Keywords
Exfoliative Dermatitis, Signal detection, Meropenem, Adverse drug reactions, VigiBase™
Cutaneous adverse drug reactions (CADRs) account for nearly one-third of all adverse drug reactions (ADRs), and severe reactions while they are rare, can dramatically affect patients’ quality of life or even cost them their lives. While clinical trials may prove medicine’s effectiveness, they cannot give a thorough picture of the drug’s safety profile. Spontaneous surveillance and data mining techniques provide a promising complementary technique for post-marketing monitoring to detect safety signals.
The objective of this research was to assess the data obtained and uploaded to VigiBase about adverse medication responses affecting the skin and surrounding structures, with a specific focus on identifying any possible signals linked with Meropenem that are not currently indicated on the medicine label.
A retrospective study involved clinical review and data mining of patients who suffered cutaneous reactions reported to national Pharmacovigilance centers in Iraq and other countries from January 2010 to December 2021; a total of 4,510 reports were found in Iraq and filtered according to several criteria to obtain a safety signal with the most significant impact on public health. To improve the signal quality, all global cases were included in evaluating the detected signal, excluding duplicate and incomplete reports.
In a total of 65 cases that contained Meropenem as the suspected cause of exfoliative dermatitis (ED), only 53 cases met the inclusion criteria. Assessment of the included cases proved the detection of a new unlabeled signal that links Meropenem to ED. The mean affected age was 59 years, and males outnumbered females (30:23). The combination showed to be statistically significant (IC025, 2.961; PRR025, 8.227; ROR025, 8.244), and causality assessment showed a possible relation in more than 70% of the cases.
Spontaneous reporting systems, despite their limitations, are vital to pharmacovigilance systems and the identified signal needs further research.
Exfoliative Dermatitis, Signal detection, Meropenem, Adverse drug reactions, VigiBase™
Skin is the largest organ of the adult human body and serves several essential functions.1,2 Drugs may cause undesirable or unfavorable effects that may limit their use and effectiveness, known as adverse drug reactions (ADRs),3 and the risk of ADRs ranges from almost nothing to severe with substantial financial and quality of life costs.4 One of the most commonly used and general classifications of ADRs is the World Health Organization (WHO) classification, also known as the mnemonic classification.5
Cutaneous adverse reactions (CADRs) are prevalent and account for about 10% to 30% of all ADRs and affect 2-3% of hospitalized patients.6 CADRs can be fatal if sufficient emergency care is not delivered.7 While there are several risk factors for developing CADRs, antibiotics are responsible for 40 to 60% of instances,5,8 and according to the WHO, around 2% of dermatologic CADRs are deemed severe.9 Severe CADRs (SCARs) include Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), erythroderma or exfoliative dermatitis (ED), and drug reaction with eosinophilia and systemic symptoms (DRESS).10
Pharmacovigilance (PV) plays a crucial role in determining the safety profile of medications over their complete life cycle through two primary approaches, spontaneous surveillance and active surveillance.11 While spontaneous reporting systems (SRS) have their limitations, they are vital for the early discovery of novel reactions in terms of their clinical nature, severity, or frequency.12 The WHO utilizes VigiBase as its worldwide database, which relies on the collection of spontaneous reports to facilitate the timely identification of possible safety signals.13
VigiLyze and VigiGrade are a tool and method, respectively, that are integrated with other tools and methods with VigiBase. VigiLyze is an online resource that provides a quick and clear overview of VigiBase, while VigiGrade represents report quality, it provides a completeness score for the amount of clinically relevant information provided in an individual case safety report (ICSR).14–16
A signal emerges from information gathered from one or more sources. It is a hypothesis suggesting a potential causal relationship or a new aspect of a known relationship between an active ingredient and an event or a set of related events. The hypothesis should have enough likelihood of being true to justify further investigation.17,18
Signal management encompasses a series of activities aimed at evaluating potential new or modified risks associated with the utilization of a medicinal product. These activities encompass signal prioritization, which involves ongoing assessment of risk impact; signal detection, which involves qualitative analysis utilizing disparity and case report analysis; signal validation; and signal inspection.19
The objective of this research was to assess the data obtained and uploaded to VigiBase about adverse medication responses affecting the skin and surrounding structures, with a specific focus on identifying any possible signals linked with Meropenem that are not currently indicated on the medicine label.
This manuscript presents a descriptive retrospective study to analyze CADRs in patients from Iraq and other countries to detect new safety signals. VigiLyze, a powerful web-based tool developed by the Uppsala Monitoring Center (UMC) accessed at here, provides easy access to VigiBase with powerful search and analysis features.16 VigiLyze, was utilized to examine drug-event combinations (DECs) and their associated information within the timeframe of 2010 to 2021. Specifically, the program was configured to view the relevant reactions by applying search filters, reaction SOC “Skin and subcutaneous tissue disorders” and the time frame stated above. This allowed for the retrieval of ADR reports that were relevant to the specified 12-year period. The search within VigiLyze returned 4,257,857 global reports after applying the search filters, 4,510 of which are reports documenting cases of patients from Iraq. All relevant ICSRs were downloaded for further analysis. To screen the reports, a primary inclusion criterion was DECs with positive IC025 value, excluding all cases caused by vaccines and medical devices.
The secondary inclusion criteria were:
1. Reports of serious or severe DECs20
2. ICSRs with a completeness ratio higher than 50%
The secondary exclusion criteria were:
1. Duplicate reports
2. Common or known side effects as listed in Summary of Product Characteristics (SmPC)
3. Reports missing important information or incorrect description of the reaction
A detected signal would be validated and assessed using WHO-UMC causality assessment (shown in Table 1),21 Bradford Hill criteria,22 WHO seriousness criteria,23 Schumock and Thornton preventability scale,24 and Hartwig’s Severity Assessment Scale.25
Statistical parameters are calculated and provided by VigiLyze were the starting point, namely information component (IC), proportional reporting ratio (PRR), and reporting odds ratio (ROR).26 IC is a logarithmic measure that reflects the strength of dependency in a DEC.27
To improve signal accuracy, a multi-step filtration approach was employed. Firstly, to reduce false positive signals, the lower band of the 95% confidence interval (CI) of IC (IC025) was utilized, and DECs with IC025 values of negative or 0 were excluded, along with DECs related to vaccines and medical devices (1st filtration). Next, a qualitative analysis was performed, omitting non-severe and expected (known) CADRs (2nd filtration),20 by depending on literature and SmPC of each reaction and active ingredient, respectively, in order to exclude mild and common skin reactions. To enhance signal quality, duplicated reports, and reports with less than 50% completeness ratio (vigiGrade) were excluded manually (3rd filtration).14
Afterwards, the signals that were detected were assessed for their possible implications on public health using a mathematical technique suggested by Waller et al.,28 the formulae are listed in Table 2a and impact categories are in Table 2b.
| Parameter | Formula | Criteria |
|---|---|---|
| Evidence Score (E) | a × b × c | 1 – 9: Weak |
| 10 – 100: Strong | ||
| Public Health Score (P) | d × e × f | 1 – 9: Minor |
| 10 – 100: Major |
| Evidence Score (E) | |||
|---|---|---|---|
| Strong | Weak | ||
| Public Health Score (P) | Major | A | B |
| Minor | C | D | |
Signals that have a substantial influence were subjected to additional validation via the use of the WHO-UMC causality evaluation and the Bradford Hill criteria.29,30 This comprehensive approach aims to refine the identified signals and provide reliable pharmacovigilance and patient safety insights.
As there were only three cases in Iraq for Meropenem–Erythroderma DEC, all global cases were included in the research to avoid bias and minimize signal noise. All steps are summarized in Figure 1.
The Institutional Ethical Committee deemed this study exempt from ethical review due to its reliance on secondary data analysis and absence of direct engagement with human subjects.
The data for the current study was analyzed using descriptive statistics. The statistical analysis technique used Microsoft Excel Professional Plus 2021 (RRID: SCR_016137) for the Microsoft Windows operating system. The equations used and their criteria for significance are shown in Table 3.
Meropenem – Erythroderma was the DEC with the highest impact on public health, found to be category “A” according to Waller et al., tool,28 a high-priority signal that needs further investigation, calculation of evidence and public health scores is shown in Table 4. In the 11 years of study, a total of 65 cases were found in the global database, and only 53 met the inclusion criteria. Table 5 shows the country distribution of the reports.
| a | b | c | d | e | f | |
|---|---|---|---|---|---|---|
| Meropenem-ED | 60 | 0.8 | 1 | 80 | 0.7 | 0.5 |
| Country of primary source | Number | Percentage |
|---|---|---|
| Thailand | 28 | 52.8% |
| China | 17 | 32.1% |
| Iraq | 3 | 5.7% |
| Malaysia | 2 | 3.8% |
| Korea (the Republic of) | 1 | 1.9% |
| Singapore | 1 | 1.9% |
| Nigeria | 1 | 1.9% |
There was a slight male predominance in the affected patients with a M:F ratio of 1.3:1 (Figure 2) and an average age of 58.5 ± 25.5 years (range newborn through 95 years), the largest share of cases was of the (45-64) years age group (Figure 3).
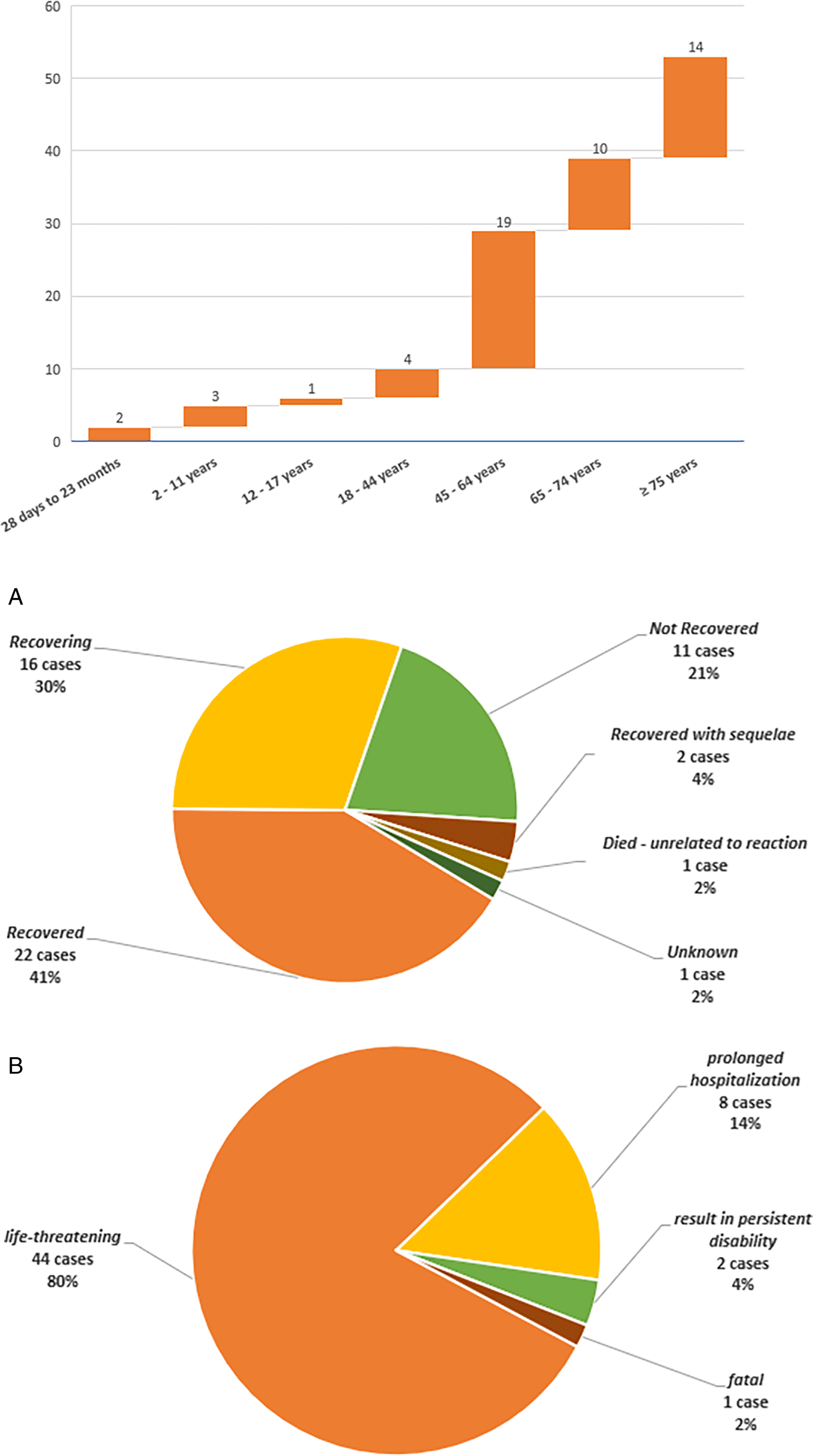
(A) Cases outcome as reported in ICSRs. (B) Cases seriousness assessment according to WHO criteria.
Statistical parameters, IC025, ROR025, and PRR025 were calculated manually for the included cases to estimate the strength of the association, values are listed in Table 6.
| Parameter | Corrected value calculated manually | Significance |
|---|---|---|
| IC025 | 2.961 | Highly significant |
| PRR025 | 8.227 | Highly significant |
| ROR025 | 8.244 | Highly significant |
The use of quantitative analysis has shown a robust correlation between the administration of Meropenem and the occurrence of exfoliative dermatitis. This association has been substantiated by the meticulous examination of individual cases. Tables 7 and 8 validate the discovered signal using the WHO-UMC causality criteria and Bradford Hill criteria, respectively.
| WHO causality assessment | Cases | Percentage |
|---|---|---|
| Probable | 16 | 30% |
| Possible | 22 | 42% |
| Unlikely | 15 | 28% |
| Criterion | Findings |
|---|---|
| Strength of the Association | The association is statistically highly significant: IC025 > 0, ROR025 and PRR025 > 1 |
| Consistency | This is the first study linking ED to Meropenem |
| Specificity | In 29 cases, patients have received only Meropenem |
| Temporality | The time to onset (TTO) of erythroderma symptoms ranged from a few hours to 120 days after receiving Meropenem, with an average of 10 days |
| Biological Gradient | Not Found |
| Biologic Rationale (Plausibility) | Drug hypersensitivity reaction (DHR), a type-B ADR34 |
| Coherence | Drugs come second as the leading cause of ED35 |
| Experimental Evidence | None |
| Analogy | Imipenem (another Carbapenem) has ED listed as a rare skin side effect in its SmPC36 |
In the present study, ED led to the death of one of the patients and caused disability in two other patients, cases outcome is listed in Figure 3A, while the seriousness assessment was weighed up according to WHO criteria23 and listed in Figure 3B.
Since Exfoliative dermatitis is a type-B ADR, it is not predictable nor dose-dependent and was found to be not preventable after evaluation using Schumock and Thornton scale37 and to assess the severity, Hartwig’s Severity Assessment Scale38 was used with results listed in Table 9.
Erythroderma is one of the rarely encountered and diagnostically challenging conditions.39,40 Erythroderma, often known as ED, is a syndrome with a non-specific clinical picture and is considered as a dermatological emergency associated with a significant mortality rate among hospitalized patients.41,42
In Iraq, there were only 3 cases of Meropenem-erythroderma DEC, and from what was clear from all the 4,510 reports of Iraqi patients that have been reviewed, some syndromes were recorded as separate symptoms and while, even a minority, there were some mistermed reactions and ICSRs missing essential details that are vital in analyzing the case at hand. Moreover, underreporting significantly impedes the Pharmacovigilance system worldwide, drastically reducing the number of reports submitted to VigiBase.43,44
From 65 cases of Meropenem–Erythroderma DEC, only 53 were included, as 12 ICSRs had a completeness ratio of less than 50% which led to their exclusion. In more than 50% of the cases, Meropenem was the sole administered drug, and 28 patients had a positive dechallenge; together with the results of the various assessment tools that were used and the solid statistical evidence, there’s strong evidence of a relationship between Meropenem and ED.
Several studies that discussed and researched genetic susceptibility to SCARs showed higher susceptibility in Thai and Chinese populations due to specific Human Leukocyte Antigens (HLAs),45–49 and that might explain why Thailand and China ranked first and second in the number of reports.
In the present study, male preponderance was seen, and the average age was in the 5th decade; these findings agree with other studies.10,50,51
Drug-induced ED is rapid in onset and progresses faster than ED caused by primary skin disease with faster resolution.35,52 In the current study, the average time for the onset of symptoms was 10 days, ranging between 0 days (the day Meropenem started) to 30 days, and this is comparable with Sindi et al. findings.40 There was a single female patient whose beginning was documented as occurring 120 days before. However, it remains unclear if she began experiencing signs and symptoms after this four-month period or whether she had entirely recovered, with the reported date being her final follow-up appointment.
Histopathologic and laboratory data that may aid in the diagnosis of the cases were not available, in addition to previous allergy and medical history that could explain if there is a possible drug-drug or drug-disease interactions, as these can insidiously modulate how the body reacts to different drugs by various mechanisms causing aggravation of pre-existing dermatoses or forms an antigenic complex that triggers an immune response.53,54
Due to the voluntary nature of ADR reporting, the main limitations faced in our research were underreporting and incomplete reporting, primarily caused by a lack of sufficient pharmacovigilance knowledge and practice or finding the reporting process a time-consuming and fairly complicated procedure. Additionally, some healthcare providers do not report because of the fear of legal consequences, which was also seen in several studies.55–58
Although Erythroderma is a rare reaction, death may occur if the patient has not received rapid management and the underlying trigger was treated, this can be challenging due to the variable course and non-specific symptoms of erythroderma.
This study found a strong link between Meropenem and ED supported by statistical evidence and data from analyzing case series.
The influence of genetic and ethnic variations on drug hypersensitivity reactions (DHRs) is significant, as it alters the body’s response to various medications, resulting in a wide range of symptoms or modifying the vulnerability of certain groups.
Exfoliative dermatitis; new safety signal detection regarding Meropenem in VigiBase™: A study based on WHO database, https://doi.org/10.5281/zenodo.8336302.
This project contains the following underlying data:
- Meropenem exfoliative dermatitis cases details table.xlsx (contains demographic data and results of different assessments for each case)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY).
The entire data set would be supplied following a reasonable request made to the corresponding author at: ahmed21289@uomustansiriyah.edu.iq
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)