Keywords
Colorectal cancers, Chemotherapy, Surgery, Liver metastases, Regression, Histology, Prognosis, Survival
Pathological response of liver metastases (LM) from colorectal carcinoma (CRC) to neoadjuvant therapy is a known prognostic factor associated with recurrence and survival. The aim of this study was to compare the performance of two prognostic scores in patients who underwent surgery for colorectal cancer liver metastases (CRCLM), specifically in stage IV disease.
We conducted a study on patients with stage IV colorectal cancer who received preoperative chemotherapy (CT) followed by liver metastasis (LM) resection between 2015 and 2021. Among these patients, 57% had synchronous metastases (diagnosed at the same time as the primary tumor), while the remaining cases were metachronous (diagnosed after the primary tumor). Pathological response was evaluated using both the Rubbia-Brandt tumor regression grade (TRG) and the Blazer scoring system. We then assessed the performance of these two prognostic scores based on homogeneity (using the likelihood ratio, LR+), monotonicity, and discriminative ability (using the area under the receiver operating characteristic [ROC] curve, AUC).
70 cases were included in the study. Mean age was 56 years. The sex ratio (males/females) was 2.2. Forty patients were stage IV (57%) with synchronous all CRCLMs. The overall survival, all stages combined, was 85.5% at 12 months, 41.7% at 24 months and 19.3% at 36 months. The median survival was better in case of major response (TRG1/TRG2) evaluated at 40.1 and 41.1 months after diagnosis. In cases of partial response (TRG3), the median survival was 32.1 months. In cases with no response (TRG4/TRG5), survival was estimated at 29.9 and 18.5 months. The Rubbia-Brandt TRG had the highest LR+ (10.95). The LR+ of the Rubbia-Brandt score was greater than 10, so it was a test with very strong contribution. The LR+ of the Blazer score was between 5 and 10, it was a test with strong contribution. The Rubbia-Brandt TRG had the highest linearity value (10.73). With a higher AUC of the ROC curve (0.8), the Rubbia-Brandt TRG was better at predicting survival than the Blazer score.
Surgical resection is the gold standard for CRCLM, with improved prognosis from neoadjuvant chemotherapy. Pathological response to CT is a key prognostic factor, and the Rubbia Brandt TRG system enhances survival predictivity when combined with ypTN stage.
Colorectal cancers, Chemotherapy, Surgery, Liver metastases, Regression, Histology, Prognosis, Survival
In this revised version of the manuscript, several key clarifications and modifications were made to enhance clarity and precision.
First, the sex ratio now includes a required comparator, as this information cannot be inferred without assumptions. Additionally, the percentage of males (or alternatively females) has been provided for clearer interpretation.
In the abstract, both the background and methods sections were updated to clarify that all patients are stage IV, specifying the breakdown between synchronous and metachronous cases, and removing the ambiguous phrase "all stages combined."
For survival data, the term “median survival” has replaced “mean survival” where applicable to better reflect the data. Conclusions were also added to the abstract to summarize findings effectively.
Moreover, data normality has been assessed, as per prior feedback, and the manuscript now reflects this adjustment.
Figures 1 and 2 were corrected to resolve a previous mix-up, and two additional figures were included, one of which is a Kaplan-Meier curve stratified by regression grades, offering a visual representation of survival data.
Lastly, references were updated to ensure accuracy and relevance to the manuscript.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Colorectal cancer (CRC) ranks third globally in terms of the most commonly diagnosed cancer and second as the leading cause of cancer-related deaths.1 CRC progression typically involves metastatic spread, with the liver being the most frequently affected site. Synchronous liver metastases (LM) occur in approximately 20% of patients, while nearly 50% will develop them during the course of their illness.2 Surgical resection is considered the most effective treatment option for LM, but only a subset of patients are candidates for resection, depending on factors such as tumor size, number, location, and liver function. Neoadjuvant chemotherapy (CT) significantly improves the prognosis of resectable cases and can make initially unresectable lesions amenable to surgery.3 The pathological response of LM to neoadjuvant therapy is a crucial prognostic factor for recurrence and survival. The Rubbia-Brandt et al. score4 was one of the earliest established to evaluate tumor response to therapy, and other scores, such as the one proposed by Blazer et al., have also been suggested.5 However, no standardized scoring system currently exists, and studies comparing the performance of different scores are still lacking. This study aims to assess tumor response in liver resection specimens histologically, based on the Rubbia-Brandt and Blazer scores after neoadjuvant treatment, and compare the prognostic performance of these two scoring systems.
The Ethics Committee for Mongi Slim Hospital La Marsa has examined the study and protocol of the following project: “Liver Metastases From Colorectal Carcinoma: Performance of Pathological Response Scores”. It was made and presented by Dr. MALLEK Ines (Department of anatomopathology). The project does not raise any particular ethical problem.
The ethics approval was given prior to the start of the study in 2021, in French, as Tunisia is a mainly French speaking country. An updated ethics approval was also provided in English for the purposes of the f1000 submission.
The approval was registered under number 43/2021 in French version and 27/2023 in English version. Participated in this meeting: PR Lamia BEN JEMAA, Ethics Committee President Mongi Slim Hospital of Marsa and PR Mohamed Sami MEBAZAA.
Study design: This study was a retrospective, and longitudinal analysis of a single-center series of patients with CRCLM who underwent surgery after neo-adjuvant treatment. All cases were collected from the department of Pathology of the University hospital in north Tunisia (Hospital Mongi Slim) between January 2015 and June 2021.
Study population: We included patients who met the following criteria: Diagnosis of CRCLM and underwent surgical treatment after neo-adjuvant CT and availability of a detailed anatomical-pathological report. We excluded patients who: Had cancer in another organ, underwent surgery without neo-adjuvant CT. Additionally, patients whose hospital records were unusable or could not be found, and cases with non-usable slides or tissue blocks were excluded from the study. Patients who received other neo-adjuvant treatments (hepatic intra-arterial CT or percutaneous radiofrequency) were also excluded.
Data collection: We collected epidemiological, clinical, and biological data, as well as information on primary CRC, CRCLM, types of neo-adjuvant therapy initiated, type of surgical procedure performed, follow-up, and outcome for all patients included in the study.
Pathological study: Based on the pathology report, we recorded the location, number, and size of the CRCLM. All slides were reviewed by two senior pathologists. We assessed the degree of tumor response or Tumor Regression Grading (TRG) according to: The Rubbia-Brandt score4 and the Blazer score.5 We also recorded the following tumor characteristics: The state of the resection margins (R0 if safe or R1 if less than 1mm), the presence or absence of vascular emboli, and endobiliary extension.
Statistical analysis: The data was entered using SPSS® version 24.0. Descriptive and analytical studies were conducted. Mortality was assessed by actuarial survival curves using the Kaplan Meier model. The comparison of survival curves was performed by the Log Rank test. The performance of each score was assessed by the following criteria: homogeneity, monotonicity, and discriminatory capacity. The significance level was set at 0.05.6,7
We included 70 patients in our study. 48 were male (69%), giving a sex ratio (males/females) of 2.2. The average age of the patients was 56 years. In 54 cases (77%) it was a colon cancer, in 16 cases (23%) rectal cancer. In 64 cases (92%), it was adenocarcinoma without other specifications (SAI). We found five cases of mucinous adenocarcinoma (7%), and only one case of adenosquamous carcinoma. The SAI adenocarcinomas were all low grade. The most frequent stage at diagnosis was stage IV with synchronous metastases, all of which were in the liver (40 cases, 57%). All patients underwent carcinological surgical resection according to the initial tumour site.
Time to onset of liver metastases: Forty patients (57%) had at least one synchronous LM. Thirty patients (43%) had metachronous LM. The mean time to onset of metachronous LM was 10 months.
Neo-adjuvant treatment modalities: Of our patients, 47 received CT alone (67%) and 23 received CT plus targeted therapy (33%). The most represented CT regimen was FOLFOX (Folinic acid + 5FU + Oxaliplatin), used in 63 patients (90%). The average number of courses administered was estimated at 6 courses (extremes between 2 and 12 courses).
Surgical treatment of liver metastases: CRCLMs were bi-lobar in 63% of cases (44 cases) and uni-lobar in 37% (26 cases). Anatomical hepatectomy (lobectomy/segmentectomy) was performed in 28 cases (40%) and non-anatomical (metastasectomy or wedge resection) in 42 cases (60%).
Pathological characteristics of liver metastases: The average number of LMs was three (extremes of 1 to 12 per patient) and the mean lesion size was 25 mm (range 2-130 mm). The LMs were in 43% (30 cases) in the right lobe and in 20% (14 cases) in the left lobe. They were bi-lobar in 37% of cases (26 cases). According to Rubbia-Brandt score, the CRCLMs were classified into TRG 1 in eight cases (11%), TRG 2 in eight cases (11%), TRG 3 in 17 cases (24%), TRG 4 in 30 cases (43%), TRG 5 in seven cases (10%). And according to Blazer, five patients (7%) had a complete pathological response, 34 patients (49%) had a minor response and 31 (44%) had a major response. The resection margins of LM were R0 in 59 resection pieces (77%) and invaded (R1) in 11 cases (16%). We also found vascular emboli in six patients (9%). Only one patient had endo-biliary extension. Moreover, Six of the 45 patients had lymph node metastases.
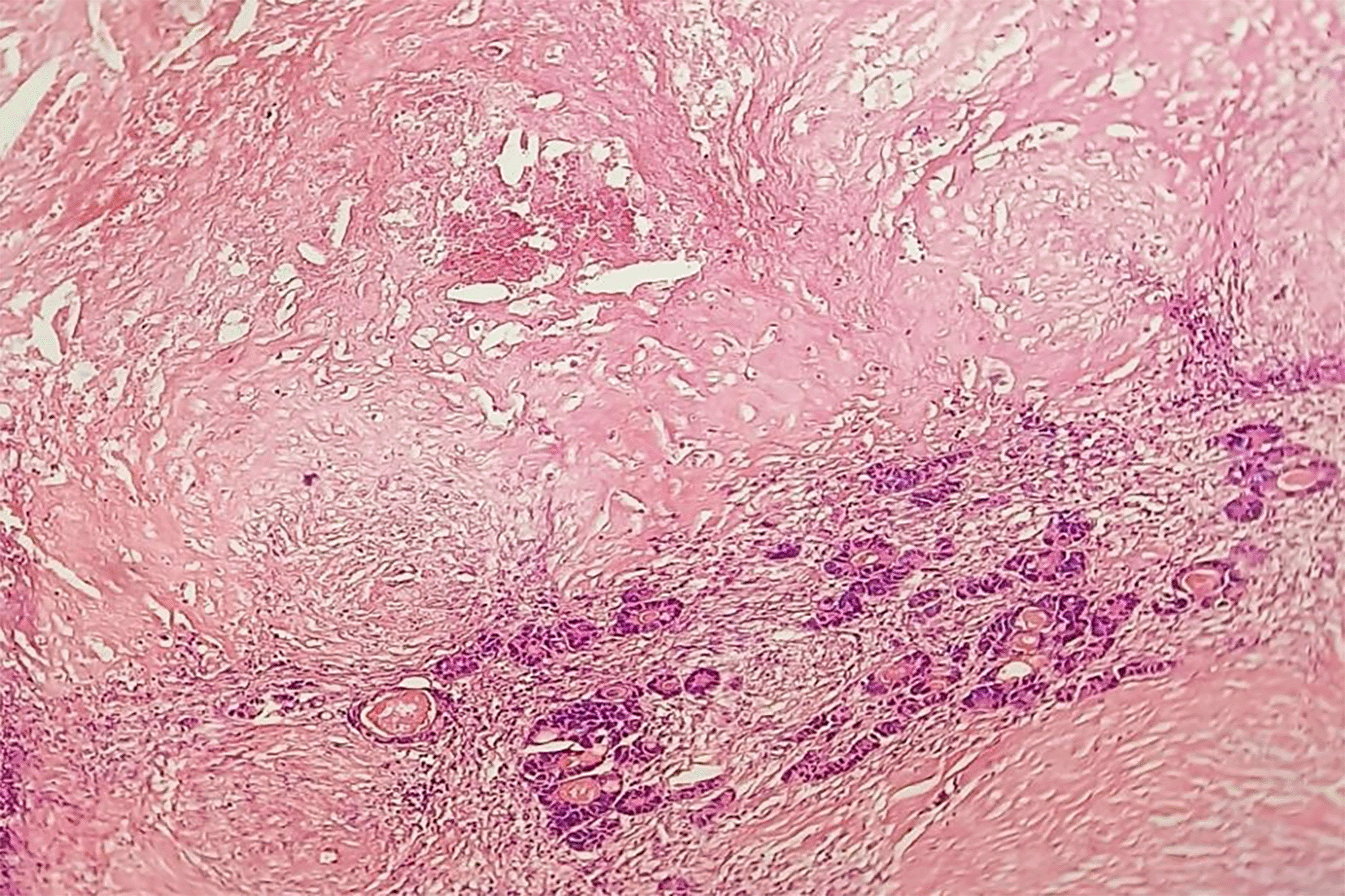
Predominant fibrosis with scattered residual tumour cells: According to Rubbia-Brandt, partial response (TRG 3) and according to Blazer, this is a major pathological response (presence of 1 to 49% residual tumour cells) (HEx20).
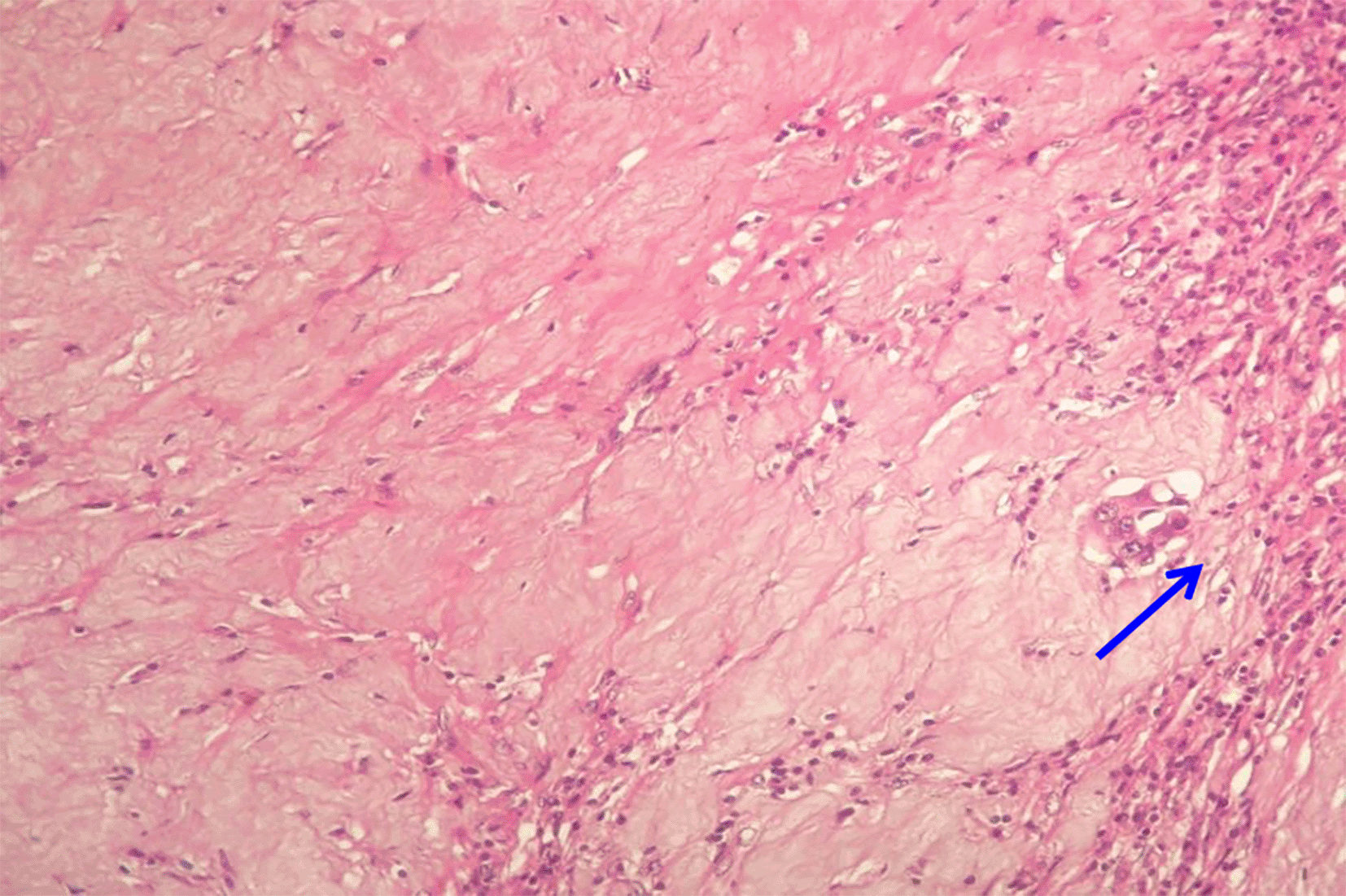
Abundant fibrosis and rare residual carcinomatous structures (arrow). According to Blazer, this is a major pathological response and TRG2 according to Rubbia-Brandt (HEx25).
Microscopic examination of the liver parenchyma remote from the CRCLMs showed the presence of chemo-induced lesions in 42 patients (60%): 18 cases (26%) of vascular lesions (sinusoidal obstruction syndrome), 13 cases of steatosis (19%), one case of steatohepatitis and 10 cases with associated lesions (14%).
Overall survival for all stages was 85.5% at 12 months, 41.7% at 24 months and 19.3% at 36 months. There was a significant difference in survival between the different grades for Rubbia- Brandt TRG (p=0.03) but not for Blazer TRG (p=0.269). For Rubbia-Brandt TRG, the median survival was better in the case of a major response (TRG 1/TRG 2) assessed at 40.1 and 41.1 months after the initial diagnosis. In the case of partial response (TRG 3), the median survival was 32.1 months. In cases of no response (TRG 4/TRG 5), survival was estimated at 29.9 and 18.5 months. For Blazer, the median survival was greater for complete response, estimated at 41.1 months after initial diagnosis. For the major response group, survival was estimated at 38.2 months. For the minor or no response group, survival was 29.3 months. When discussing homogeneity, the likelihood ratio χ2 (LR) for The Rubbia- Brandt TRG had the highest LR+. Rubbia-Brandt has a score of 10.953 and Blazer has a score of 7.246. The RV+ of the Rubbia-Brandt score was greater than 10, so it is a score with very strong diagnostic contribution. The RV+ of the Blazer score was between 5 and 10, so it is a score with strong diagnostic input. When looking at monotonicity with the linear trend χ2, of the two scores, the Rubbia-Brandt TRG had the highest linearity value. Rubbia-Brandt has a score of 10.738 and Blazer has a score of 4.446. Looking at the Discriminatory capacity, we can see a sensitivity and specificity of scores for survival prediction. The graphical representation of the predictive capacity of each score for survival is the AUC of the ROC curve is as follows the Figure 1. The Rubbia-Brandt score was a good performing score as its AUC under the ROC curve was 0.8. The Blazer score was a poorly performing score as its AUC under the ROC curve was 0.6. The Rubbia-Brandt TRG score was better at predicting survival than the Blazer score (p=0.003).
In this study, 70 patients were included with an average age of 56 years and a sex ratio (males/females) of 2.2. 57% of patients were stage IV at diagnosis with synchronous LM. The Rubbia-Brandt TRG and the Blazer score were used to classify patients' pathological responses. The Rubbia-Brandt TRG classified 11% of LMs as TRG 1, 11% as TRG 2, 24% as TRG 3, 40% as TRG 5, and 10% as TRG 5. The Blazer score showed that 7% of CRCLMs had a complete pathological response, 49% had a minor response, and 44% had a major response. The overall survival rate for all stages was 85.5% at 12 months, 41.7% at 24 months, and 19.3% at 36 months. Patients who had a major response had better median survival at 40.1 and 41.1 months, while patients with partial response had a median survival of 32.1 months. Patients with no response had a survival of 29.9 and 18.5 months. The Rubbia-Brandt TRG score had a higher RV+ value and linearity value compared to the Blazer score. The Rubbia-Brandt TRG score also performed better in predicting survival with an AUC of 0.8, while the Blazer score had an AUC of 0.6. The study concluded that the Rubbia-Brandt score is a good tool for assessing pathological response of CRCLM after neoadjuvant therapy and can be used in daily practice.
The retrospective nature of the study with some incomplete clinical or complementary data, and heterogeneity in treatment protocols may have affected the therapeutic response, the results of both scores and the prognosis of patients. The inter-observer variability for each of the two scores was not studied.
Preoperative factors such as the time of onset of LM and the impact of neoadjuvant CT on overall patient survival were found to be significant factors. The number and size of CRCLMs were also important, with recent studies showing that tumors larger than 10 cm had a poor prognosis.8 The quality of surgical resection was found to be an important prognostic factor.9 Pathological factors of invasion were also found to be associated with a significant reduction in overall survival [68,69]. Tumor regression after neo-adjuvant treatment was assessed using the Response Evaluation Criteria in Solid Tumors radiology system [73]. However, it was shown that better survival is not always associated with a good radiological response, especially after targeted therapy whose mechanism of action is mainly cytostatic rather than cytotoxic [72]. Pathological response on the surgical specimen was found to be an accurate means of assessing response to systemic therapy.10,11 The presence of systemic treatment-induced liver injury was also found to have an important prognostic impact.3
In 2006, Rubbia Brandt and colleagues elaborated the first pathological response score for CRCLM4 based on Mandard's12 score for oesophageal and rectal cancers. The study included 525 CRCLM resected from 181 patients comparing a group of 112 patients who received different neo-adjuvant CT regimens (5FU, FOLFOX, FOLFIRI and FOLFOXIRI) versus a control group of 69 patients who underwent surgical resection only. Assessment of pathological tumour regression was scored by two independent pathologists using a semi-quantitative five-category system, which assesses the relative proportion of tumour cells and fibrosis. Thus, the Rubbia-Brandt TRG was as follows: TRG1: Extensive fibrosis, no tumour cells, TRG 2: Abundant fibrosis interspersed with few residual tumour cells, TRG 3: Predominantly fibrosis with more residual tumour cells, TRG 4: Predominantly tumour cells over fibrosis, TRG5: Tumour cells without fibrosis. This score did not include necrosis, as it would be a rather spontaneous phenomenon related to the tumour and not to the cytotoxic effects of CT. In the series studied by Rubbia-Brandt et al,4 pathological regression was significantly better (p<0.0001) in patients who received neo-adjuvant CT compared to the control group (surgical treatment only). In our study, eight LMs (11%) were classified as TRG1, eight LMs were classified as TRG2 (11%), 17 LMs were classified as TRG3 (24%), 30 LMs were classified as TRG4 (40%) and finally seven LMs (10%) were classified as TRG5.
The prognostic value of a “partial response” would be important. Indeed, several meta- analyses have argued that near-complete regression has a better prognosis than partial regression, as applied in gastro-oesophageal cancers.14,15 This system has the advantage of being congruent on the system used for primary CRC, recommended by the American Joint Committee. This allows easy comparison of pathological regression between the primary and metastatic sites. In the original study by Rubbia-Brandt et al,4 the five-year overall survival was 41. The rate of response in patients with LM classified as TRG 1-2 and 38% for LM with TRG 3, compared to 9% in patients with LM without pathological response TRG 4-5. Overall survival at 5 years was significantly better in LM with complete or near- complete response (TRG 1-2) as well as in LM with partial response (TRG3) compared to LM without response (p = 0.0003 and p =0.0019 respectively).
There is an individualisation of a 'complete response' group and the system must be designed to identify a ‘strong response’ to CT, to which the ‘complete response’ belongs. Indeed, a difference between the ‘complete response group’ and the ‘low viable cell group’ can be attributed to treatment sensitivity and a lack of sampling on macroscopy. In addition, TRG2 corresponding to the presence of ‘rare’ residual tumour cells would lack precision. Indeed, do ‘rare’ tumour cells correspond to isolated cells or to small groups of tumour cells? A percentage with precise cuts-off to quantify residual tumour cells would be useful to limit this subjectivity and improve reproducibility5 ( Figure 2). Then, the use of two components to develop the TRG: tumour cells and fibrosis. In practice, the use of a single component in the scoring scale should be more widely and easily applicable to clinical practice. This prompted a Canadian team to propose a tumour regression score: Pathological Response Grade (PRG), defined by a single criterion; the percentage of viable tumour cells correlated with survival. Patients with a strong PRG (<10% viable tumour cells in all resected lesions) had significantly better survival and significantly longer recurrence-free survival (an independent predictor of survival).16 Nevertheless, larger series would be needed to prospectively validate this system. Finally, the treatments used in the Rubbia-Brandt et al. study only take into account the use of FOLFOX and FOLFIRI protocols without bevacizumab and cetuximab. Targeted therapies have been shown to be intrinsically effective.17
In 2008, Blazer et al. conducted a retrospective study of 305 patients who received induction the therapy (FOLFOX or FOLFIRI, with or without bevacizumab) followed by CRCLM resection. The pathological regression study was semi- quantitative based on the percentage of viable tumour cells remaining. The score is made up of three groups: Complete pathological response: absence of residual tumour cells, Major pathological response: presence of 1 to 49% residual tumour cells, Minor pathological response: presence of more than 50% residual tumour cells.
Patients without pathological regression (100% residual tumour cells) are included in the minor response group. If multiple metastases exist, the pathological response is the average of the scores of the individual tumour nodules. Of our 70 patients, five patients (7%) had a complete pathological response, 34 patients (49%) had a minor response and 31 (44%) had a major response. Overall survival was significantly different between the three response groups.
In the original study by Blazer et al,5 the 5-year overall survival was 75% for complete responders and 56% for major responders, compared to 33% for minor responders.
The 5-year overall survival was significantly better in patients with a complete response compared to those with a major response (p=0.037). The difference in overall survival between patients with a major response and those with a minor response was also significant (p=0.028).
In our study, the complete pathological response group had the best survival but no significant difference with the other two groups.
The Blazer score has several limitations. Firstly, subjectivity and inter-observer variability have been widely reported,13 indicating inconsistent interpretations among different observers. Secondly, the score relies on estimating the initial tumor area, which can be challenging to determine accurately. Thirdly, when identifying the ‘major response’ group, it considers the presence of a lower percentage of viable tumor cells as the defining factor. However, the threshold of 50% viable tumor cells seems less specific as a marker for a pathological response to CT, especially considering that necrosis or fibrosis can be seen in LM without any CT treatment.4
An ideal grading system should assess the therapeutic response and prognosis in a reproducible and accurate way. To date, the optimal score for assessing pathological response remains a matter of debate. To our knowledge, no study has compared the performance of pathological regression scores of operated RCCs. We studied the predictive capacity of the two scores for survival based on the ROC curve with AUC measurement. We found that the Rubbia Brandt ROC system was 0.8, higher than the Blazer score which was 0.6. Thus, the Rubbia-Brandt TRG is a score with a better performance. Similarly, the homogeneity and linear capacity of the two regression scores were studied. We found that the RV+ Rubbia Brandt score4 was higher than 10. It is therefore a test with a very strong contribution. The Blazer score5 had an RV+ between 5 and 10. It is therefore a test with a strong contribution. Of the two scores, the Rubbia-Brandt TRG had the highest linearity value (10.73). Although both scores were predictive of median survival, the Rubbia-Brandt TRG system performed better in our study. It is a more detailed five-level score, which looks at the response of the tumour to the treatment given, reflected by pathological changes, especially fibrosis. This is significantly correlated with better survival.18 The three-level Blazer score would be more stable in terms of consistency but the addition of levels in the score could provide additional prognostic information. We note an important limitation between these two systems. This is the category ‘almost complete regression’ which can be very subjective. Indeed, TRG 3 according to Rubbia-Brandt has been defined as “Presence of residual tumour cells scattered within predominantly fibrous territories”.4 This category is equivalent to “major regression, <50% residual tumour cells” according to the Blazer score5 ( Figure 3). Rubbia-Brandt and Blazer systems may be inaccurate in predicting prognosis, especially in N+ or R1 patients. Consideration of regional N status and margin status could improve the prognostic ability of these grading systems that assess LM only. These criteria must be taken into account and reported in the pathology reports. A final point is that these two scores do not seem to take into account intra-tumour heterogeneity. Indeed, for patients with multiple LM, the highest TRG is taken into account, which does not reflect the heterogeneity observed among metastatic lesions. The TRG of Rubbia-Brandt thus ignores the regression status of all but the worst of the tumours.19 Blazer's score considers the average response of all tumours, whereas there is evidence that at least one of several LMs with complete pathological response is associated with a better prognosis.20 This highlights that intra-tumour response variations should not be neglected. Thus, detailing each score for each LM seems more accurate.
A strong and valid pathological regression system will be integral to establishing a standard in the analysis of resected CRCLM. Prospective studies with large cohorts are needed to ensure standardisation of macroscopic management protocols, determine the reliability and inter-observer variability of any monitoring pathological system notation, to accurately define and validate the prognostic value of a pathological regression score, integrate pathological regression systems and other pathological parameters into a standardised treatment algorithm for CRCLM.
A difficult aspect of conducting a multi-institutional study will be the standardisation of treatment. According to our results, the use of the Rubbia Brandt TRG should be integrated into the daily practice of pathologists for a better management of CRCLM liver resection specimens after neo-adjuvant therapy. Its predictive value for survival has been widely proven.
The Rubbia Brandt TRG system can complement the post neoadjuvant pTN stage (ypTN) and other pathological criteria that have a strong correlation with survival. Their combination is thus more predictive of survival. It would be interesting to complete our work with prospective, larger sample, multicentre studies, taking into account the clinical, radiological and evolutionary data of the patients.
In conclusion, surgical resection remains the gold standard treatment for CRCLM, and the prognosis is significantly improved with the use of neoadjuvant chemotherapy (CT). Pathological response to neo-adjuvant therapy is a crucial prognostic factor correlated with recurrence and survival. The Rubbia Brandt TRG system can complement the ypTN stage and other pathological criteria to improve the predictivity of survival.
Figshare. Data Liver Metastases From Colorectal Carcinoma: Performance Of Pathological Response Scores. DOI: https://doi.org/10.6084/m9.figshare.23620656.v1.21
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC BY 4.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Trialist investigating pre-operative and adjuvant therapy in CRCLM resection surgery patients
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Harrell F: Evaluating the Yield of Medical Tests. JAMA: The Journal of the American Medical Association. 1982; 247 (18). Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: medical image analysis, colorectal liver metastases
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
References
1. Cervantes A, Adam R, Roselló S, Arnold D, et al.: Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up.Ann Oncol. 2023; 34 (1): 10-32 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: clinical epidemiology, transplantation, liver cancer, surgery
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, et al.: Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery.Ann Oncol. 2007; 18 (2): 299-304 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Pathology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
|
Version 3 (revision) 29 Aug 25 |
read | ||||
|
Version 2 (revision) 29 Nov 24 |
read | read | |||
|
Version 1 28 Nov 23 |
read | read | |||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)