Keywords
Colorectal cancers, Chemotherapy, Surgery, Liver metastases, Regression, Histology, Prognosis, Survival
Pathological tumor regression after neoadjuvant chemotherapy is a major prognostic factor in colorectal cancer liver metastases (CRCLM). However, the comparative predictive value of existing histological response scoring systems remains unclear.
We retrospectively reviewed 70 patients with stage IV CRCLM who underwent liver resection after neoadjuvant chemotherapy between 2015 and 2021. Pathological response was assessed using two scoring systems: the five-tier Tumor Regression Grade (TRG) by Rubbia-Brandt and the three-tier system proposed by Blazer. Survival analyses were performed using Kaplan–Meier curves, log-rank tests, and Cox regression. Prognostic performance was evaluated through likelihood ratio χ2 tests, trend tests, and area under the ROC curve (AUC) with 95% confidence intervals (CI).
The median follow-up was 32 months. Median overall survival was 40.1 months in patients with TRG 1–2, 32.1 months for TRG 3, and 18.5 months for TRG 4–5 (p = 0.03). The Rubbia-Brandt TRG score showed good prognostic discrimination with an AUC of 0.80 (95% CI: 0.69–0.90). In contrast, the Blazer score showed no statistically significant survival difference among categories (p = 0.26) and yielded a lower AUC of 0.60 (95% CI: 0.48–0.73). Both the likelihood ratio χ2 (7.41 vs. 2.68) and trend test (p = 0.03 vs. p = 0.09) favored the Rubbia-Brandt system.
In this series, the Rubbia-Brandt TRG score demonstrated superior prognostic performance compared to the Blazer score for assessing survival after liver resection for CRCLM. These findings support the integration of pathological response scoring into postoperative risk stratification, in combination with classical staging elements. Further validation in larger prospective cohorts is warranted.
Colorectal cancers, Chemotherapy, Surgery, Liver metastases, Regression, Histology, Prognosis, Survival
This revised version of the article includes several key revisions aimed at enhancing clarity and the clinical relevance of findings. First, we clarified the definitions and categorization criteria of the Rubbia-Brandt and Blazer scoring systems, emphasizing their structural differences and implications for interpretation. Second, the Results section now includes updated survival data with clearer stratification by TRG subgroups and corresponding median overall survival estimates. Third, the Discussion has been expanded to provide a more comprehensive interpretation of the prognostic implications of the two grading systems, incorporating relevant literature to contextualize the findings. Finally, the Conclusions have been refined to more directly highlight the clinical value of the Rubbia-Brandt score in risk stratification and postoperative management. These revisions improve the rigor and accessibility of the manuscript for both clinical and academic audiences.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Colorectal cancer (CRC) ranks third globally in terms of the most commonly diagnosed cancer and second as the leading cause of cancer-related deaths.1 CRC progression typically involves metastatic spread, with the liver being the most frequently affected site. Synchronous liver metastases (LM) occur in approximately 20% of patients, while nearly 50% will develop them during the course of their illness.2 Surgical resection is considered the most effective treatment option for LM, but only a subset of patients are candidates for resection, depending on factors such as tumor size, number, location, and liver function. Neoadjuvant chemotherapy (CT) significantly improves the prognosis of resectable cases and can make initially unresectable lesions amenable to surgery.3 The pathological response of LM to neoadjuvant therapy is a crucial prognostic factor for recurrence and survival. The Rubbia-Brandt et al. score4 was one of the earliest established to evaluate tumor response to therapy, and other scores, such as the one proposed by Blazer et al., have also been suggested.5 However, no standardized scoring system currently exists, and studies comparing the performance of different scores are still lacking. This study aims to assess tumor response in liver resection specimens histologically, based on the Rubbia-Brandt and Blazer scores after neoadjuvant treatment, and compare the prognostic performance of these two scoring systems.
The Ethics Committee for Mongi Slim Hospital La Marsa has examined the study and protocol of the following project: “Liver Metastases From Colorectal Carcinoma: Performance of Pathological Response Scores”. It was made and presented by Dr. MALLEK Ines (Department of anatomopathology). The project does not raise any particular ethical problem.
The ethics approval was given prior to the start of the study in 2021, in French, as Tunisia is a mainly French speaking country. An updated ethics approval was also provided in English for the purposes of the f1000 submission.
The approval was registered under number 43/2021 in French version and 27/2023 in English version. Participated in this meeting: PR Lamia BEN JEMAA, Ethics Committee President Mongi Slim Hospital of Marsa and PR Mohamed Sami MEBAZAA.
Study design: This study was a retrospective, and longitudinal analysis of a single-center series of patients with CRCLM who underwent surgery after neo-adjuvant treatment. All cases were collected from the department of Pathology of the University hospital in north Tunisia (Hospital Mongi Slim) between January 2015 and June 2021.
Study population: We included patients who met the following criteria: Diagnosis of CRCLM and underwent surgical treatment after neo-adjuvant CT and availability of a detailed anatomical-pathological report. We excluded patients who: Had cancer in another organ, underwent surgery without neo-adjuvant CT. Additionally, patients whose hospital records were unusable or could not be found, and cases with non-usable slides or tissue blocks were excluded from the study. Patients who received other neo-adjuvant treatments (hepatic intra-arterial CT or percutaneous radiofrequency) were also excluded.
Data collection: We collected epidemiological, clinical, and biological data, as well as information on primary CRC, CRCLM, types of neo-adjuvant therapy initiated, type of surgical procedure performed, follow-up, and outcome for all patients included in the study.
Pathological study: Based on the pathology report, we recorded the location, number, and size of the CRCLM. All slides were reviewed by two senior pathologists. We assessed the degree of tumor response or Tumor Regression Grading (TRG) according to: The Rubbia-Brandt score4 and the Blazer score.5 We also recorded the following tumor characteristics: The state of the resection margins (R0 if safe or R1 if less than 1mm), the presence or absence of vascular emboli, and endobiliary extension.
Statistical analysis: The data was entered using SPSS® version 24.0. Descriptive and analytical studies were conducted. Mortality was assessed by actuarial survival curves using the Kaplan Meier model. The comparison of survival curves was performed by the Log Rank test. The performance of each score was assessed by the following criteria: homogeneity, monotonicity, and discriminatory capacity. The significance level was set at 0.05.6,7
We included 70 patients in our study. 48 were male (69%), giving a sex ratio (males/females) of 2.2. The average age of the patients was 56 years. In 54 cases (77%) it was a colon cancer, in 16 cases (23%) rectal cancer. In 64 cases (92%), it was adenocarcinoma without other specifications (SAI). We found five cases of mucinous adenocarcinoma (7%), and only one case of adenosquamous carcinoma. The SAI adenocarcinomas were all low grade. The most frequent stage at diagnosis was stage IV with synchronous metastases, all of which were in the liver (40 cases, 57%). All patients underwent carcinological surgical resection according to the initial tumour site.
Time to onset of liver metastases: Forty patients (57%) had at least one synchronous LM. Thirty patients (43%) had metachronous LM. The mean time to onset of metachronous LM was 10 months.
Neo-adjuvant treatment modalities: Of our patients, 47 received CT alone (67%) and 23 received CT plus targeted therapy (33%). The most represented CT regimen was FOLFOX (Folinic acid + 5FU + Oxaliplatin), used in 63 patients (90%). The average number of courses administered was estimated at 6 courses (extremes between 2 and 12 courses).
Surgical treatment of liver metastases: CRCLMs were bi-lobar in 63% of cases (44 cases) and uni-lobar in 37% (26 cases). Anatomical hepatectomy (lobectomy/segmentectomy) was performed in 28 cases (40%) and non-anatomical (metastasectomy or wedge resection) in 42 cases (60%).
Pathological characteristics of liver metastases: The average number of LMs was three (extremes of 1 to 12 per patient) and the mean lesion size was 25 mm (range 2-130 mm). The LMs were in 43% (30 cases) in the right lobe and in 20% (14 cases) in the left lobe. They were bi-lobar in 37% of cases (26 cases). According to Rubbia-Brandt score, the CRCLMs were classified into TRG 1 in eight cases (11%), TRG 2 in eight cases (11%), TRG 3 in 17 cases (24%), TRG 4 in 30 cases (43%), TRG 5 in seven cases (10%). And according to Blazer, five patients (7%) had a complete pathological response, 34 patients (49%) had a minor response and 31 (44%) had a major response. The resection margins of LM were R0 in 59 resection pieces (77%) and invaded (R1) in 11 cases (16%). We also found vascular emboli in six patients (9%). Only one patient had endo-biliary extension. Moreover, Six of the 45 patients had lymph node metastases (Figures 2 and 3).
Microscopic examination of the liver parenchyma remote from the CRCLMs showed the presence of chemo-induced lesions in 42 patients (60%): 18 cases (26%) of vascular lesions (sinusoidal obstruction syndrome), 13 cases of steatosis (19%), one case of steatohepatitis and 10 cases with associated lesions (14%).
Overall survival for all stages was 85.5% at 12 months, 41.7% at 24 months and 19.3% at 36 months. There was a significant difference in survival between the different grades for Rubbia- Brandt TRG (p=0.03) but not for Blazer TRG (p=0.269). For Rubbia-Brandt TRG, the median survival was better in the case of a major response (TRG 1/TRG 2) assessed at 40.1 and 41.1 months after the initial diagnosis. In the case of partial response (TRG 3), the median survival was 32.1 months. In cases of no response (TRG 4/TRG 5), survival was estimated at 29.9 and 18.5 months. For Blazer, the median survival was greater for complete response, estimated at 41.1 months after initial diagnosis. For the major response group, survival was estimated at 38.2 months. For the minor or no response group, survival was 29.3 months. When discussing homogeneity, the likelihood ratio χ2 (LR) for The Rubbia- Brandt TRG had the highest LR+. Rubbia-Brandt has a score of 10.953 and Blazer has a score of 7.246. The RV+ of the Rubbia-Brandt score was greater than 10, so it is a score with very strong diagnostic contribution. The RV+ of the Blazer score was between 5 and 10, so it is a score with strong diagnostic input. When looking at monotonicity with the linear trend χ2, of the two scores, the Rubbia-Brandt TRG had the highest linearity value. Rubbia-Brandt has a score of 10.738 and Blazer has a score of 4.446. Looking at the Discriminatory capacity, we can see a sensitivity and specificity of scores for survival prediction. The graphical representation of the predictive capacity of each score for survival is the AUC of the ROC curve is as follows the Figure 1. The Rubbia-Brandt score was a good performing score as its AUC under the ROC curve was 0.8. The Blazer score was a poorly performing score as its AUC under the ROC curve was 0.6. The Rubbia-Brandt TRG score was better at predicting survival than the Blazer score (p=0.003).
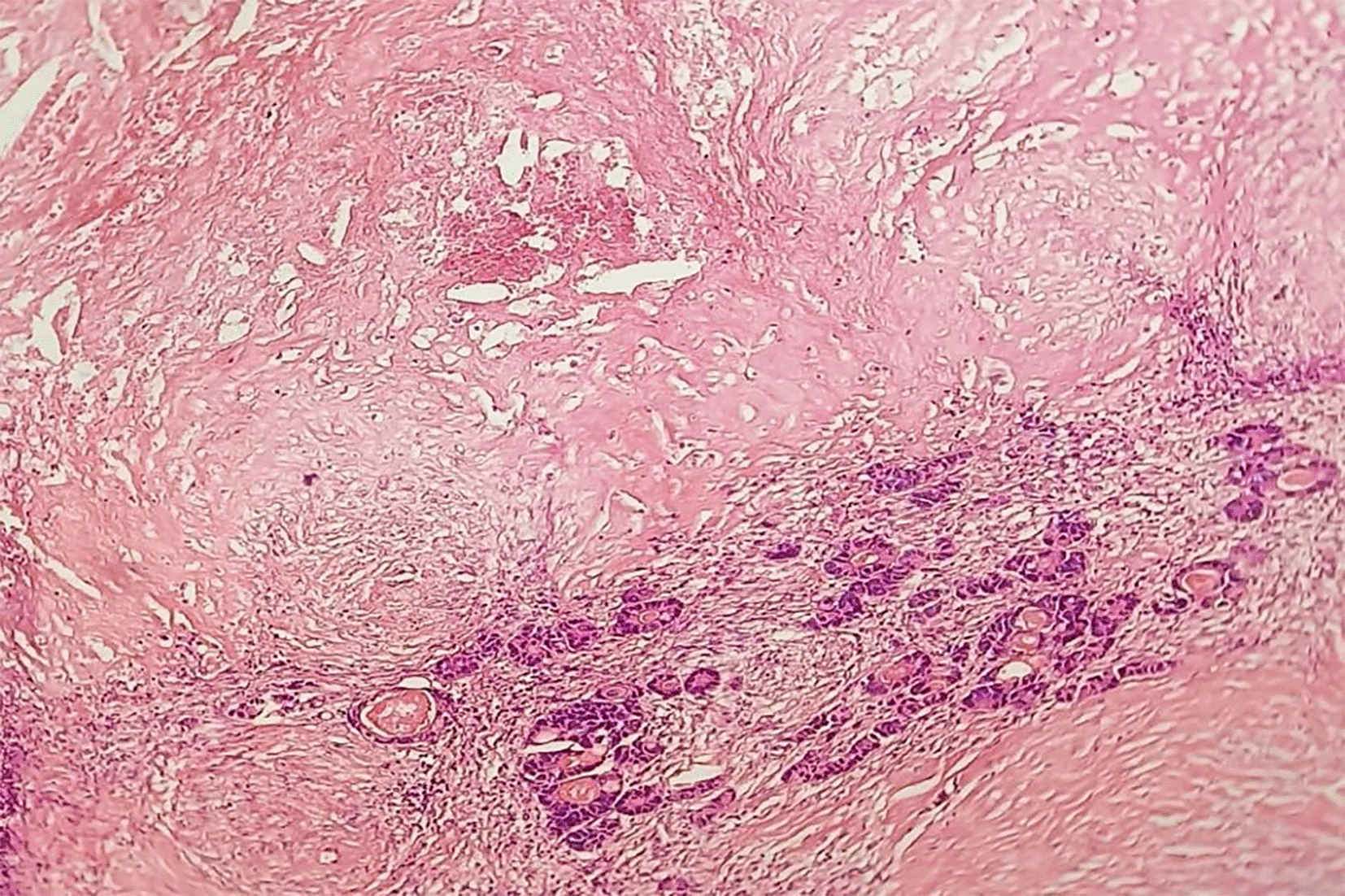
Predominant fibrosis with scattered residual tumour cells: According to Rubbia-Brandt, partial response (TRG 3) and according to Blazer, this is a major pathological response (presence of 1 to 49% residual tumour cells) (HEx20).
In this retrospective study of 70 patients with colorectal cancer liver metastases (CRCLM), the average age was 56 years with a male-to-female ratio of 2.2. A total of 57% of patients presented with synchronous liver metastases (LM) at diagnosis. Pathological response was assessed using two scoring systems: the five-tier Rubbia-Brandt Tumor Regression Grade (TRG) and the three-tier Blazer score. According to the Rubbia-Brandt TRG, 11% of metastases were classified as TRG 1, 11% as TRG 2, 24% as TRG 3, 40% as TRG 4, and 10% as TRG 5. Using the Blazer score, 7% of cases had a complete pathological response, 44% showed a major response, and 49% a minor response. Overall survival was 85.5% at 12 months, 41.7% at 24 months, and 19.3% at 36 months. As expected, patients with a major or complete pathological response had longer median survival than those with partial or no response. The Rubbia-Brandt TRG demonstrated superior predictive performance for survival, with an area under the curve (AUC) of 0.8 on ROC analysis, compared to 0.6 for the Blazer score. It also exhibited higher diagnostic contribution (RV+ >10) and better linearity (10.73). These results suggest that the Rubbia-Brandt TRG, through its detailed five-level assessment based on tumor cell regression and fibrosis, may be more accurate in reflecting treatment response and correlating with survival.
Limitations: This study presents several limitations. Its retrospective design, combined with incomplete clinical data and treatment heterogeneity, may have influenced therapeutic responses and survival outcomes. The absence of inter-observer variability assessment for both TRG systems is another important limitation.
Prognostic factors in CRCLM: Multiple prognostic variables are known to impact outcomes in CRCLM. These include the timing of LM occurrence, response to neoadjuvant chemotherapy (CT), number and size of metastases (tumors >10 cm are associated with poor prognosis)8, and quality of surgical resection.9 Histopathological invasion factors also correlate with reduced overall survival.10,11 While radiological assessment using RECIST criteria remains standard,12 studies show it does not always align with survival, particularly after targeted therapy with cytostatic effects.13 In contrast, pathological evaluation of the resection specimen more accurately reflects systemic therapy response.13,14 Additionally, chemotherapy-induced liver injury significantly affects prognosis.3
Rubbia-Brandt TRG system: First proposed in 2006, the Rubbia-Brandt score4 was adapted from Mandard’s system12 for rectal and esophageal cancers. It defines five TRG categories based on the ratio of residual tumor cells to fibrosis: TRG1 (complete fibrosis), TRG2 (predominant fibrosis with rare tumor cells), TRG3 (fibrosis with more tumor cells), TRG4 (predominant tumor cells), and TRG5 (no fibrosis, only tumor). Necrosis is excluded, as it may reflect spontaneous tumor degeneration rather than chemotherapy-induced regression. In the original study,4 patients receiving neoadjuvant CT had significantly better pathological regression than those undergoing surgery alone (p < 0.0001). Five-year survival was 41% for TRG 1–2, 38% for TRG 3, and only 9% for TRG 4–5. In our cohort, we observed similar TRG distributions and confirmed the prognostic value of near-complete and partial responses. Importantly, this system aligns with the American Joint Committee on Cancer (AJCC) recommendations for primary tumors, enabling direct comparison between primary CRC and metastases. Nonetheless, TRG2 remains imprecise due to the subjective notion of “rare residual tumor cells.” Introducing defined percentage thresholds could improve reproducibility.15 Moreover, using two parameters (tumor cells and fibrosis) may complicate routine implementation. Alternative approaches like the PRG (Pathological Response Grade), which relies solely on the percentage of viable tumor cells, have shown prognostic utility16 but require further validation. Another limitation is the lack of integration of modern targeted therapies in the original Rubbia-Brandt study. Agents like bevacizumab or cetuximab, widely used today, may alter tumor response patterns.17
Blazer score: In 2008, Blazer et al.5 proposed a simplified three-tier regression system based on residual viable tumor cells: complete response (0%), major response (1–49%), and minor response (≥50%). The score averages responses across multiple metastases, which may dilute heterogeneity. In our study, complete response was observed in 7% of patients, major in 44%, and minor in 49%. Blazer et al. reported 5-year survival rates of 75%, 56%, and 33% for complete, major, and minor responders, respectively. While we observed better survival in complete responders, statistical differences between groups were not significant. This score, while simple, has limitations. Its reliance on estimating initial tumor area introduces variability, and its 50% threshold may lack sensitivity. Additionally, fibrosis or necrosis can occur in untreated tumors, complicating interpretation.4
Performance of the scores: No prior studies have directly compared the performance of pathological regression scores for CRCLM. Our findings suggest that the Rubbia-Brandt TRG outperforms the Blazer score in prognostic accuracy. With an AUC of 0.8, higher RV+, and greater linearity, it offers more granularity in assessing tumor regression and survival correlation.18 However, both systems present challenges, especially in interpreting intermediate categories. The “almost complete regression” category is subjective. TRG 3, defined as “residual tumor cells scattered in fibrotic tissue”,4 overlaps with the Blazer “major response” (<50% viable tumor),5 yet their clinical implications may differ. Importantly, neither score accounts adequately for intra-tumoral heterogeneity. The Rubbia-Brandt system considers only the worst lesion, while the Blazer score averages across all metastases. Evidence suggests that the presence of at least one LM with complete regression is associated with improved prognosis.19,20 Thus, reporting individual TRG scores for each lesion may enhance prognostic precision.
Perspectives and recommendations: A robust and standardised pathological response system is essential for evaluating resected CRCLM. Future prospective studies with large cohorts should aim to harmonise macroscopic sampling protocols, assess inter-observer reproducibility, and integrate TRG with other histopathological variables in predictive algorithms. Given its superior performance in our study, the Rubbia-Brandt TRG should be incorporated into routine pathology reports for resected CRCLMs following neoadjuvant therapy. Used alongside ypTN staging, it provides valuable prognostic information and could inform clinical decision-making.21 Multicentric studies are warranted to validate these findings and further refine pathological assessment strategies.
In conclusion, surgical resection remains the gold standard treatment for CRCLM, and the prognosis is significantly improved with the use of neoadjuvant chemotherapy (CT). Pathological response to neo-adjuvant therapy is a crucial prognostic factor correlated with recurrence and survival. The Rubbia Brandt TRG system can complement the ypTN stage and other pathological criteria to improve the predictivity of survival.
Figshare. Data Liver Metastases From Colorectal Carcinoma: Performance Of Pathological Response Scores. DOI: https://doi.org/10.6084/m9.figshare.23620656.v1.21
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC BY 4.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: HBP-surgery
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Trialist investigating pre-operative and adjuvant therapy in CRCLM resection surgery patients
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Harrell F: Evaluating the Yield of Medical Tests. JAMA: The Journal of the American Medical Association. 1982; 247 (18). Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: medical image analysis, colorectal liver metastases
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
References
1. Cervantes A, Adam R, Roselló S, Arnold D, et al.: Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up.Ann Oncol. 2023; 34 (1): 10-32 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: clinical epidemiology, transplantation, liver cancer, surgery
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, et al.: Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery.Ann Oncol. 2007; 18 (2): 299-304 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Pathology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
|
Version 3 (revision) 29 Aug 25 |
read | ||||
|
Version 2 (revision) 29 Nov 24 |
read | read | |||
|
Version 1 28 Nov 23 |
read | read | |||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)