in 1980 to establish contrast echocardiography as a recognized diagnostic method.33 Ophir and Parker (1989) provided a summary of UCM’s application in medical imaging.34 Free gas bubbles, encapsulated gas bubbles, colloidal suspensions, emulsions, and aqueous solutions were the five categories of agents that were categorized according to their physical characteristics. Producing the “perfect” contrast media that would satisfy the following requirements was still a major difficulty in those days. Such as, distribution of the substance inside the myocardial or heart chambers, which is indicative of regional blood flow; agent’s capacity to endure after an intravenous infusion during an imaging test; containing microbubbles with a diameter of less than 8 mm (smaller than red blood cells), allowing passage via the pulmonary system and the body’s smallest capillaries; good safety profile, physiological inert; and strong, regulated, and echogenic acoustic interaction.
In 1984 (Feinstein et al. 1984), cavitation was used to create microbubbles after inserting the tip of a sonicator horn into a solution of human serum albumin.17 This solved the problem of creating stable encapsulated microbubbles that could survive passage through the heart and the pulmonary capillary network. After a peripheral venous injection, these microbubbles could be seen in the left heart. Due to the creation of functionalized microbubbles,35 or microbubbles with one or more targeted moieties inserted into the phospholipid encapsulation,36 non-invasive imaging of pathophysiological events has recently been demonstrated to be viable with ultrasound. Target sites have focused on internal vasculature processes such inflammation,37 angiogenesis,38 and thrombus formation39 since microbubbles are purely intravascular. See Table 1.
The development of the first microbubbles that met the majority of the requirements for an intravenous UCM also sparked intense research by doctors, scientists, and the makers of ultrasound equipment to explain the physical phenomena and apply what they learned to therapeutic settings.
There were several technologies looked at to stabilize the microbubbles. For the purpose of lowering surface tension and stabilizing the gas core against quick dissolution, thin shells consisting of protein, polymer, or phospholipids were utilized. Unfortunately, due to the high solubility of air in water, the first-generation agents still had poor stability and relatively short circulation times. By substituting perfluorinated gases with low solubility in water, such as sulphur hexafluoride, perfluoropropane, or perfluorobutane for air during circulation, persistence during circulation was dramatically improved, resulting in sufficient persistence of the agent in the blood circulation for clinical use.41
There are many ultrasound-sensitive sub-micron agents currently being researched. This research is motivated by the enhanced-permeability and retention effect,42 whereby small nanometer sized particles locally extravasate from leaky blood vessels and accumulate in the perivascular space of solid tumors. Phase-shift droplets,43 nanobubbles,44 gas vesicles,45 echogenic liposomes,46 and polymeric nanoparticles47 are a few of the more common examples. Although research into the physics of acoustic droplet vaporization is still ongoing, it is most probable that both intrinsic and external elements play a role in the process.
Vibrating microbubbles’ nonlinear nature is essential to their efficiency as an ultrasonic contrast agent. These emissions allow for the separation of bubble signals from the surrounding (about linear) tissue from those within tiny vessels. Thus, certain microbubble imaging modes were created concurrently with the advancements in UCM and as a result of a better knowledge of non-linear microbubble behavior; these are now used in the majority of clinical ultrasound systems.48–50 The first methods of bubble identification were harmonic imaging, which involved gathering and filtering energy from the receive signal at the second harmonic, which is twice the driving frequency. Because the second harmonic signal produced by microbubbles is substantially greater than the second harmonic signal produced by tissue, it has a higher signal-to-noise ratio than the fundamental energy. The success of low mechanical index (MI) (0.1) contrast-specific imaging, which is primarily employed for real-time perfusion and intra-cavitary measurements, is particularly explained by the non-linear shell behavior. Furthermore, a number of diagnostic imaging procedures and/or quantification strategies are founded on the distinct and extremely sensitive attribute of microbubble destruction.51 See Figure 1.
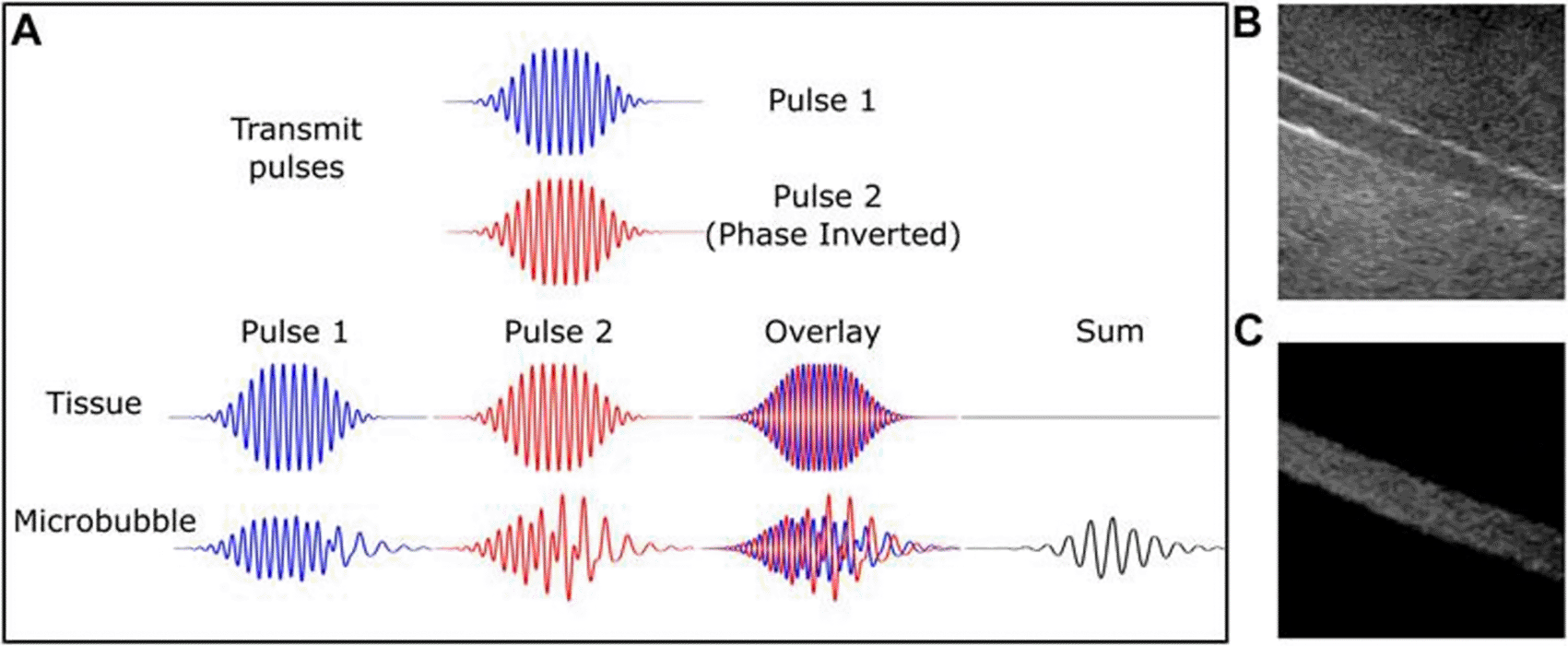
Pulse inversion diagram (A). Two 180-degree pulses produce tissue echoes that are out of phase. Microbubbles are nonlinear; hence, this is not true. Microbubbles produce a strong echo, while linear tissue almost totally cancels it off. B-mode and contrast-specific imaging of an 8-mm artery phantom exhibit microbubble-specific imaging's improved vascular contrast. The Philips iU22 scanner, C5-2 probe, and DefinityTM contrast medium were used to record this. This source provides this number. Microbubble-specific imaging sequences exclude linear scattering tissue and capture a nonlinear contrast agent signal. Yusefi, H., & Helfield, B. (2022), https://www.frontiersin.org/files/Articles/791145/fphy-10-791145-HTML/image_m/fphy-10-791145-g002.jpg, CC BY 4.0.28
In other words, the reflection pattern from the bubble to the ultrasound signal is significantly altered by UCM. They start by greatly boosting the backscattered signal.23 UCM resonate linearly in response to acoustic pressure. Acoustic pressure increases cause nonlinear vibrational patterns to manifest.52 Only at higher mechanical indices (MI) do tissues create harmonic resonances, making it easy to distinguish between the signal’s tissue or UCM origin. Multiples of the natural frequencies are received using filter devices, allowing for some background (non-UCM) signal reduction. Microbubbles are disrupted by high pressure levels, which results in strong signals and signals with various properties.
Understanding the interaction between ultrasonic waves and gaseous microbubbles was made much easier by using the process utilized to explain the set of echoes first discovered on M-mode echocardiograms. The strong compressibility of the gas core appears to be particularly significant since it produces frequency-dependent volume pulsations with a clear maximum at the resonance frequency, which is inversely proportional to the size of the microbubbles.24,53 However, In the following circumstances, UCM is advised by cardiologic guidelines: if the left ventricular (LV) cavum does not have two continuous segments, If the original spectrum signals are insufficient, to enhance Doppler evaluations, when periodic evaluation of the ejection fraction is necessary given the reduced variability caused by UCM, and in the case of Takotsubo myopathy, left ventricular (LV) aneurysms, and intracavitary thrombi.52,54,55
Acoustic color Doppler is an imaging method that overlays color-coded maps of tissue velocity on grey-scale images of tissue anatomy. It combines anatomical information acquired from ultrasonic pulse-echo techniques with velocity information derived from ultrasonic Doppler techniques. The technique is most frequently used to visualize blood flow through the heart, arteries, and veins, but it can also be used to visualize the movements of solid tissues like the walls of the heart. vectors. Almost all commercial ultrasound equipment now provides color Doppler imaging, which has been proven to be very useful in determining blood flow in a variety of clinical circumstances. Although the technique for getting velocity information is quite similar to the technique for getting anatomical information, there are a number of reasons why it is technically more difficult. It also has a few flaws, the biggest of which is that, in conventional systems, the velocities measured and subsequently displayed are the components of the flow velocity directly towards or away from the transducer, whereas the method’s ideal output would provide data on the magnitude and direction of the three-dimensional flow vectors.56–61
In conjunction with color or power Doppler, stimulated acoustic emission is employed in high mechanical index (MI) imaging. A high MI ultrasound impulse is used to deflate the microbubbles, and the signal that is received is a complicated mixture of ultrasound waves that causes a Doppler shift.62 It is especially helpful when a UCM with tissue specificity, is in its late stages.20 Color Doppler imaging has been shown that despite the poor spatial resolution, real-time imaging was possible due to the tiny size of the picture window.63
Recently, the use of ultrasound color Doppler has been shown to monitor bubbles during ultrasound therapy. An active ultrasound imaging method called color Doppler uses the phase shifts between the echoes of imaging pulses to measure velocities. In the aforementioned investigation, an increase in the color Doppler signal was connected with the formation of cavitation bubbles on their own under a high-pressure ultrasonic beam. The rise was utilized to evaluate tissue fractionation and was related to the mobility of the surrounding tissue brought on by cavitation within the focused location. Although this method is helpful for high-pressure therapy, it does not reveal how net bubbles flow through the field.64,65
All currently marketed UCM are made up of an inert gas enclosed in a shell. The gas determines solubility and most of the acoustic qualities of the bubbles, while the shell mostly affects the viscoelastic properties, such as stability and durability.66 Perfluorocarbon bubbles, which range in size almost 10 m and are real blood pool agents, allow transit through the pulmonary vascular system, which is necessary for entry to the systemic circulation.52 Soft shell materials have better nonlinear oscillations and are made of phospholipids or other surfactants.67 There are also protein-shelled microbubbles that contain an albumin shell around perfluoropropane gas.
Contrast agent microbubble vibration basics and its applications in common contrast-imaging are outlined in this paper. Over the span of the last fifty years, UCM imaging has made substantial progress. Previously, the primary clinical emphasis was on echocardiography, while myocardial perfusion was considered the ultimate goal and an attractive market for contrast echo. The primary purpose of echocardiography was to enhance the visibility of the LV by making it more opaque, hence improving the clarity of the LV endocardial border. UCM imaging is a safe and effective method for many clinical applications, and its use is growing. Due to increased clinical awareness of ultrasound’s advantages as well as collaborative research projects between physicists, chemists, engineers, and clinicians on the study of microbubble behavior, signal processing methods, contrast agent synthesis, and device development, this imaging technology has achieved tremendous success to date.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Medical Imaging
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Medical Imaging
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Medical Imaging
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 04 Jun 24 |
||
|
Version 2 (revision) 11 Mar 24 |
read | read |
|
Version 1 07 Nov 23 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)