Keywords
ASCVD, CBC, HbA1c, Inflammatory markers, Lipid profiles
This article is included in the Research Synergy Foundation gateway.
ASCVD, CBC, HbA1c, Inflammatory markers, Lipid profiles
Type 2 diabetes mellitus (T2DM) has a significant impact on health issues globally. Persistent and extensive inflammation has accelerated T2DM-related complications and mortality.1 Increased hemoglobin A1c (HbA1c) as a hyperglycemia marker is associated with higher cardiovascular mortality.2 Hyperglycemia induces cellular immunity activation and triggers the production of pro-inflammatory cytokines, which accelerates the progression of T2DM complications.3 T2DM is the foremost contributor to atherosclerotic cardiovascular disease (ASCVD). Higher HbA1c levels are strongly linked to an increased risk of cardiovascular disease (CVD).4 The American Heart Association/American College of Cardiology (AHA/ACC) Blood Cholesterol 2018 Guideline recommends risk stratification for patients with clinical ASCVD who are considered very high-risk (VHR) patients. VHR is established by having a history of two or more major ASCVD events or one major ASCVD event and two or more high-risk conditions.5
T2DM pathology is significantly correlated with disruptions in the metabolic and vascular systems.6 Relating to hematology parameters, alterations in the component of complete blood count (CBC) from erythrocyte indices such as hemoglobin (Hb), mean cell/corpuscular volume (MCV), mean cell/corpuscular hemoglobin (MCH), mean cell/corpuscular hemoglobin concentration (MCHC), and red blood cell distribution width (RDW), along with alterations in leucocyte indices, have been considered as prognostic biomarkers for cardiovascular diseases (CVDs) in patients with T2DM.7 Inflammatory markers derived from hematology parameters such as the neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), monocyte-lymphocyte ratio (MLR), mean platelet value (MPV)-platelet ratio (MPR), RDW-platelet ratio (RPR) have been associated with higher inflammation in T2DM.8 White blood cell (WBC) count, NLR, PLR, and MLR have all been identified as important biomarkers in acute coronary syndrome (ACS).9 Lipid parameters have also been linked with inadequate glycemic control in T2DM. The levels of total cholesterol (TC), low-density lipoprotein (LDL), triglycerides (TG), and LDL to HDL ratio were found as prospective biomarkers in T2DM.10
In recent times, CBC and inflammatory markers derived from hematology parameters along with lipid profile tests have been presented as novel biomarkers and as predictors of chronic condition outcome for CVD.11 In T2DM with CVD, CBC and inflammatory markers derived from the CBC examination along with a lipid profile test may reveal the advancing inflammation in patients with T2DM. Patients with T2DM ASCVD have profound metabolic abnormalities that correlate with intensive inflammation and an increased risk of cardiovascular worsening. However, to the best of our knowledge, the relationship between all of these parameters has not been studied in T2DM ASCVD risk groups or those with T2DM who experienced first-time ACS. This study aimed to evaluate the associations of glycosylated hemoglobin with alterations in the CBC, lipid profiles, and inflammatory markers derived from neutrophils, monocytes, platelets, and lymphocytes in T2DM ASCVD.
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics and Research Committee of the National Cardiovascular Center Harapan Kita Jakarta Indonesia on April 21st, 2021, with decision letter number: LB.02.01/VII/548/KEP 035/2021. All the participants involved in this study had previously given their informed consent and filled out the informed consent forms before they were enrolled.
This cross-sectional study was conducted between September 2021 and July 2022 in the National Cardiovascular Center Harapan Kita by using a purposive sampling method. Overall, this study included 75 patients, including those diagnosed with T2DM ASCVD who were very high risk (VHR), subjects with T2DM who were high risk (HR), and patients with T2DM with first ACS-ST-elevation myocardial infarction (ACS-STEMI) incidence. A total of 25 subjects with T2DM-HR and 25 subjects with T2DM-VHR were from the Outpatient Department of the National Cardiovascular Center Harapan Kita, and 25 subjects with T2DM-ACS were from the Emergency Department.
Diagnostic criteria for ASCVD VHR, HR, and ACS were as follows: ASCVD VHR was defined by having a history of ≥2 major ASCVD events [recent ACS, ischemic stroke, symptomatic peripheral artery disease (PAD), history of myocardial infarction (MI)] or one major ASCVD event and ≥2 high-risk conditions [age ≥65 years old, diabetes, hypertension, smoking, familial hypercholesterolemia, chronic kidney disease, congestive heart failure, persistently elevated low-density lipoprotein cholesterol (LDL-C), or prior coronary artery revascularization].5 While ASCVD HR were patients who possessed more than three major risk factors [age, family history, cigarette smoking, high blood pressure, low high-density lipoprotein (HDL)], diabetes, chronic kidney disease stage 3B or 4, and LDL-C > 190 mg/dL.12 ECG and cardiac troponin were used to diagnose ACS in patients with T2DM. Patients with diabetes were confirmed by their history (diagnosed with T2DM and treated for T2DM) and by evaluating their HbA1c results. NLR, MLR, PLR was enumerated by dividing absolute neutrophil, absolute monocytes, and platelet count by absolute lymphocytes count. Inclusion criteria were ensured by the patient’s data history, HbA1c assessment, ASCVD criteria by American Heart Association/American College of Cardiology (AHA/ACC) Blood Cholesterol Guideline,5 and ACS by electrocardiogram (ECG) and cardiac biomarkers. Exclusion criteria included patients with T2DM-ASCVD who refused to give informed consent or refused to undergo laboratory examinations, patients with incomplete data, patients with COVID-19 infection, and patients with pulmonary disease.
Patients with T2DM from the Outpatient Department and Emergency Department who gave their informed consent were then evaluated for sociodemographic factors and laboratory examinations. Blood samples were collected and measured for CBC, HbA1c, lipid profile (total cholesterol, high-density cholesterol, and low-density cholesterol), and IL-6 examinations. CBC was measured using SYSMEX XN3000 hematology analyzer, lipid profiles were measured using a Cobas Pro chemical autoanalyzer, and human IL-6 was measured by using ELISA kit (Quantikinine R&D Systems) with Thermo Scientific Multiscan Sky Microplate Spectrophotometer.
Purposive sampling methods were used in our study to collect participants. We tried to selectively gather participants according to the ASCVD VHR, HR, and ACS diagnostic criteria. We calculated sample size by using an analytic correlation test with a coefficient correlation of r (r=0.5), a one-sided test, a 5% significance level test (α=0.05), and a power of 80% (β=0.20). The required sample size was approximately 24 (n=24) for each ASCVD group, but we added up to 25 so that the total sample became 75.
The laboratory data were collected and analyzed using the IBM SPSS Statistics (RRID:SCR_016479) version 22.0 software (IBM Corp.) and presented in the form of tables or figures. To determine the differences between groups, ANOVA with Tamhane’s T2 post hoc analysis was performed for normal distribution variables and data are presented as mean ± standard deviation, while non-normal distribution variables were analyzed using non-parametric Kruskal Wallis and presented as median with a range. Categorical variables were analyzed using Chi-squared tests and presented as percentages with the number of subjects. Analysis to determine the associations between glycosylated hemoglobin with the alterations in CBC, lipid profiles, and inflammatory markers derived from neutrophils, monocytes, platelets, and lymphocytes in T2DM ASCVD was evaluated using the Pearson correlation coefficient and Spearman's rank correlation coefficient tests, which was declared significant if p<0.05. Logistic regression analysis was used to analyze the relationship between hematology, lipid profiles, and inflammatory parameters with T2DM ACS. An analysis of receiver operating characteristic (ROC) curve was carried out to assess the accuracy of parameters for predicting the presence of ACS.
The characteristics of participants and hematology parameters, lipid profile, and IL-6 from T2DM HR, T2DM VHR, and T2DM ACS are demonstrated in Table 1.58 This study included 57 male (76%) and 18 female (24%) participants, and 41 subjects in middle age group (54.66%) between 45-60 years old. Our results have shown some significant differences in levels of RDW (p<0.05), total leucocyte count (p<0.001), differential leucocyte count, basophils (p<0.05), eosinophils (p<0.001), segmented neutrophils (p<0.001), lymphocytes (p<0.001), monocytes (p<0.05), NLR (p<0.001), MLR (p<0.05), PLR (p<0.05), absolute lymphocytes (p<0.05), and monocyte count (p<0.05), total cholesterol (p<0.001), LDL (p<0.001), HDL/total cholesterol ratio (p<0.001), HbA1c (p<0.001), and IL-6 (p<0.001).
T2DM, Type 2 diabetes mellitus; ASCVD, atherosclerotic cardiovascular disease; HR, high risk; VHR, very high risk; ACS, acute coronary syndrome.
| Parameters | T2DM ASCVD | P-value | ||
|---|---|---|---|---|
| HR (n=25) | VHR (n=25) | ACS (n=25) | ||
| Age (years) | 0.94 | |||
| 44-60 | 13 (52.0%) | 14 (56.0%) | 14 (56.0%) | |
| 61-75 | 12 (48.0%) | 11 (44.0%) | 11 (44.0%) | |
| Sex | 0.215 | |||
| Male | 20 (80.0%) | 16 (64.0%) | 21 (84.0%) | |
| Female | 5(20.0%) | 9 (36.0%) | 4 (16.0%) | |
| Hemoglobin (g/dL) | 13.1±1.74 | 13.16±1.68 | 13.77±1.88 | 0.366 |
| Hematocrit (%) | 38.10±5.17 | 38.33±4.68 | 40.30±5.12 | 0.237 |
| Erythrocyte (106/μL) | 4.42±0.66 | 4.46±0.53 | 4.74±0.70 | 0.156 |
| Mean Corpuscular Volume (MCV) (fL) | 87.2 (72.6–95.7) | 85.6 (77–97.7) | 85.2 (71.1–95.8) | 0.279 |
| Mean Corpuscular Hemoglobin (MCH) (pg) | 30.5 (23.6–34) | 29.70 (25.9–34.1) | 29.80 (23.5–33.5) | 0.063 |
| Mean Corpuscular Hemoglobin Concentration (MCHC) (%) | 34.5±1.1 | 34.3±1.2 | 34.1±0.9 | 0.443 |
| Red cell Distribution Width (RDW) (%) | 13.4 (11.7–17.2) | 13.1 (11.9–17.5) | 12.7 (11.7–15.8) | <0.05* |
| Leucocyte (/μL) | 8,720 (4,760–50,970) | 7,730 (4,950–14,970) | 13,360 (6,430–24,880) | <0.001** |
| Basophil (%) | 0.5 (0–1.4) | 0.6 (0.3–1.2) | 0.3 (0.1–0.8) | <0.05* |
| Eosinophil (%) | 3.5 (1–10.3) | 3.5 (0–10.1) | 0.5 (0–5.3) | <0.001** |
| Segmented neutrophil (%) | 56.6 (39.7–79.4) | 58.3 (32.5–73.8) | 78.4 (43.4–91) | <0.001** |
| Lymphocyte (%) | 29.90 (11.10–48.80) | 27.90 (16.60–53.10) | 15.50 (5.20–45.70) | <0.001** |
| Monocyte (%) | 7.75±2.02 | 7.78±2.12 | 6.00±2.01 | <0.05* |
| Neutrophil-Lymphocyte Ratio (NLR) | 1.91 (0.81–7.2) | 2.1 (0.6–4.2) | 5.1 (1.0–16.4) | <0.001** |
| Monocyte-Lymphocyte Ratio (MLR) | 0.26 (0.14–0.7) | 0.26 (0.13–0.6) | 0.34 (0.2–1.7) | <0.05* |
| Platelet-Lymphocyte Ratio (PLR) | 98.8 (41.3–226.7) | 125 (60.1–212.8) | 160.6 (47.8–367.6) | <0.05* |
| Absolute Lymphocyte (/μL) | 2,569 (966–6,116) | 2,212 (1,090–3,993) | 1,950 (677–4,316) | <0.05* |
| Absolute Monocyte (/μL) | 685.24±153.31 | 616.20±201.36 | 803.16±358.05 | <0.05* |
| Platelet (103/μL) | 277.1±73.7 | 290±76.3 | 309.4±85.5 | 0.349 |
| Cholesterol total (mg/dL) | 136 (100–197) | 178 (100–290) | 210 (94–703) | <0.001** |
| Cholesterol Low-density Lipoprotein (LDL) (mg/dL) | 80.64±23.63 | 111.32±44.26 | 132.72±43.75 | <0.001** |
| Cholesterol High-density Lipoprotein (HDL) (mg/dL) | 42 (24–53) | 36 (25–68) | 37 (14–179) | 0.447 |
| Cholesterol total/HDL ratio | 3.4 (2.2–6.7) | 4.5 (2.9–7.8) | 5.2 (1.4–50.2) | <0.001** |
| HbA1c (%) | 6.20 (5.10–7.90) | 7.00 (5.70–10.70) | 9.80 (6.60–12.00) | <0.001** |
| IL-6 (pg/mL) | 2.7 (1.4–14.9) | 3.3 (2.4–8.3) | 8.7 (2.5–402.5) | <0.001** |
Patients with T2DM ACS showed decreased levels of RDW, basophils, eosinophils, lymphocytes, monocytes, and absolute lymphocytes. Compared with T2DM HR and VHR, T2DM ACS showed increased levels of leucocytes, segmented neutrophils, NLR, MLR, PLR, absolute monocytes, total cholesterol, LDL, HDL/total cholesterol ratio, HbA1c, and IL-6.
ROC analysis revealed that NLR values greater than 3.97 were 84% sensitive and 94% specific for determining T2DM ACS (AUC 0.846, p<0.001), MLR values greater than 0.32 were 68% sensitive and 80% specific for determining T2DM ACS (AUC 0.734, p<0.05), PLR values greater than 158.96 were 60% sensitive and 84% specific for determining T2DM ACS (AUC 0.695, p<0.05), IL-6 values greater than 5.8 were 72% sensitive and 88% specific for determining T2DM ACS (AUC 0.837, p<0.001) (Figure 1).
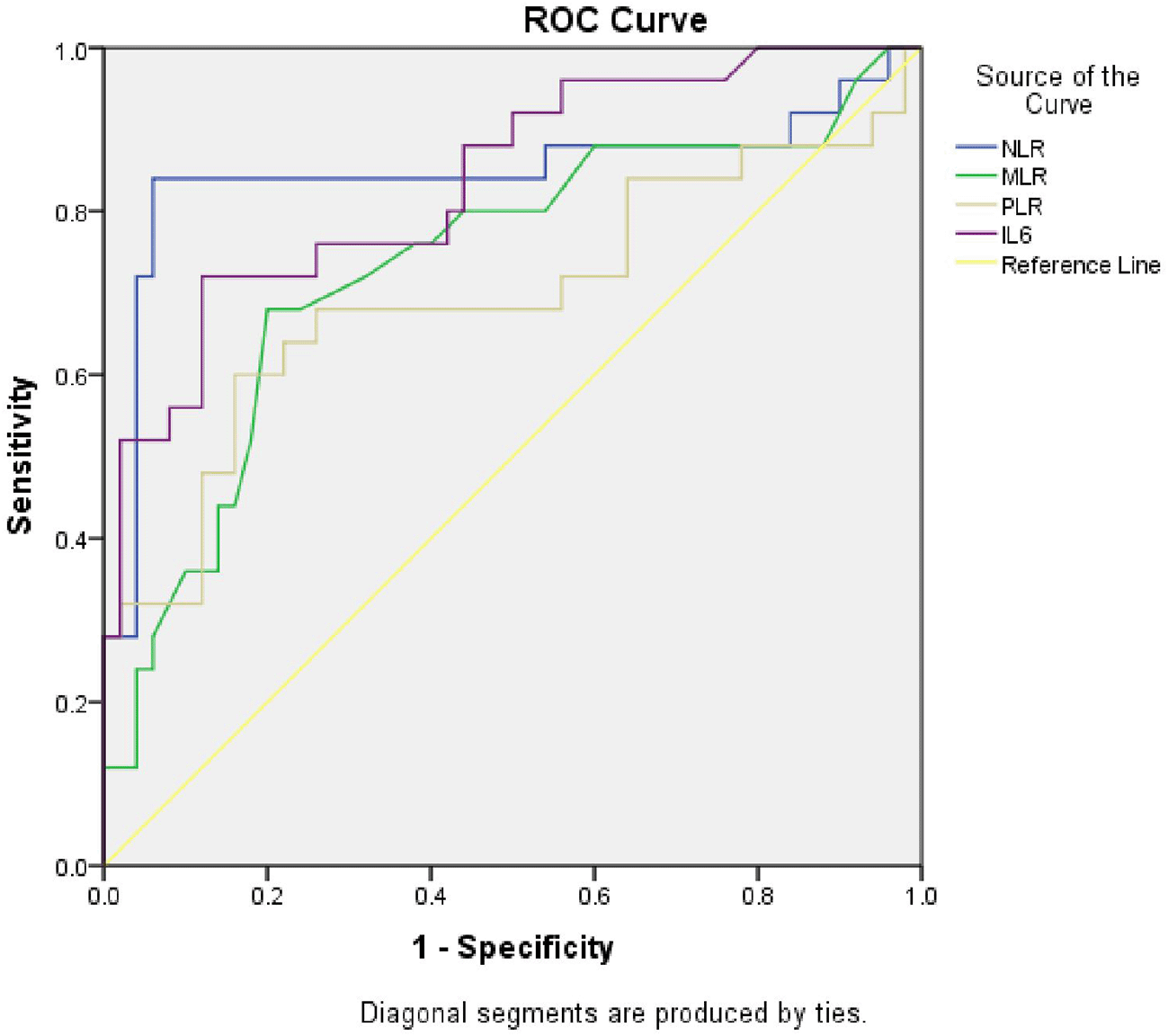
ROC, Receiver operating characteristic; NLR, neutrophil-lymphocyte ratio; MLR, MPV-lymphocyte ratio; PLR, platelet-lymphocyte ratio; T2DM, Type 2 diabetes mellitus; ACS, acute coronary syndrome.
The correlation analysis between HbA1c and other parameters in T2DM ASCVD demonstrated that HbA1c was correlated significantly and positively with leucocytes (p<0.05), segmented neutrophils (p<0.001), NLR (p<0.05), PLR (p<0.05), total cholesterol (p<0.05), LDL (p<0.05), total cholesterol/HDL ratio (p<0.05), and IL-6 (p<0.001). Meanwhile, HbA1c was correlated significantly and negatively with eosinophils (p<0.05), lymphocytes (p<0.05), monocytes (p<0.05), and absolute lymphocytes (p<0.05) (Table 2).
HbA1c, hemoglobin A1c; CBC, complete blood count; T2DM, Type 2 diabetes mellitus; ASCVD, atherosclerotic cardiovascular disease.
| Parameters | Number of patients with T2DM ASCVD | Correlation coefficient | P-value |
|---|---|---|---|
| Hemoglobin | 75 | 0.084 | 0.472 |
| Hematocrit | 75 | 0.099 | 0.400 |
| Erythrocyte | 75 | 0.1499 | 0.203 |
| Mean Corpuscular Volume (MCV) | 75 | -0.082 | 0.487 |
| Mean Corpuscular Hemoglobin (MCH) | 75 | -0.113 | 0.336 |
| Mean Corpuscular Hemoglobin Concentration (MCHC) | 75 | -0.087 | 0.458 |
| Red cell Distribution Width (RDW) | 75 | -0.227 | 0.051 |
| Leucocyte | 75 | 0.312 | <0.05* |
| Basophil | 75 | -0.116 | 0.321 |
| Eosinophil | 75 | -0.267 | <0.05* |
| Segmented neutrophil | 75 | 0.402 | <0.001** |
| Lymphocyte | 75 | -0.340 | <0.05* |
| Monocyte | 75 | -0.252 | <0.05* |
| Neutrophil-Lymphocyte Ratio (NLR) | 75 | 0.355 | <0.05* |
| Monocyte-Lymphocyte Ratio (MLR) | 75 | 0.221 | 0.057 |
| Platelet-Lymphocyte Ratio (PLR) | 75 | 0.249 | <0.05* |
| Absolute Lymphocyte | 75 | -0.235 | <0.05* |
| Absolute Monocyte | 75 | 0.108 | 0.356 |
| Platelet | 75 | 0.108 | 0.355 |
| Cholesterol total | 75 | 0.284 | <0.05* |
| Cholesterol Low-density Lipoprotein (LDL) (mg/dL) | 75 | 0.340 | <0.05* |
| Cholesterol High-density Lipoprotein (HDL) (mg/dL) | 75 | -0.030 | 0.798 |
| Cholesterol total/HDL ratio | 75 | 0.279 | <0.05* |
| IL-6 | 75 | 0.536 | <0.001** |
To determine the strength of association between all parameters with T2DM ACS, multivariate regression analysis was performed and presented in Table 3. Monocytes, MLR, leucocytes, eosinophils, and absolute monocytes were found to be strong risk factors for T2DM ACS presence (p<0.05).
ACS, acute coronary syndrome.
The major outcomes of our study are as follows: (i) Patients with T2DM ACS showed significant differences in hematology markers involving RDW, total leucocyte count, differential leucocyte count, NLR, MLR, PLR, total cholesterol, LDL cholesterol, total cholesterol/HDL ratio, HbA1c, and IL-6; (ii) HbA1c in T2DM ASCVD groups were correlated significantly and positively with leucocytes, segmented neutrophils, NLR, PLR, total cholesterol, LDL, total cholesterol/HDL ratio, and IL-6; HbA1c was correlated significantly and negatively with eosinophils, lymphocytes, monocytes, and absolute lymphocyte count; (iii) Monocytes, MLR, leucocytes, eosinophils, and absolute monocyte count were found to be a strong predictors for T2DM ACS.
In our study, RDW was found to be lower in T2DM ACS, who also had an elevated HbA1c level. Meanwhile, RDW was higher in T2DM HR and VHR with lower HbA1c levels (Table 1). Similar to our findings, the REACTION study also demonstrated that increased RDW was associated with optimal glycemic targets in T2DM.13 Alamri et al.,14 also demonstrated that RDW was found to be negatively correlated with being outside the glycemic target range. Increased RDW could be the result of chronic inflammation, which affects erythropoiesis and decreases the deformability and half-life of red blood cells (RBCs). T2DM and atherosclerosis have a strong association as they share some deleterious mechanisms, including hyperglycemia, dyslipidemia with higher atherogenic LDL, increased oxidative stress, and high-level inflammation.15 Under conditions of excessive reactive oxygen species, higher lipoprotein oxidation heightens inflammation and cardiovascular complications.16 Increased oxidative stress leads the erythrocyte to suffer higher eryptosis, which affects erythrocyte deformability and threatens microcirculation.17 Excessive glucose in T2DM increases viscosity and impairs erythrocyte mobility. The effects of glucose adhesiveness in erythrocytes triggers more clumping that generates higher friction for erythrocytes, leading to erythrocyte damage and the production of debris that leads to sedimentation and vascular plaque.18 An atherosclerosis-induced inflammatory state triggers an increased level of RDW due to inadequate erythropoiesis, which produces immature and large erythrocytes and slows erythrocyte clearance.19 Higher RDW in STEMI patients has been associated with increased in-hospital mortality.20 Increased RDW has also been found to be associated with increased major adverse cardiovascular events (MACEs) in patients with ACS.21 Meanwhile, lower RDW has been linked to a lower risk of cardiovascular mortality and MACEs following ACS.19
Our study demonstrated increased levels of total and differential leucocytes (Table 1) and HbA1c was also found to be significantly positively correlated with leucocytes and segmented neutrophils (Table 2). An increased leucocyte count in diabetes with cardiovascular disease was also found in the EPIC-NL cohort study. Lassale et al.,11 revealed that a higher leucocyte count could be beneficial for identifying future CVD risk. Narjis et al.,22 also demonstrated that an increased leukocyte count was significantly correlated with instability of glycemic levels in diabetes. Kawabe et al.,23 demonstrated that a higher leucocyte count was a predictor for AMI, heart failure (HF), and all-cause death in patients with T2DM with coronary artery disease (CAD). Increased leucocytes disrupt the capillary blood vessels due to their higher viscosity, larger diameter, and larger volume, causing endothelial cell impairment. A higher leucocyte count is associated with increased MACEs. Classification and regression tree (CART) analysis displayed leucocyte count as one of the measurements that determine increased MACE risk in patients with ACS.
Increased neutrophils and higher NLR in our study was correlated with glycometabolic status (Table 2). Increased NLR in T2DM with CAD was also demonstrated by Qiao et al.,24 Increased NLR was an independent predictor for survival in patients with T2DM and CAD. He et al.,25 also found that poor prognosis in patients with T2DM was associated with increased neutrophil count and HbA1c. Increased neutrophil activation leads to atherosclerotic plaque destabilization and higher platelet activation and P-selectin expression that trigger thrombus formation. Pro-inflammatory cytokines and catecholamines produced in the ACS in response to hyperglycemia reduces lymphocyte production and tissue distribution, resulting in lymphocyte apoptosis. A higher NLR with hyperglycemia increases the progression of diabetic complications and causes more severe and complex coronary lesions.
Our study found that eosinophils were decreased in T2DM ASCVD (Table 1) and HbA1c was significantly negatively correlated with circulating eosinophils, lymphocytes, and monocytes (Table 2). Zhu et al.,26 also found a similar finding that the peripheral eosinophil count was inversely correlated with insulin resistance and diabetes. Eosinophils’ movements from the circulating artery into adipose tissue enhances the production of IL-4 and IL-13. Increased IL-4 and IL-13 levels enhance glucose control and suppress inflammatory gene expression. Gao et al.,27 demonstrated that lower percentages of eosinophils in leukocytes (PELs) was strongly related with acute coronary arterial thrombotic event and severe CAD. The reduction of eosinophil and lymphocyte counts were associated with higher HF incidence in the short term.28 Eosinophils drive the formation of atherosclerotic plaque and increased thrombosis. The interaction between eosinophils with platelets leads to the development of eosinophil extracellular traps, which heighten the stability of thrombus.29 Guner et al.,30 also demonstrated that lower eosinophil count in STEMI patients on admission was associated with increased MACE. Reduced eosinophil count in circulation soon after AMI was due to massive migration and progressive infiltration of blood eosinophils into the infarcted myocardium. Increased Eotaxin-1 (eosinophil-specific chemoattractant) after MI mediates the trafficking and infiltration of eosinophils into the infarcted area. Along with increased eosinophil cationic protein, eosinophil activation and degranulation play important roles in pro-coagulant states that trigger edema, microvascular obstruction, and necrosis.31 Increased eosinophils in infarcted myocardium provides beneficial functions. Eosinophils generate IL-4 production and cationic protein mEar1 that reduce cardiomyocyte death, fibroblast activation, and neutrophil adhesion.32
In our study, the monocyte percentage in T2DM ACS was lower than that in T2DM HR and VHR; however, the leucocyte count was found to be higher in T2DM ACS (Table 1). As a result, the absolute monocyte count (calculated as leucocyte count multiplied by monocyte percentage) was also higher in T2DM ACS. Our findings revealed that monocyte count was similar between T2DM ASCVD groups and HbA1c was inversely correlated with monocyte count (Table 2). According to Reijrink et al.,33 the monocyte count in patients with diabetes and CAD was not significantly different from that of healthy subjects. However, patients with T2DM have an altered monocyte subset with lower non-classical monocytes and higher monocytes expressing proangiogenic tunica intima endothelial kinase 2 (Tie2). People with T2DM also have higher levels of angiopoietin-2, which produces proinflammatory mediators and increases adhesion molecules. Increased proangiogenic monocytes and angiopoietin-2 in T2DM have contributed to plaque vulnerability in T2DM. Marsh et al.,34 found that reduced non-classical monocytes in pre-reperfusion time up to 90 minutes post-percutaneous coronary intervention (PCI) correlated with extensive infarct size and decreased left ventricular ejection fraction (LVEF) in STEMI patients. However, due to splenic and bone marrow release, monocyte populations increased again 24 hours after reperfusion.
Our results demonstrated that all T2DM ASCVD groups had MLR >0.25 (Table 1). According to Ji et al.,35 MLR with cut-off value >0.25 has a prediction value for more severe coronary lesions. Increased MLR is strongly linked with more intense coronary stenosis and more severe lesions in CAD. Monocyte activation leads to macrophage differentiation and the production of proinflammatory cytokines, ROS, and matrix metalloproteinase, which are important for plaque development and plaque rupture. Zhai et al.,36 demonstrated that MLR has been shown to be associated with the severity and prognosis of cardiovascular disease. Increased MLR has a strong association with cardiac intensive care unit (CICU) in-hospital mortality. Lower lymphocyte count in cardiovascular disease is the result of dysregulated immune responses that trigger lymphocyte apoptosis. Increased monocyte count has been linked with the severity of inflammatory-mediated atherosclerosis and the extent of MI.36 MLR is a more specific and efficient method of determining the severity of a coronary artery lesion. Increased MLR is associated with higher levels of inflammatory reactions, a higher degree of coronary stenosis, and an increased risk of atherosclerotic plaque rupture. MLR provides a more specified and coherent evaluation to determine coronary lesion severity and to predict MACE.37 MLR has also served as an independent predictor for mortality in patients with CAD after PCI. Higher MLR has been found to be strongly correlated with enhanced cardiac mortality and major adverse cardiovascular and cerebrovascular events (MACCEs) in patients with CAD after PCI.38
Our study showed that PLR was significantly higher in T2DM ACS (Table 1) and significantly positively correlated with HbA1c (Table 2). A similar finding was also displayed by Hudzik et al.,39 who found that PLR was a good predictor for late and in-hospital mortality in STEMI patients with diabetes. According to Atak et al.,40 increased PLR in patients with diabetes was significantly positively correlated with HbA1c. Platelets contribute to increased blood clot formation and enhanced inflammatory responses, while lymphocytes assist in controlling inflammatory-related pathways. Higher PLR is associated with worse cardiovascular outcomes in STEMI and LV dysfunction patients.41 Hyperglycemia has a huge impact on platelets. Hyperglycemia has induced higher platelet activation and increased platelet-immune cell aggregation that contributes to the release of inflammatory cytokines and chemokines, hastening the progression of ASCVD in T2DM.42 Hyperglycemia alters the level and glycosylation pattern of platelets. Higher glycosylation levels lead to increased platelet reactivity and heightens thrombosis risk in T2DM with CVD.43 Increased PLR has been found in severe atherosclerosis CAD patients. PLR reflects a higher plaque burden and correlates with atherosclerosis severity in ACS or CAD patients.44 PLR was also found as a reliable indicator for CAD severity predictor. PLR is closely linked with higher CRP level, which suggests that increased PLR reflects heightened inflammation and a higher prothrombotic state.45 PLR serves as a more economical marker to predict worsened Functional Capacity (FC) after PCI in patients with stable CAD.46
In our study, level of total cholesterol (TC), LDL, and TC/HDL ratio were significantly different between groups, with higher levels in patients with T2DM ACS (Table 1). TC, LDL, and TC/HDL ratios were positively correlated with HbA1c (Table 2). Hussain et al.,47 also found significant correlations between HbA1c, TC, and LDL. Patients with glycemic levels outside the target range (HbA1c > 7.0%) had significantly increased levels of TTC and LDL. A study by Artha et al.,10 also demonstrated that LDL-C increased in higher HbA1c levels. Long-term hyperglycemia with insulin resistance triggers higher levels of very low-density lipoproteins (VLDL) and ApoC-III production and increased chylomicrons absorption in the gastrointestinal tract. Chylomicron or VLDL then exchange into LDL cholesteryl esters via cholesteryl-ester-transfer-protein (CETP), which generates small dense LDL. Elevated LDL and triglycerides are associated factors that contribute significantly to cardiovascular disease progression in T2DM.48 TC/HDL ratio was a valuable predictor that should be considered for high-risk patients with T2DM ASCVD. Patient with lower LDL-C but higher TC/HDL ratio were found to be at higher risk of ASCVD progression.49 Another study on patients who underwent surgical coronary revascularization has shown that increased LDL-C levels significantly predicted future cardiac events after coronary artery surgical revascularization.50
Our results demonstrated that IL-6 was increased significantly in T2DM ACS (Table 1) and correlated significantly with HbA1c (Table 2). Increased IL-6 levels early after STEMI are reflecting a condition of bigger infarct size and deteriorated cardiac functions.51 Akujuru et al.,52 found increased NLR and IL-6 in patients with T2DM. IL-6 has been linked to the development of diabetic complications by suppressing glycogen synthesis and insulin-mediated lipogenesis, lowering GLUT4 expression, and disrupting glucose transport. T2DM also triggers constant neutrophil activation that severely affected insulin resistance. Phapale et al.,53 demonstrated that higher high-sensitivity C-reactive protein (hsCRP), IL-6, TNF-α, and HbA1c in patients with diabetes and CAD was the result of increased cytokine production by monocytes/macrophages that exaggerated β-cell injury. Patients with unstable glycemic levels experience higher stenosis severity. In CVD, IL-6 is strongly linked with carotid plaque formation and development. IL-6 is secreted by immune cells such as monocytes and macrophages, and a higher level of IL-6 is correlated with an enhanced risk of CVD.54
The logistic regression analysis demonstrated that monocyte count was a risk factor for T2DM ACS (Table 3). Yang et al.,55 also found similar findings that circulating monocyte count was an independent mortality predictor in patients with T2DM with macrovascular complications. Edgar et al.,56 revealed that hyperglycemic conditions have induced “trained immunity” in macrophages and hematopoietic stem cells (HSCs), which subsequently aggravated atherosclerosis progression. Hyperglycemia triggers aerobic glycolysis, which affects chromatin accessibility and leads to higher RUNX1 binding sites. RUNX1 was found to be important in diabetic atherogenesis-regulating processes. Thiem et al.,57 also demonstrated that hyperglycemic conditions have generated a “trained phenotype” in monocytes. Immunological memory in monocytes promotes inflammation and is involved in diabetes associated complications through epigenetic regulation.
Our study showed that T2DM ASCVD groups have significantly different levels of RDW, total leucocyte count, differential leucocyte count, absolute lymphocyte count, absolute monocyte count, NLR, MLR, PLR, total cholesterol, LDL, HDL/total cholesterol ratio, HbA1c, and IL-6. T2DM ASCVD groups also demonstrated that HbA1c was correlated significantly with leucocytes, segmented neutrophils, NLR, PLR, total cholesterol, LDL, total cholesterol/HDL ratio, and IL-6, eosinophils, lymphocytes, monocytes, and absolute lymphocyte count. Logistic regression analysis demonstrated that monocytes have the strongest predictive value for T2DM ACS. Different levels of total leucocyte count and differential leucocyte count not only have been implicated in stable ASCVD and progression to ACS in T2DM but could also be used as beneficial predictors for T2DM progression. Hematology examinations could provide affordable and valuable markers to predict atherosclerosis and inflammatory progression in T2DM ASCVD.
Our single-centered cross-sectional study had a relatively small number of patients with T2DM ASCVD. However, this study showed the importance of hematology examination and hematology-derived inflammatory biomarkers, which demonstrated the alteration of the CBC, lipid profile, and inflammatory cytokines in a different type of ASCVD, including new ACS STEMI, ASCVD VHR, and ASCVD HR. In the future, we suggest that more T2DM ASCVD studies could be conducted together with other cardiovascular centers, and could gain more data not only from ACS STEMI but also from other types of ACS to enhance data from other T2DM cardiovascular complications.
Figshare: Untitled Item. https://doi.org/10.6084/m9.figshare.22191907. 58
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)