Keywords
breast cancer, postmastectomy radiation, scar boost radiation, locoregional control, overall survival
breast cancer, postmastectomy radiation, scar boost radiation, locoregional control, overall survival
In Thailand, breast cancer is the most common cancer among women, accounting for 22.8% of all cancer incidences per year (estimated 22,158 new cases in 2020). It is the third most common cancer overall in the country, with 37.8 cases per 100,000 population.1 The main treatment for breast cancer is surgery, with breast-conservative surgery and mastectomy being the two main surgical techniques. Adjuvant treatments include radiotherapy and systemic therapy, and most breast cancer patients receive combined modality treatments.
According to many prospective trials and meta-analysis, for the patients who underwent mastectomy, postmastectomy radiotherapy (PMRT) as an adjuvant treatment has shown benefits in terms of chest wall local control (LC), locoregional control (LRC), and overall survival (OS).2–9
The National Comprehensive Cancer Network (NCCN) Clinical Practice Guideline in Oncology for Breast Cancer recommends PMRT after total mastectomy for patients with certain risk factors, including tumor size greater than 5 cm, margins less than 1 mm, positive margins, lymph node positivity, and multiple high-risk features such as central/medial tumors or tumors greater than 2 cm with either grade 3, ER-negative, or lymphovascular invasion (LVI). The recommended radiation dose is 45-50.4 Gy at 1.8-2.0 Gy/fraction, with alternative doses of 40 Gy at 2.67 Gy/fraction or 42.5 Gy at 2.66 Gy/fraction for patients not undergoing breast reconstruction. Scar boost radiation, delivered using electrons or photons, may be added for an additional 10-16 Gy at 1.8-2.0 Gy/fraction, with or without bolus.10
Even with PMRT, 5-15% of patients still encountered local recurrence (LR) in the chest wall.2–6 A scar boost radiation is often considered in high risk patients such as those at an advanced stage, with LVI, and positive margin to potentially improve chest wall LR.11 In real-world clinical practice, the use of scar boost radiation is often seen. A survey conducted in South Korea indicated that 45.7% of the respondents routinely use scar boost radiation after PMRT.12
However, the benefits of scar boost radiation following PMRT have not been evaluated in prospective trials. Only a limited number of retrospective studies have been conducted with a few studies showing improvement in chest wall LC11,13 while the others did not show additional benefits of scar boost radiation.14–17
This study aims to determine the benefit of scar boost radiation on chest wall LC in post-mastectomy breast cancer patients, as well as to identify risk factors for chest wall LR and locoregional recurrence (LRR). Additionally, our study will compare LR, LRR, OS, and radiation toxicities between patients who received scar boost radiation and those who did not.
This study was granted ethical approval by the Khon Kaen University Ethics Committee for Human Research on 20 December 2021 (Reference No. HE641644). Patient informed consent was waived by the ethics committee.
In this retrospective study, the medical records of female patients diagnosed with invasive ductal or lobular carcinoma of the breast who underwent PMRT after total mastectomy at Srinagarind Hospital between January 1st, 2012, and January 31st, 2015, were reviewed. Patients who had received prior PMRT, experienced chest wall recurrence during treatment, or had insufficient data for analysis in their medical records were excluded from the study. Clinical, pathological, surgical and radiotherapy data were collected and managed using REDCap electronic data capture tools hosted at Khon Kaen University.18,19
The following formulae were used to calculate for required sample size in this study.20
Where, N = sample size
E = number of events
= event proportion of control (no scar boost group)
= event proportion of treatment (scar boost group)
r = number of treatment per 1 control
Zα = 1.96
Zβ = 0.84
According to the study by Panoff et al.,11 the 60-month rate of LR was 5.7% in scar boost group and 12.7% in no scar boost group. These results were used for calculation. The initial sample size was N = 490. With the estimation of 10% loss to follow-up patients, the adjusted sample size was N = 546.
The primary outcome was chest wall LC, which was assessed as the absence of chest wall LR, as evidenced by clinical, pathological, or imaging findings. PMRT was defined as radiation to the chest wall administered following total mastectomy which can be either conventional or hypofractionated schedule. Scar boost radiation was defined as additional radiation delivered to chest wall scar area after PMRT which can be either electron or photon. The secondary outcomes were the factors associated with LR, LRR and OS.
Statistical analysis was performed using Fisher’s exact test and Wilcoxon rank-sum test to compare categorical and continuous variables, respectively. Variables that were associated with a p-value of less than or equal to 0.05 on univariate analysis were incorporated into a Cox proportional hazard model for multivariate analysis, using the backward stepwise method. The relative risk of tumor recurrence was calculated for each variable with a 95% confidence interval, and a p-value less than or equal to 0.05 was considered statistically significant. The LR, LRR and OS rates were estimated using Kaplan-Meier analysis, and the equality of Kaplan-Meier curves were tested using the log-rank test. All data analyses were performed using R Statistical Software (version 4.0.3; R Core Team 2022).21,22
The medical records of 561 post-mastectomy female patients treated at the Division of Radiation Oncology, Srinagarind Hospital, between 2012-2015 were evaluated.23 Of these, 12 patients were excluded due to prior PMRT, secondary malignancy from pathological diagnosis, recurrent tumors during radiation, or inadequate data in medical records. As a result, 549 patient records were included in the study.
The median follow-up time was 21 months (range 1-128 months), and the median age was 51 years (range 28-87 years). The patients were divided into two groups based on whether they received scar boost (31.51%) or not (68.49%). Tumor size was recorded, with the median across both groups being 4 cm (range 0-15 cm). Pathological type was primarily ductal carcinoma (98.5%), with lobular carcinoma accounting for 1.5%. Histological grading was performed, with 18 patients (3.3%) being grade 1, 245 patients (44.6%) being grade 2, and 214 patients (39.0%) being grade 3. The presence of LVI was recorded as positive in 191 patients (34.8%), negative in 115 patients (20.9%), and unknown in 243 patients (44.3%). The surgical margin was recorded as negative in 467 patients (85.1%), closed in 21 patients (3.8%), and positive in 39 patients (7.1%). The distance from the surgical margin was recorded, with a mean of 0.85 cm (range 0.09-3.5 cm). The location of the cancer was recorded as left in 305 patients (55.6%) and right in 244 patients (44.4%). The median number of metastatic nodes was 4 (range 0-49). The presence of extranodal extension (ENE) was recorded as negative in 222 patients (40.4%), positive in 83 patients (15.1%), and unknown in 244 patients (44.4%). Staging was performed according to the American Joint Committee on Cancer (AJCC) 8th edition. Hormonal status (ER and PR), HER-2 status and Ki-67 were recorded. Surgical mastectomy techniques, surgical axilla staging techniques, the number of nodal dissections, and breast reconstruction were recorded. Patients received either neoadjuvant, perioperative, or adjuvant chemotherapy. Chest wall radiation details, including duration, machine used, technique, bolus presence, dose, and scar boost were also recorded. The baseline characteristics of the patients are summarized in Table 1 and supplementary document.
A total of 84 patients had distant metastasis and 34 patients had LRR, with 7 of them presenting recurrences in the scar area, 3 presenting recurrences outside of the scar area, 10 presenting recurrences in an unspecified chest wall area, and 14 presenting regional node recurrences (as shown in Table 2). The Kaplan-Meier survival analysis showed that the estimated 10-yr chest wall LC rate was 82.14% (95% CI 68.12-99.04%) in scar boost group versus 93.18% (95% CI 89.58-96.92%) in no scar boost group (log-rank test p-value 0.8400), with the estimated 10-yr LRC rate of 76.70% (95% CI 63.13-93.18%) versus 89.76% (95% CI 85.52-94.21%) in scar boost and no scar boost groups respectively (log-rank test p-value 0.6013). Further analysis with Cox proportional hazard model indicated that there was no significant difference in chest wall LR between the no scar boost group and the scar boost group (HR 0.87, 95% CI 0.35-2.19, p-value 0.73) as shown in Figure 1. The median time of chest wall LR was 21 months. With regards to LRR (Figure 2), there was also no significant difference between the scar boost group and the no scar boost group (HR 1.20, 95% CI 0.60-2.37, p-value 0.606).
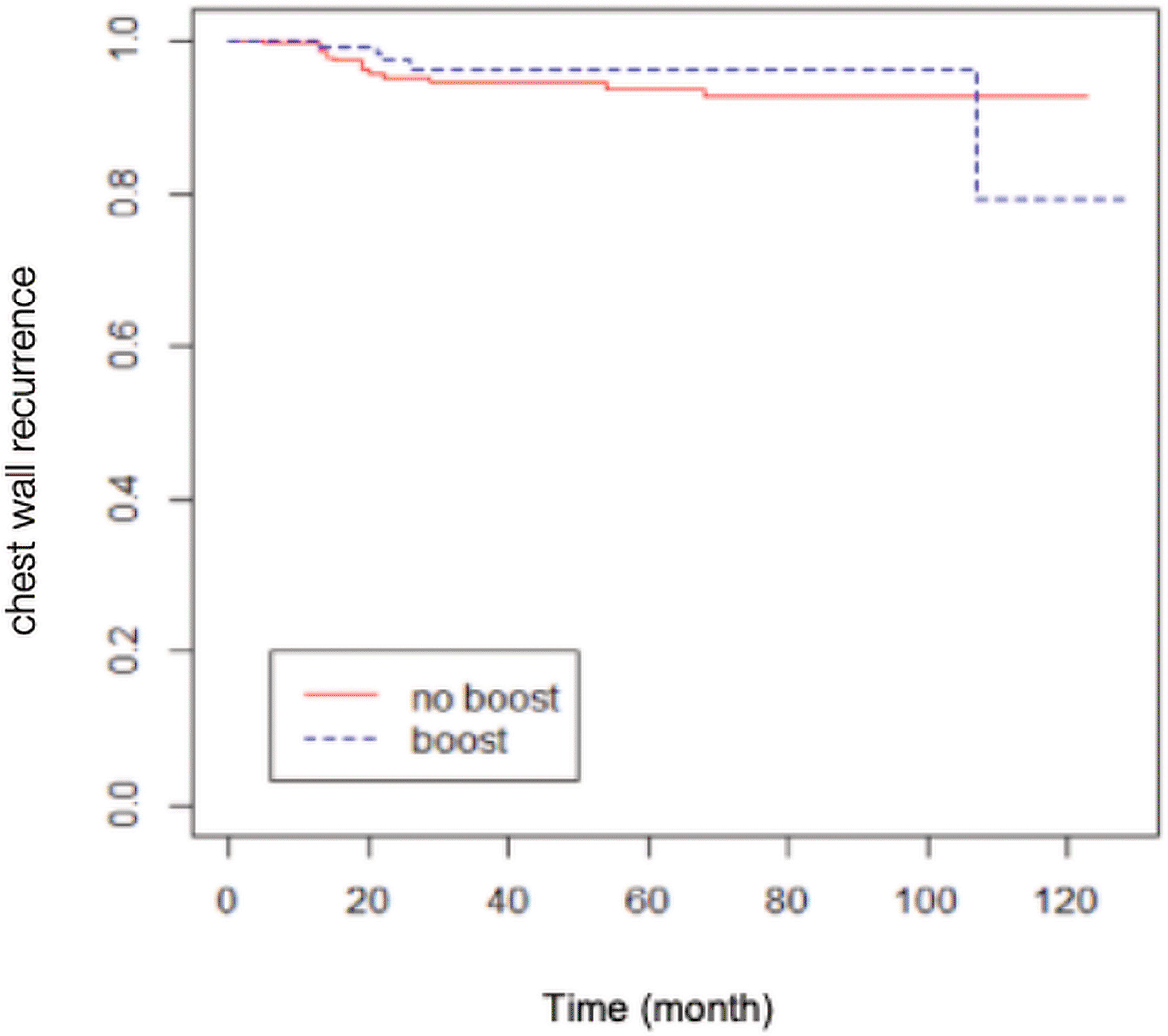
(HR 0.87, 95% CI 0.35-2.18, p-value 0.73).
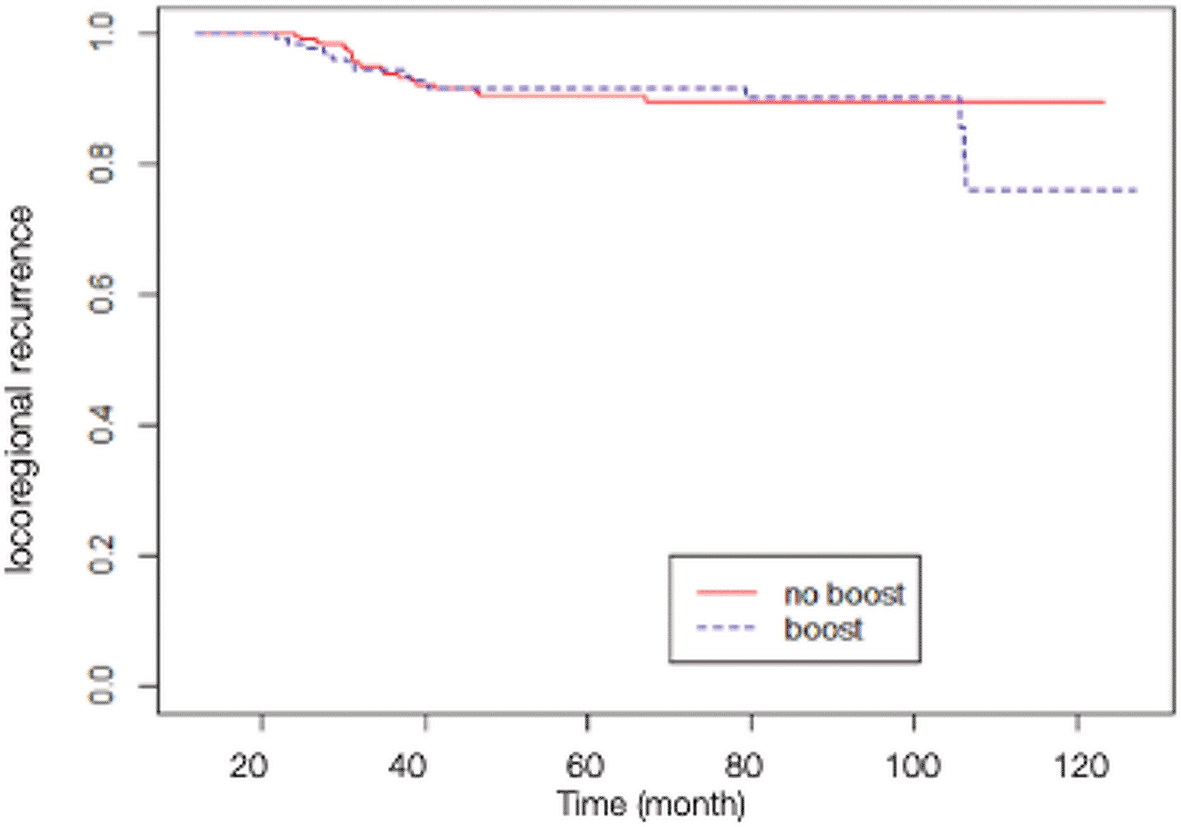
(HR 1.20, 95% CI 0.60-2.37, p-value 0.61).
Regarding survival outcomes, the estimated 10-yr OS rate from the Kaplan-Meier method was 55.65% (95% CI 47.89-64.67%) in scar boost group versus 53.83% (95% CI 48.67-59.54%) in no scar boost group (log-rank test p-value 0.3618). The Cox regression analysis showed no significant difference in OS between the scar boost group and the no scar boost group (HR 1.05, 95% CI 0.09-11.54, p-value 0.97) as shown in Figure 3.
In the subset analysis (Tables 3, 4), multivariate analysis determined that lobular carcinoma type was a significant predictor of both chest wall LR (HR 11.32, 95% CI 3.24-39.54) and LRR (HR 5.65, 95% CI 1.71-18.61). Additional unfavorable factors, including skin invasion and positive supraclavicular nodes, were identified as contributing to an increased risk of LRR (HR 2.80, 95% CI 1.26-6.21 and HR 4.51, 95% CI 1.37-14.79, respectively).
| No recurrence (N = 529) | Recurrence (N = 20) | HR | 95% CI | p-value | |
|---|---|---|---|---|---|
| Histologic type | 11.32 | 3.24-39.54 | <0.001 | ||
| - Ductal carcinoma | 524 (96.86%) | 17 (3.14%) | |||
| - Lobular carcinoma | 5 (62.50%) | 3 (37.50%) |
In terms of complications, the study found that there was a statistically significant higher incidence of grade 1 acute skin toxicity and late skin fibrosis in the scar boost group compared to the no scar boost group (21.39% versus 5.85%, p-value <0.001 and 7.51% versus 1.86%, p-value 0.0095 respectively). Grade 1 radiation pneumonitis was also significantly higher in the scar boost group (9.82% versus 1.86%, p-value <0.001). The complications are summarized in Table 5.
The present study was designed to investigate the benefit of scar boost radiation following PMRT. We aimed to provide a clearer understanding of the impact of scar boost radiation on patient outcomes, and to determine the risk factors for chest wall LR and LRR as well as radiation toxicities. To our knowledge, there were limited evidences regarding scar boost radiation following PMRT in female breast cancer.
A large retrospective cohort of 4747 women from California Cancer Registry conducted in 2014 by Mayadev et al. found no difference in breast cancer survival (BCS) or OS with the addition of chest wall scar boost.14 The results were aligned with our findings that there was no statistically significant difference in 10-yr OS between both groups. This issue may be explained by the fact that the modern systemic therapy plays a more important role in survival benefit. Hence, the addition of local treatment such as scar boost radiation cannot translate into higher OS.
A retrospective analysis by Panoff et al. in 2012 revealed a tendency towards improved LRR in the scar boost group, however, the study did not perform a sub-analysis between chest wall and regional lymph node recurrence.11 Hence, our study aimed to clarify the likelihood of chest wall and regional lymph node recurrence between the no scar boost and scar boost groups, and we found that the recurrence rate was not significantly different in either the chest wall or regional lymph node for both groups. Our finding was consistent with a retrospective study by Shah et al. in 2015 which showed that scar boost did not improve LC.15
Another retrospective study by Abouegylah et al. in 2018 found that patients with nodal metastasis were more likely to experience LRR, however, it did not sub-analyze the location of lymph node metastasis.16 Meanwhile, our study found that supraclavicular lymph node metastasis significantly increased the risk of LRR, while other regional lymph node metastasis was not associated with LRR.
Regarding high-risk features, such as positive margins, closed margins, LVI, T3 or T4 tumors, our study found that these features were not associated with chest wall LR or LRR, which is consistent with the findings of studies by Abouegylah et al., Albert et al. and Naoum et al.13,16,17 Nevertheless, we remarked that a higher proportion of patients with closed margins, positive margins, and T4 tumors received scar boost radiation in our study. Additionally, our study found that the histologic type of lobular carcinoma was associated with increased risk for both chest wall LR and LRR, although the sample size may not have been sufficient for a meaningful calculation.
Moreover, our study showed that scar boost radiation significantly increased the incidence of skin toxicity (grade 1-3), skin fibrosis (grade 1), and radiation pneumonitis (grade 1). This finding is in line with the results of a study by Naoum et al. in 2019, which showed that boost radiation was associated with infection, skin necrosis, and implant exposure in breast reconstruction patients.17
The limitations of this study include its retrospective nature and the fact that it was conducted in a single center, with old pathological reports differing from current standards and missing data in medical records due to aging of documents.
The results of this study indicate that there is no evidence supporting the notion that scar boost radiation following PMRT can improve chest wall LR, LRR and OS in female breast cancer. Patients with skin invasion and supraclavicular lymph node metastasis were found to have an increased risk of LRR, and lobular carcinoma was a risk factor for both chest wall LR and LRR. Scar boost radiation was found to increase the incidence of skin toxicity (grade 1-3), skin fibrosis (grade 1), and radiation pneumonitis (grade 1). It is suggested that omitting scar boost radiation may reduce skin and lung toxicities. Patients with lobular carcinoma may benefit from scar boost radiation. Further prospective studies should be conducted to confirm these results and determine the subset of patients who may derive benefit from scar boost radiation.
Open Science Framework: Benefit of Scar Boost Radiation Following Postmastectomy Radiation in Female Breast Cancer: A Retrospective Analysis, https://doi.org/10.17605/OSF.IO/ZJ6GU. 23
This project contains the following data:
Open Science Framework: Benefit of Scar Boost Radiation Following Postmastectomy Radiation in Female Breast Cancer: A Retrospective Analysis, https://doi.org/10.17605/OSF.IO/ZJ6GU. 23
This project contains the following data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors would like to express our gratitude to Ms. Kaewjai Thepsuthammarat, Biostatistician at Clinical Epidemiology Unit, Faculty of Medicine, Khon Kaen University, for providing valuable biostatistical consultation and conducting the statistical analysis of the study. Her expertise and contributions were essential to the completion and interpretation of the data. The authors appreciate her dedication and commitment to the success of the study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: epidemiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 28 Nov 23 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)