Keywords
CuNiTi archwire, nickel ion release, copper ion release, surface topography, deflection, unloading force, chlorhexidine, and natrium fluoride.
Copper (Cu), nickel (Ni), chromium (Cr) ion release, and surface topography change from the orthodontic wire are the initial processes of corrosion that may affect the mechanical properties of the archwire. In this study, we aim to evaluate the effect of CHX, NaF, and chitosan on the corrosion of CuNiTi wire nickel and copper ions released, surface roughness change, and archwire deflection.
Ninety samples of CuNiTi Tanzo™ archwires were divided into five groups according to their immersion solution: Artificial Saliva, CHX, NaF, CHX-NaF, and chitosan group. Each group was further divided into three subgroups (n=6) corresponding immersion time, i.e., two, four, and six weeks. The corrosion of the samples was analyzed with an atomic absorption spectrophotometer (AAS), scanning electron microscope (SEM), and universal testing machine (UTM).
The amount of nickel ion releases was increasing, but the copper ion releases were reduced by the time of observations. The highest nickel ion was released in the CHX-NaF group and the lowest in the chitosan group for six-week immersion. It also corresponded to the surface topography by SEM analysis which showed the most extended cracks and deep pits in the CHX-NaF group and a smoother surface in the chitosan group. Copper ion release showed the highest ion release in the NaF group and the lowest release in the chitosan group. The unloading force of CuNiTi archwire deflection remains the same at week two and week four for all mouthwashes.
The use of mouthwashes that contained CHX, NaF, and chitosan could further alter the passive layer and cause higher nickel and copper ion release and increased CuNiTi archwire surface structure porosity. But there is no distinction between mouthwashes to release the unloading force within two until four weeks.
CuNiTi archwire, nickel ion release, copper ion release, surface topography, deflection, unloading force, chlorhexidine, and natrium fluoride.
Added:
1. A sentence which recommend to do the intra or inter-relialibilty test
2. Mentioned about McDougall's ingredients of artificial saliva, and the mechanical properties of Copper-Nickel-Titanium archwire to the discussion part.
See the authors' detailed response to the review by SHAZA HAMMAD
See the authors' detailed response to the review by Asma Ashari
See the authors' detailed response to the review by Andrzej Zielinski
Nickel-titanium alloy archwire offers greater flexibility and resistance to deformation and also exhibits excellent biocompatibility and corrosion resistance (Mitchell, 2013). Further addition of copper (Cu) in Cu nickel (Ni) titanium (Ti) archwires produces a more constant force on the teeth (Singh, 2007; Bhalajhi, 2012; Phulari, 2017). However, in the oral environment, archwires are constantly exposed to various stresses from masticatory forces, loading appliances, temperature fluctuations, and varieties of ingested food and saliva (Chaturvedi and Upadhayay, 2010).
The electrochemical mechanism of corrosion plays an essential role in metal corrosion when in contact with an electrolyte fluid, for example, saliva or mouthwashes. When two different alloys are in contact with a fluid electrolyte, the alloy with lower electrode potential will become the anode and produce oxidation that released several ions into the solution (Anusavice et al., 2012). Corrosion creates two issues; it can alter the physical properties of archwires (Mane et al., 2012; Geramy, Hooshmand and Etezadi, 2017; Doddamani, Ghosh and Tan, 2018) and cause a local or systemic condition due to allergic reactions and biological side effects (Lü et al., 2009; Pazzini et al., 2009; Suárez et al., 2010; Hafez et al., 2011; Castro et al., 2015). The metal products released during corrosion are nickel, chromium, and copper. Nickel is classified as a chemical carcinogen (IARC Working Group, 1990). In addition, it is a powerful medium for an immune reaction which can lead to a hypersensitivity reaction, contact dermatitis, gingivitis, gingival hyperplasia, periodontal stomatitis, periodontitis, burning mouth syndrome, angular cheilitis, cytotoxicity, mutagenic reaction (Hafez et al., 2011; Heravi et al., 2015; Lü et al., 2009). Meanwhile, copper is required by the body, but an excessive amount can produce cytotoxicity in the form of allergies at the point of contact and metal deposits in organs (Farrukh, 2011; Mahalaxmi, 2013).
Deflection of the archwire also plays an essential role in affecting tooth movement during orthodontic treatment (Aghili et al., 2017; Khatri and Mehta, 2014). Deflection of the archwire is defined by the ability of the archwire to transmit forces to the dentoalveolar to promote tooth movement (Parvizi and Rock, 2003; Sarul et al., 2013).
Dental hygienist usually prescribes mouthwashes to patients with low oral hygiene and a high risk of caries to prevent the formation of microbial plaque (Anuwongnukroh et al., 2017; Castro et al., 2015). The use of fluoride mouthwashes helps the enamel layer remineralize and protects it from the acidic environment (Roveri et al., 2009; Sivapriya et al., 2017), but the production of hydrofluoric acid (HF) may have a destructive impact on the archwires. HF degrades the protective oxide layers on the surface, which leads to corrosion (Castro et al., 2015; Hafez et al., 2011; Lü et al., 2009; Marques et al., 2012; Suárez et al., 2010). But there are several published studies on the corrosion resistance of NiTi alloys in saliva and NaF solutions even with increasing concentrations of fluorides (Heravi, Hadi Moayed and Mokhber, 2015; Mirhashemi, Jahangiri and Kharrazifard, 2018; Fatene et al., 2019).
Chlorhexidine also has high effectiveness in preventing the formation of dental plaque and is also effective in decreasing gingival inflammation (Metin-Gürsoy and Uzuner, 2014; Deriaty, Nasution and Yusuf, 2018). Several authors have evaluated a significant lowering in corrosion resistance in stainless steel or NiTi archwires in chlorhexidine mouthwashes compared to other mouthwashes (Danaei et al., 2011; Deriaty et al., 2018; Habar and Tatengkeng, 2020). Several studies also showed degradation in the performance of an elastomeric chain (Sufarnap et al., 2021), and more significant surface corrosion was observed under the scanning electron microscope (SEM) in wires from chlorhexidine mouthwashes (Mane et al., 2012; Doddamani, Ghosh and Tan, 2018; Chitra, Prashantha and Rao, 2020).
Chitosan is a natural polysaccharide resulting from the deacetylation of chitin. Chitosan has a broad antibacterial content and a low level of toxicity, so it is often used as a mouthwash for plaque control (Chen and Chung, n.d.; Fei Liu et al., 2000). A study by Uraz et al. showed no significant difference in the use of chitosan mouthwash compared to chlorhexidine mouthwash plus chitosan in decreasing plaque index (Uraz et al., 2012).
Several studies were conducted on the corrosion resistance, deflection, and ion release of NiTi orthodontic archwires in chlorhexidine or fluoride mouthwashes. However, there were limited studies observing nickel ion release, copper ion release, deflection test, and surface structure in mouthwashes containing chlorhexidine and fluoride, and chitosan in CuNiTi archwire, and as such this became the objective of this study. We hypothesized the differences found at each immersion solution at each time observation to the surface structure, deflection, and nickel and copper ion release. This study is a continuation of the Devi et al. (2022) study.
The research type was an experimental study using a post-test control design. There were ninety (90) Tanzo (American Orthodontics®) CuNiTi 4cm long archwires, sized 0.016×0.022 inches. Samples were divided into five groups according to the immersion solution, i.e., control group, CHX group, NaF group, CHX-NaF group, and chitosan group. The samples were further divided into three subgroups (n=6) corresponding to the duration of immersion, two, four, and six weeks. The sample size was determined with the formulation from Irmawantini, 2017:
(n – 1).(r – 1) ≥ 15
(n – 1).(5 – 1) ≥ 15
4n ≥ 19 n = 4.75 (minimum)
Each group has 18 samples divided into three subgroups (n=6) according to the immersion time: two, four, and six weeks. Group 1: immersed in artificial saliva as a control group (produced by the Oral Dental Hospital of Universitas Sumatera Utara Pharmacies followed by McDougall’s recipe of minerals: NaHCO3, Na2HPO4.12H2O, NaCl, KCl, CaCl2 anhydrous, MgCl2 anhydrous, and H2O) (Khan et al., 2021); Group 2: immersed in artificial saliva and 0.1% chlorhexidine gluconate mouthwash (Minosep, Minorock, Indonesia; as the CHX group); Group 3: immersed into artificial saliva and 0.05% NaF (Merck KGaA, Darmstadt, Germany, as the NaF group); Group 4: immersed into artificial saliva, 0.05% NaF, and 0.12% chlorhexidine gluconate (PerioKin®; as the CHX-NaF group); Group 5: immersed into artificial saliva and 2% chitosan (prawn shells was formulated at the Laboratory of Research Centre (Faculty of Mathematics and Science, Universitas Sumatera Utara as the chitosan group). Ninety samples were made in total, and they were all incubated at 37°C.
Archwires were simulated in the mouth environment, all samples were immersed in 10 ml saliva within observation time (2,4, and 6 weeks), and mouthwashes were simulated two times a day for one minute. All samples immersed corresponding to the subgroup 2, 4, and 6 weeks; Minosep® mouthwash, 0.05% NaF, PerioKin®, and chitosan mouthwash were added into the test tubes in group 2 to 5 simulated respectively for 28 minutes at two weeks subgroup, 56 minutes at four weeks subgroup, and 84 minutes at six weeks subgroup. The samples were agitated with a vortex for one minute before being incubated. After being immersed at each time point, wires were removed from the solutions, washed with distilled water, and dried for further surface roughness topography analysis with the SEM machine. Test tubes were sealed again with aluminum foil and placed at room temperature to prepare the analysis.
The research was conducted at the Faculty of Pharmacies Laboratory, Universitas Sumatera Utara, where the samples were incubated at 37°C; nickel and copper ion release sample’s immersed solution were analyzed at Balai Standardisasi dan Pelayanan Jasa Industri (Baristand) Medan using atomic absorption spectrometry (AAS, Shimadzu AA7000); the surface structure of CuNiTi wires was tested with a scanning electron microscope (SEM, Hitachi TM3000, Tabletop Microscope, Japan) at 2000× magnification on three sites at Integrated Research Laboratory- Universitas Sumatera Utara; and deflection test with the Universal Testing Machine (UTM, Tensilon RTF 1350) at the Impact Fracture Research Center (IFRC) Laboratory, Faculty of Engineering, Universitas Sumatera Utara.
The SEM images were taken 3 times for each sample. The Region of interest (ROI) of the images captured within the roughest area. The AAS analysis had been taken one time with the Relative Percent Difference (%RPD) below 5%. While the UTM machine results also came with one-time measurements from the machine. Calibrators had been taken 5 times to get the optimal normal curve. The sample which had improper results or outranged the standard or normal curved had been recalculated. The results needed to analyse with the inter- or intra-reliability tests.
Statistical analysis was performed using Statistical Package for Social Science (SPSS) 26.0 edition with Shapiro-Wilk for normality test (p≤0.05). The data obtained were analyzed statistically using the Kruskal-Wallis test to compare the amount of unloading force, nickel, and copper release in weeks two, four, and six.
Mean levels of nickel and copper released in each group for every time observation were significantly different, and the data are shown in Tables 1 and 2, respectively (Sufarnap, 2022). Scanning electron microscope (SEM) images result after six weeks of immersion (the longest time) of CuNiTi’s wire surface topography are shown in Figure 1. The roughness was found in all groups, but the most extended surface defects, such as cracks and pits, were found in the CHX-NaF group, followed by more comprehensive pits in the CHX group compared to another group.
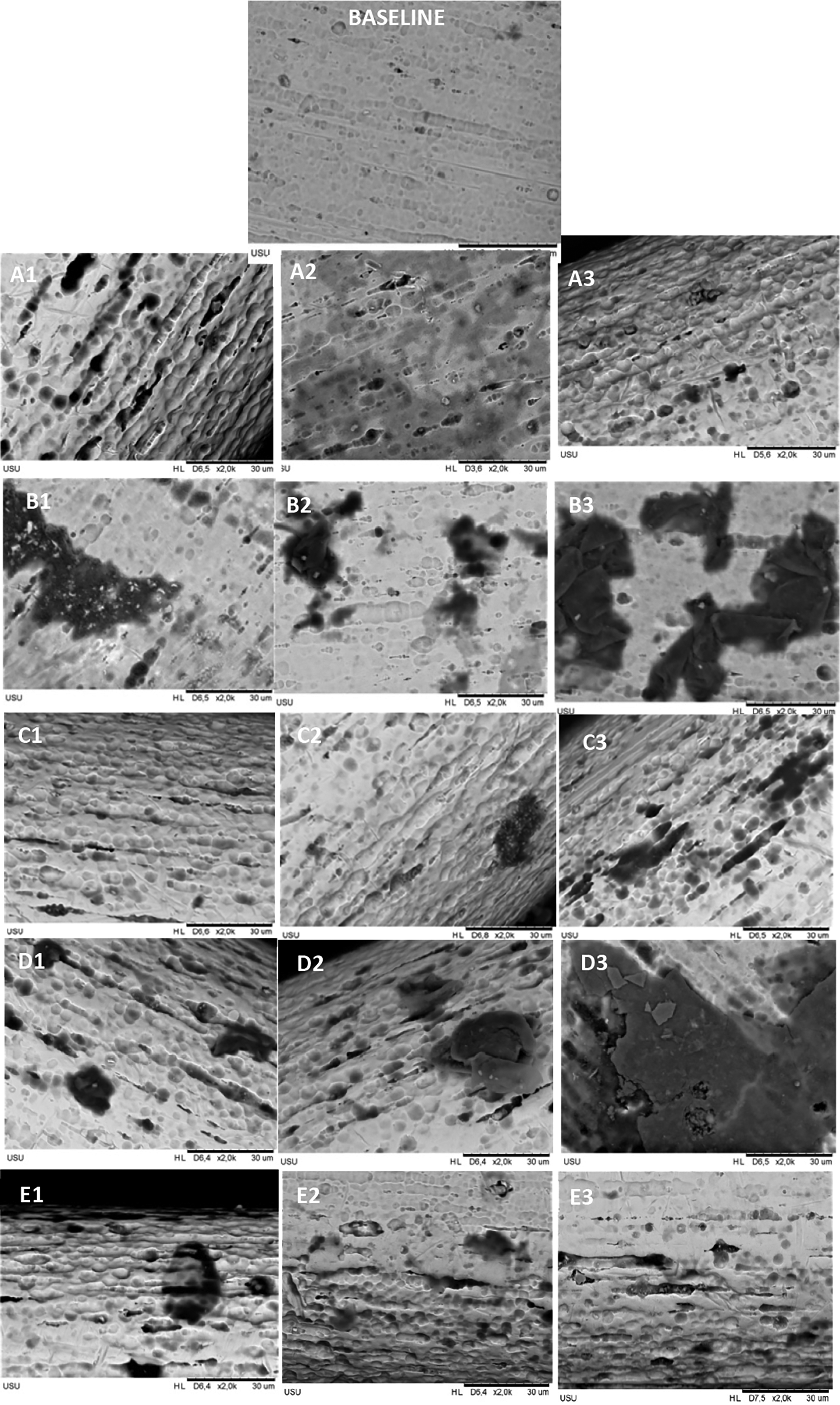
Immersed with A. Control Group B. CHX Group C. NaF Group D. CHX+NaF Group E. Chitosan Group. (1,2,3 represent 2, 4, and 6 weeks).
According to the result, nickel ions are increasingly released by the time of observation in all groups. In the beginning, the highest nickel released was at the chitosan group, but it had a slow release. The highest amount of nickel release also corresponded to the surface structure, which was the most prolonged observation in group 4 (CHX-NaF) (Figure 2) (Sufarnap et al., 2022).
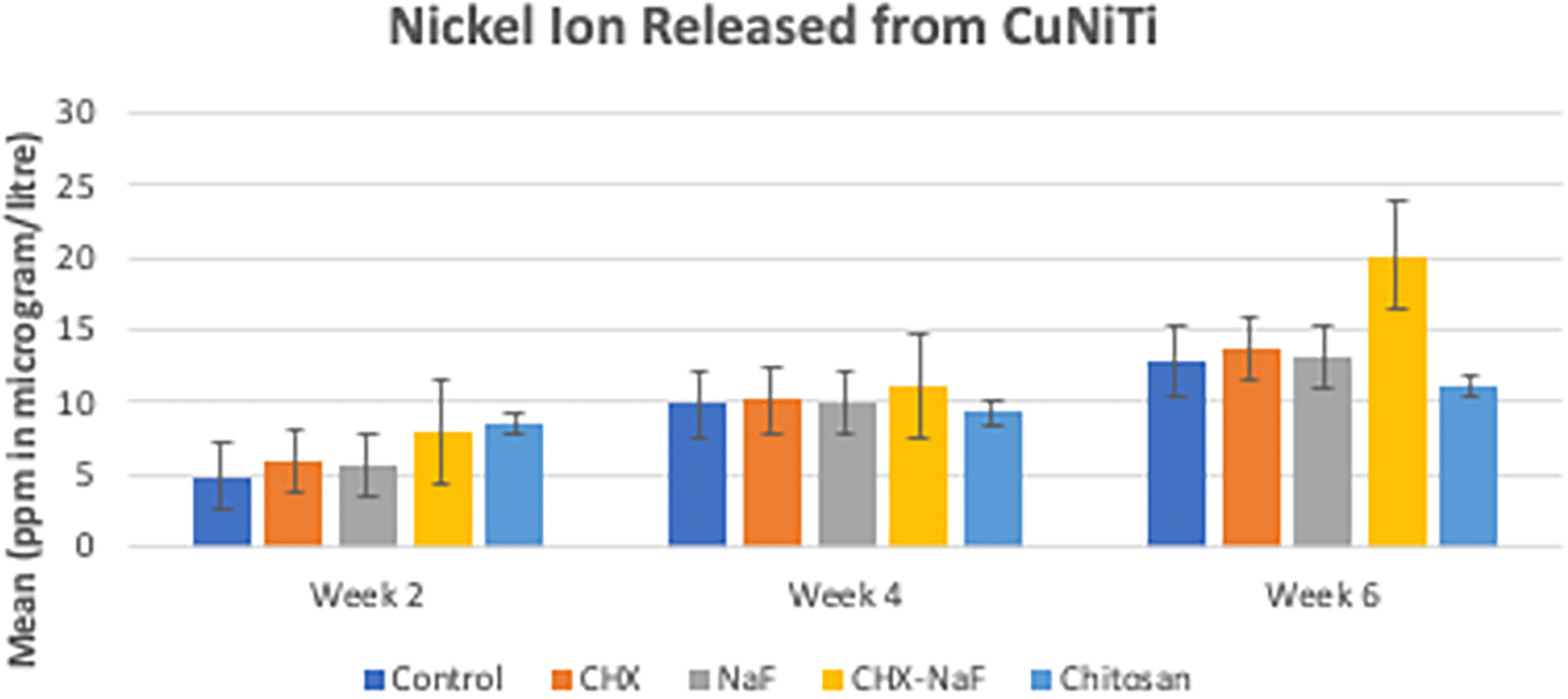
Abbreviations: NaF: Sodium Fluoride; CHX: Chlorhexidine; CHX-NaF: Chlorhexidine-Sodium Fluoride.
Copper ions released showed reduced by the time of observation at all groups. The highest copper released was found in the NaF group for all observation times (Figure 3) (Sufarnap et al., 2022). The last analysis was the mean levels of unloading forces in each group. They are shown in Table 3. Based on the results, there were no significant differences in unloading forces at two weeks and four weeks of all groups but showed a significantly different in six-week group. The data are shown in Table 3 and Figure 4 (Sufarnap et al., 2022).
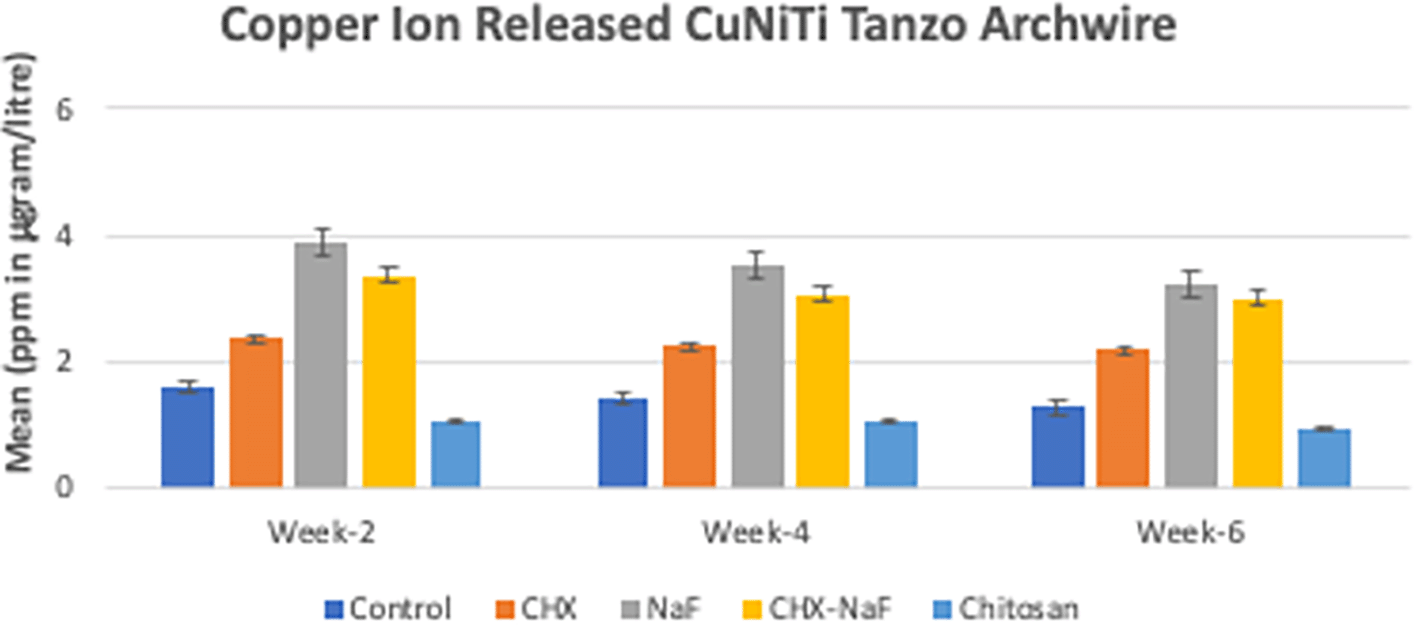
Abbreviations: NaF: Sodium Fluoride; CHX: Chlorhexidine; CHX-NaF: Chlorhexidine-Sodium Fluoride.
| Solution | Mean (N)±SD | |||
|---|---|---|---|---|
| Week two | Week four | Week six | p-value** | |
| Control Group | 47.639±2.1234 | 45.936±1.7143 | 47.207±2.2875 | 0.262 |
| CHX Group | 47.798±3.7015 | 42.532±8.8856 | 45.864±2.0239 | 0.532 |
| NaF Group | 47.95±2.32 | 48.89±1.23 | 48.14±1.34 | 0.623 |
| CHX-NaF Group | 50.141±1.3432 | 46.546±2.7308 | 46.224±1.1263 | 0.010 |
| Chitosan Group | 49.349±0.7325 | 45.428±1.7099 | 48.621±0.8625 | 0.003 |
| p-value* | 0.195 | 0.086 | 0.023 | |
The highest amount of nickel ion was 0.2020 mg from the CHX-NaF group at the six-week group, and the highest amount of copper ion was 0.03377 mg from the CHX-NaF group at week two. This showed the highest concentration of both ions was still below the warned concentration limit. The average amount of nickel intake obtained from food is 300 μg–500 μg/day (Alarifi et al., 2013; Milheiro et al., 2016). While a nickel concentration of 600 μg–2500 μg can induce an allergic reaction, and a copper concentration of 10 ppm can cause a cytotoxic reaction (Danaei et al., 2011; Milheiro et al., 2016). The, nickel and copper ions in this study were still released in the artificial saliva (control group), although it had the least amount compared to the other group. The mechanical properties of CuNiTi archwire consists 46,87% of nickel (NiO), 43,70% of Titanium (TiO2) and 6,72% of copper (CuO) (Gravina et al., 2014). These properties explained why the nickel ion released more than the copper ion release within this CuNiTi archwire.
Chlorhexidine used for a long time can generate Reactive Oxygen Species (ROS), the primary agent responsible for endogenous DNA damage (Septiani and Auerkari, 2020). Nik et al. and Omidkhoda et al. found that CHX immersion had no significant effect on NiTi archwire surface roughness, corrosion, and frictional resistance (Danaei et al., 2011; Nik et al., 2013). But some authors found a significant decrease in corrosion resistance due to CHX mouthwashes (Danaei et al., 2011; Deriaty et al., 2018; Habar and Tatengkeng, 2020), and some authors found more significant surface corrosion with SEM analysis (Mane et al., 2012; Doddamani, Ghosh and Tan, 2018; Chitra, Prashantha and Rao, 2020). This study also found the surface topography showed that the CHX group had rougher and more pitted surfaces compared to the control group or baseline topography.
In six weeks of immersion, CuNiTi wire in the CHX-NaF group has the highest amount of nickel ions released and in the NaF group, more copper ions were released than the other group. The unloading force difference is also significantly seen in the CHX-NaF and chitosan groups within different immersion durations. As a result, there were changes in CuNiTi mechanical properties after immersion of CHX-NaF, NaF, and chitosan for six weeks.
NaF content in perioKin® (CHX-NaF) mouthwash can increase the metal ions released from the CuNiTi orthodontic wire. Heravi et al. found that the NaF content in mouthwash solution would decrease the corrosion resistance of NiTi and CuNiTi wires as the NaF concentration increased (Heravi, Hadi Moayed and Mokhber, 2015; Fatene et al., 2019). Sabah and Jarjees also reported that the interaction between fluoride and titanium might cause destruction to the metal coating of the archwires and degrade the mechanical properties (Sabah, Jarjees and Awni, 2011).
The immersion of chitosan showed significant differences in Nickel and copper ion release, surface topography, and unloading force. Chitosan mouthwash has been used due to its antibacterial effect and lack of side effects. Uraz et al. has reported that chitosan 2% mouthwash had no significant difference compared to CHX 0,2% in reducing plaque index and gingivitis (Uraz et al., 2012).
The highest nickel ion released was found in the chitosan group at week two, but it increased slowly and steadily compared to other groups, and it lasted with the lowest nickel released for the longest immersion time (six-week immersion). The same result with copper ions released. It was found that the chitosan had the lowest amount of ions released from week two until six weeks. Unfortunately, nothing was found about the chitosan particles used as an inhibitory of corrosion for orthodontic archwire, but Putri et al. researched orthodontic mini-implant immersed in chitosan mouthwashes for the surface topography found that the mini implant immersed with 1.5% of chitosan mouthwash had a smoother surface compared to chlorhexidine and Sodium Fluoride mouthwash. The CuNiTi archwire surface topography in this study found that the roughness of the chitosan group also had a smoother area compared to CHX and CHX-NaF groups (Putri et al., 2021).
Furlan et al. mentioned that there were differences in the amount of nickel and copper ions released in several types of CuNiTi wire immersed in a neutral solution and an acidic solution, which were greater in the acidic solution (Furlan et al., 2018). The main limitation of this study was that it did not analyze the pH changes within each immersion solution and each observation time. This study was also done under the static condition in vitro. Further research is needed to determine the situation with in vivo experiments. The surface structure should also be better analyzed with an advanced equipment, i.e., atomic force microscopy (AFC) so that we could measure the quantity of the surface by its roughness. This will allow us to better understand the physical properties of CuNiTi wire. The inter- or intra-reliability test should appropriately had been suggested for several samples.
CuNiTi wire had an increase of nickel and reduced copper ion release parallel to the increase of immersion time, but the deflection of the wire did not show any significant differences between mouthwashes. However, after six weeks of immersion, the amount of Nickel and copper ions released was still within the safe limit. The surface roughness of CuNiTi archwire topography showed that the CHX-NaF group had the most extended cracks and deep pits. The unloading force of the CuNiTi archwire deflection remains the same at week two and week four for all mouthwashes.
OSF: Data of Corrosion of CopperNickelTitanium Archwire in Chlorhexidine, Sodium Fluoride and Chitosan Mouthwashes, https://doi.org/10.17605/OSF.IO/5QB8E (Sufarnap, 2022).
This project contains the following underlying data:
OSF: Data of Corrosion of CopperNickelTitanium Archwire in Chlorhexidine, Sodium Fluoride and Chitosan Mouthwashes, https://doi.org/10.17605/OSF.IO/5QB8E (Sufarnap, 2022).
This project contains the following extended data:
- Figure 1, 2,4. Graph of Ni-Cu Ion Release and Deflection of Tanzo CuNiTi Archwier.pdf
- Figure 3. Surface Analysis of CuNiTi Tanzo Archwire (SEM analysis).jpeg
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biomaterials engineering
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Orthodontics, clear aligners
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Orthodontics, clear aligners
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biomaterials engineering
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Orthodontic techniques, materials and appliances.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Orthodontics, clear aligners
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 30 May 24 |
read | read | |
|
Version 2 (revision) 27 Mar 23 |
read | read | read |
|
Version 1 10 Feb 23 |
read | ||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Please cite our manuscript for all readers who read our explanation and will use our methods, especially in the simulation of the mouth condition. Thank you for ... Continue reading Dear Readers,
Please cite our manuscript for all readers who read our explanation and will use our methods, especially in the simulation of the mouth condition. Thank you for being so understanding—regards by all authors.
Please cite our manuscript for all readers who read our explanation and will use our methods, especially in the simulation of the mouth condition. Thank you for being so understanding—regards by all authors.