Keywords
Autoimmune hepatitis, Somalian, liver disease, hepatopathy, liver transplantation
Autoimmune hepatitis, Somalian, liver disease, hepatopathy, liver transplantation
Autoimmune hepatitis (AIH) is a rare disease characterized by increased alanine aminotransferase (ALT) and aspartate aminotransferase (AST), hypergammaglobulinemia (elevated immunoglobulin G, IgG) and interface hepatitis or/and lobular necroinflammation in liver biopsy.1 The diagnosis of AIH is based on circulating antibodies (antinuclear antibodies (ANA), smooth muscle antibodies (SMA), liver kidney muscle antibodies (LKM)) and exclusion of other causes of liver disease including viral hepatitis. Diagnostic scoring systems from the International Autoimmune Hepatitis Group (IAIHG) have been created to established the diagnosis of AIH.2
AIH mainly affects women and is often diagnosed between the age of 40 to 70. Genome wide association studies (GWAS) associate HLA A1, B8, Cw7 and DR3 haplotype with AIH in European patients.1,3 Significant diversity in disease severity has been identified for autoimmune disorders in general and AIH before.3 Yet, data on AIH in non-European/non-Asian patients is very limited, especially for African patients. Compared to European AIH patients, non-European patients present at younger age, with a cholestatic laboratory pattern and a poorer response to standard therapy.4 A recent study from England reported a small cohort of six Somalian men with an unusual form of AIH with cholestatic features.3 These patients responded poorly to standard immunosuppressive therapy. In this context, we recently treated two Somalian patients with AIH in our tertiary care center. The aim of this study was to identify additional Somalian patients with liver disease in our electronic hospital database and analyze the cause of liver disease, the clinical course and outcome of these patients.
We screened our electronic hospital database for patients presenting to our tertiary care hospital (Hannover Medical School (MHH)) between 2008 and 2021 and with place of birth in Somalia. A total number of 17 patients (47.1% male) were identified. Of these patients, 14 had no history of liver disease or elevated transaminases at the time of presentation and were excluded from this study. Three patients met the inclusion criteria and were considered for this analysis.
All three male patients were referred from other hospitals to our Department of Gastroenterology, Hepatology, Infectious Diseases and Endocrinology for further diagnostic and therapy. Two of the three patients presented with acute onset of hepatitis, while one patient had a history of autoimmune hepatitis and a recent episode of variceal bleeding. Detailed patients’ characteristics are presented in Table 1.
A 38-year-old Somalian man was admitted to our hospital shortly after acute variceal bleeding that required transfusion of packed red blood cells. The initial diagnosis of AIH type 1 was made in another hospital five years prior to presentation. At this time, antibody screening revealed an ANA of 1:80 and a SMA 1:80 with an elevated IgG (17.2 g/l) and liver biopsy showed fibrosis but no cirrhosis and typical features of AIH including lymphoplasmacellular inflammation (Figure 1A). Immunosuppressive treatment with azathioprine (1.5 mg/kg per os once a day) was initiated. The patient denied any herbal drugs or hepatotoxic medication history and there was no family history of liver diseases or alcohol abuse.
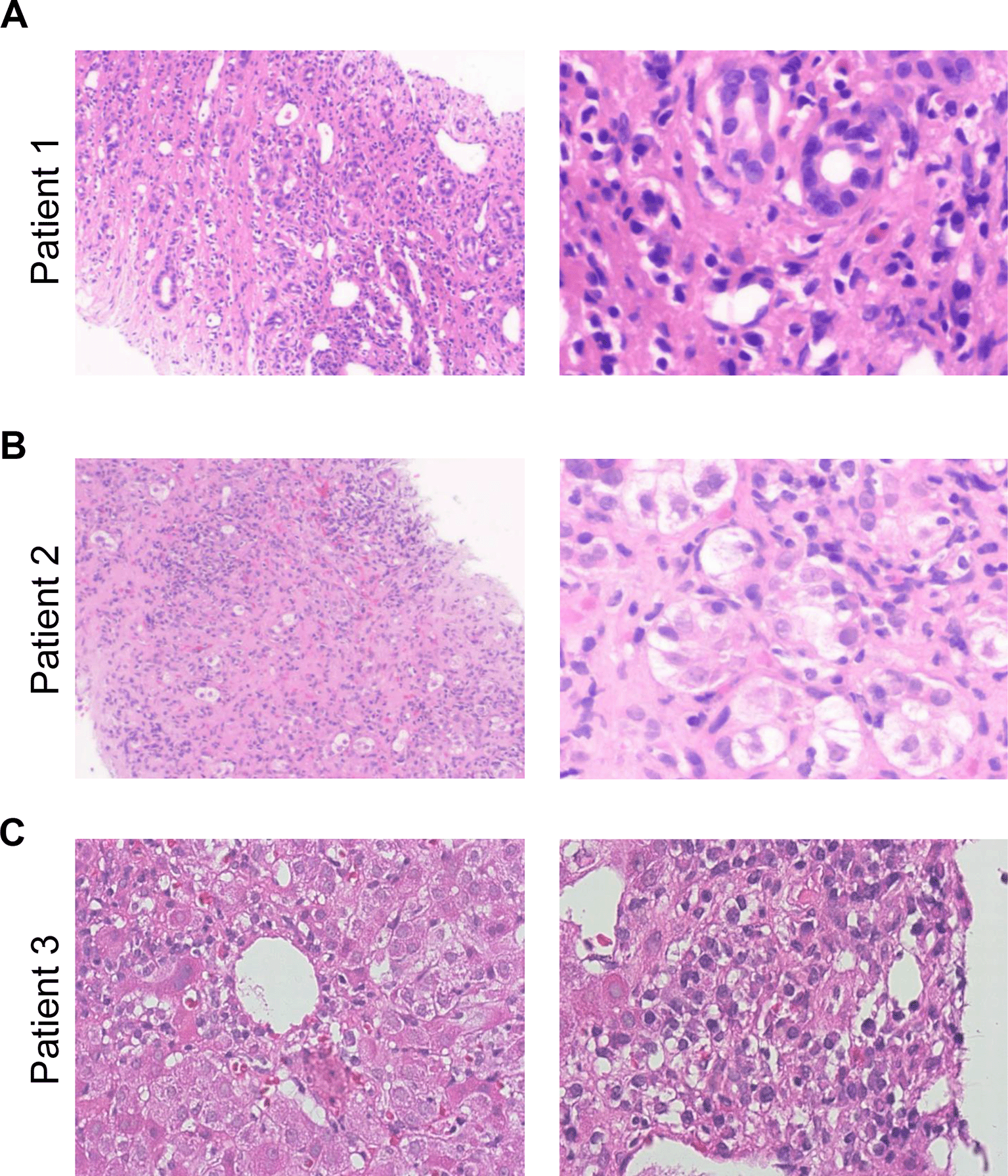
A. Liver biopsy of case no 1 shows fibrosis with incomplete cirrhosis, ductular reaction and lymphoplasmacellular inflammation. B. Liver biopsy of case no 2 shows subacute subtotal necrosis of the liver parenchyma (95%) with fibrosis, ductular reaction, hepatocyte rosettes and lymphoplasmacellular inflammation. C. Liver biopsy of case no 3 shows ballooning of hepatocytes, apoptosis and portal plasmacellular inflammation. No modifications have been made to the images.
At the current admission the patient was treated with azathioprine and showed no relevant elevation of transaminases while cholestasis parameters were slightly elevated (Table 1). Liver function corresponded to Child Pugh Score B. Total IgG was within normal range under immunosuppressive therapy. Abdominal ultrasound and computed tomography revealed signs of liver cirrhosis and a thrombosis of the mesenteric and portal vein and therapeutic anticoagulation was initiated. As the patient suffered from recurrent episodes of variceal bleeding, we implanted a transjugular intrahepatic portosystemic shunt (TIPS). During the clinical course the patient developed an increase of transaminases (AST 284 U/l, ALT 131 U/l, AP 154 U/l, GGT 95 U/l, total bilirubin 18 μmol/l) and we intensified immunosuppression by adding prednisolone at a dose of 20 mg/day (per os once a day) with good response. After TIPS placement the patient developed recurrent pleural effusion which could not be managed by diuretics alone. A pleurodesis was performed followed by the placement of a long-term indwelling pleural catheter.
In the following months the patient was readmitted several times for complications of liver cirrhosis including infection (spontaneous bacterial peritonitis, infection of pleural effusion) and hepatic encephalopathy. Esophageal varices improved after TIPS insertion. IgG was within normal range under immunosuppressive therapy. However, the general condition of the patient worsened over time. While the patient presented with another episode of acute-on-chronic liver failure and a Model of End Stage Liver Disease (MELD)-Score of 32 he was evaluated for liver transplantation. There were no contraindications for solid organ transplantation. He was listed for liver transplantation and successfully transplanted (extended right lobe) six months after he presented to our hospital for the first time.
A 53-year-old Somalian man with acute-on-chronic liver failure was admitted to our hospital for further investigation. A diagnosis of liver cirrhosis was made in another hospital some days prior to presentation. However, the cause of liver cirrhosis was still unknown. The patient reported no regularly or recent intake hepatotoxic medication/substances or alcohol abuse. There was no family history for liver diseases.
Initial laboratory showed elevated liver enzymes and cholestatic parameters, laboratory MELD was 25. Liver function corresponded with Child Pugh Score C during acute decompensation. Serological markers showed no acute viral hepatitis but status post viral hepatitis B (HBsAg negative, anti-HBc positive). Rare liver diseases such as hemochromatosis or Wilson’s disease were excluded. SMA and ANA autoantibodies were detectable (Table 1) and total IgG was highly elevated. Liver biopsy showed liver dystrophy with 95% necrosis, histological features were comparable with AIH (Figure 1B). Based on the simplified AIH score2 AIH type 1 was diagnosed and we started an immunosuppressive therapy with prednisolone (1 mg/kg body weight intravenously once a day). Under immunosuppression liver enzymes, cholestatic parameters and IgG rapidly decreased additionally confirming the diagnosis of AIH. The patient was discharged with 60 mg prednisolone per os once daily. Four weeks later the patient presented in our outpatient clinic, liver enzymes further decreased (AST 46 U/l, ALT 102 U/l, GGT 933 U/l, AP 219 U/l, total bilirubin 38 μmol/l, IgG 19.24 g/l) and liver function partly recovered (INR 1.3). Azathioprine (50 mg per os once daily) was initiated and prednisolone was tapered.
A young 24-year-old Somalian man was admitted to our hospital in 2018 for further investigation with a new onset of hepatopathy. There was no personal or family history of liver disease. He lived with his family and denied any medical or herbal medication history. Initial laboratory showed markedly elevated liver enzymes, elevated bilirubin and IgG with AP and GGT within normal range (Table 1). Laboratory studies for AIH related autoantibodies revealed a positive SMA and ANA. A liver biopsy performed five months prior to presentation in an external hospital showed portal plasmacellular inflammation consistent with drug-induced liver injury (DILI) or autoimmune hepatitis. An interferon gamma release assay showed a positive result so immunsuppressive therapy had not been started until admission to our hospital. Based on all available results, we calculated the revised AIH score5 with 15 points and made the diagnosis of AIH type 1. Immunosuppressive therapy with prednisolone (1 mg/kg bodyweight/day per os) was started, liver enzymes and bilirubin decreased and the patient was discharged.
In the subsequent months in an outpatient setting azathioprine (dose unknown) was initiated. Given that a latent tuberculosis was diagnosed isoniacide (INH) prophylaxis was co-administered (300 mg/day per os). One year later the patient presented with a flare of AIH after not taking his immunosuppressive treatment on a regular basis. Steroids were intensified (100 mg/day per os) and the dose of azathioprine was increased (200 mg/day per os), INH prophylaxis was discontinued. Another six month later the patient presented with a flare of AIH and decompensated liver cirrhosis to an external hospital. Whether the patient took his medication on a regular basis was unclear. Steroids were started (100 mg/day intravenously) and one day later he was transferred to our clinic. Liver enzymes (AST 210 U/l, ALT 221 U/l) and total IgG were elevated (50.8 g/l). Shortly after admission to our hospital the patient developed a status epilepticus for unknown reason and was admitted to our intensive care unit. In the following days he developed respiratory failure with the detection of Streptococcus pneumonia in bronchoscopic culture, PCR for M. tuberculosis was negative. Clinical condition further deteriorated (acute respiratory distress syndrome (ARDS), acidosis, intravascular hemolysis) and the patient subsequently died.
AIH is a differential diagnosis in every new onset hepatopathy. The diagnosis is based on several circulating autoantibodies, hypergammaglobulinemia, the exclusion of other causes of liver disease and liver biopsy showing typical histological findings. Two scores are used in clinical practice to determine the likelihood of AIH. While the revised AIH score includes female gender in the scoring system, the simplified AIH score does not consider gender at all.2,5 In addition, the prevalence of AIH is higher in western Europe and north America and significantly lower in Asia and Africa.1,3 Therefore, AIH is often not considered as a likely differential diagnosis in African patients with elevated liver enzymes. However, at first presentation African American patients have more advanced stages of liver fibrosis than white American patients.6 In line with this observation, data from the UK found that Black patients presented at younger age and with higher IgG levels than white patients.7 A retrospective study from a hospital in the US which serves indigent and under-resourced communities identified race/ethnicity as an independent risk factor for AIH.8 Taken together, AIH needs to be considered as a differential diagnosis not only in White women but also in patients of African origin.
In this context, D’Souza et al. published a case series that analyzed the clinical, histological and immunological features of AIH in young Somalian men.3 The authors identified six patients (all male) from Somalia (all immigrants who had lived in the UK for the last six years) by scanning their histopathology database. Similar to the aforementioned studies and our three individuals, these patients were younger at presentation (median age 37 years) than the European control group (median age 55 years). There was a trend toward more advanced liver disease at presentation in Somalian patients (median ISHAK fibrosis 2.5 compared to 2 in Europeans). Biochemical cholestasis (AP greater than twice the upper normal limit) was observed in 66% of the Somalian patients and histopathological findings consistent with cholestasis were detected in 50% of patients, in contrast there was only one European patient with evidence of biochemical cholestasis. While initial therapy with prednisolone and azathioprine was effective in 8/10 European AIH patients, only 1/6 Somalian patients had a treatment response. HLA typing in this study revealed classical autoimmune associated allotypes in the European patients (HLA A1, B8, Cw7, DR3 or DR4).1,3 In contrast, Somalian patients shared different HLA allotypes (HLA B7, DR8, DQ2 and DQ4).
Another study by Zolfino et al. identified twelve patients from Africa (six patients), Asia (five patients) and Arabia (one patient) with AIH from an AIH-patient database with a median age of 30 years at presentation.4 Of those nine (75%) had a cholestatic serum biochemistry and three had histological findings comparable to PBC or a cholangiographic evidence of PSC. Complete biochemical response to standard immunosuppressive therapy was detectable in only four patients (33%), while five patients (42%) had no response to standard therapy. Overall, four of the 12 patients underwent liver transplantation for intractable disease. Zolfino et al. compared the twelve patients to a group of 130 European patients with AIH. In contrast to Yang et al.1 the non-European patients were younger at presentation, presented with cholestatic biochemistry and morphological biliary features more frequently. In addition, they showed poorer response to standard immunosuppressive therapy.
The patients that were identified in our tertiary care hospital are comparable to the cases series mentioned before. Our Somalian patients also suffered from advanced liver disease (one required liver transplantation, another patient had progression towards decompensated liver cirrhosis in less than two years) at a young age (median age of 37). No Somalian women with AIH were identified in our tertiary care center and no relevant overlap syndrome was observed in our patients. In line with the previous report by D`Souza et al, one of our patients (no 2) showed also elevated cholestatic enzymes. The typical HLA genotypes were not detected in our cohort. The treatment response in our patients was different. Although patient 1 had a biochemical response, he subsequently developed hepatic decompensation with variceal bleeding and ascites requiring TIPS insertion and subsequently liver transplantation. Both, patients 2 and 3 had a biochemical response to standard immunosuppressive therapy. However, patient 3 was not compliant resulting in several flares of AIH with progression to liver cirrhosis in less than two years. While all three patients had possible AIH according to the revised/simplified AIH score, autoimmune hepatitis can be also be associated with the intake of certain drugs or herbal medicine.9 In this context, there are reports that Khat chewing is associated with liver disease in Somalian men,10 while others question this observation.11 Khat is a herbal product, and a study from Ethiopia observed a significant association between chewing khat and the risk for developing chronic liver disease. Unfortunately, our retrospective study could not rule out khat intake by all three patients.
An obvious limitation of this retrospective study is that there is no systematic follow up and that some patients may have been missed when the country of birth was not documented.
In summary, AIH should be considered in every new onset hepatopathy, especially when viral hepatitis is excluded. AIH in non-European patients is different. Somalian patients with AIH are younger at presentation and show a severe disease that may poorly respond to standard immunosuppressive therapy. AIH treatment of non-European patients requires a more aggressive and maybe alternative treatment. The different clinical features and treatment regimens should be considered in our multicultural society.
Written informed consent for publication of their clinical details and histological images was obtained from the patients.
All relevant data is presented in the manuscript without restrictions. De-identified data cannot be publicly shared because it contains potentially identifying and sensitive patient information. De-identified data is only available on reasonable request after an approval process involving the Ethics committee at our hospital and the corresponding author. The corresponding author can be contacted through: mederacke.ingmar@mh-hannover.de.
Figshare: Autoimmune hepatitis in young Somalian men – experience from a German tertiary care center (Case reports), https://doi.org/10.6084/m9.figshare.21547887.v4. 12
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the cases’ history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the conclusion balanced and justified on the basis of the findings?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Liver Transplantation and metabolic liver disease
Is the background of the cases’ history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the conclusion balanced and justified on the basis of the findings?
Partly
References
1. Granito A, Muratori P, Ferri S, Pappas G, et al.: Diagnosis and therapy of autoimmune hepatitis.Mini Rev Med Chem. 2009; 9 (7): 847-60 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Autoimmune liver diseases
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 15 Feb 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)