Keywords
COVID-19, pregnancy, pregnancy outcomes, risk factors, severity
This article is included in the Emerging Diseases and Outbreaks gateway.
COVID-19, pregnancy, pregnancy outcomes, risk factors, severity
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the source of the coronavirus disease 19 (COVID-19), a highly contagious and dangerous viral illness that led to a global pandemic a significant loss of human life.1 Severe acute respiratory syndrome coronavirus infection in pregnant women can cause either asymptomatic or symptomatic disease (mild, moderate, severe, and critical).2,3 Although the risk of developing severe illness is minimal, pregnant women with COVID-19 are more likely to do so than non-pregnant people. In addition, after adjusting for age, race/ethnicity, and underlying medical conditions, pregnant women had noticeably higher rates of intensive care unit (ICU) admission, mechanical ventilation, extracorporeal membrane oxygenation, and mortality.4
Contrary to pregnant and previously pregnant women without the illness, COVID-19-positive pregnant women had a higher risk of stillbirth and preterm birth.5 In addition, pregnancy-related physiological and immunological changes substantially impact the severity and effects of viral infections.6 For example, increased ACE2 expression, changes in the immune system to ensure the acceptance of the fetus, and changes in the respiratory system causing hyperventilation are all factors that could enhance vulnerability to COVID-19 infection during pregnancy.7
Compared to non-pregnant women, pregnant women with COVID-19 are less likely to experience symptoms. Being overweight or obese, being older than 35 years, and having pre-existing comorbidities (hypertension, diabetes, lung diseases) are all variables that increase the risk of developing a severe illness.8 To determine how long COVID-19 patients stay in hospitals, it is critical to examine their risk factors. According to a study from China in 2022, patients aged ≥ 45 and those with severe COVID-19 infection tended to stay longer in the hospital.9
The advantages of COVID-19 immunization, which include a decreased risk of severe illness and hospitalization for the pregnant woman and a reduced risk of hospitalization for the newborn in the first six months of life, should be discussed with expectant mothers.10 All eligible individuals, including those who are pregnant, nursing, or planning a pregnancy, should receive a COVID-19 vaccine or a series of vaccines, according to recommendations from the Society for Maternal-Fetal Medicine, American College of Obstetricians and Gynecologists, and CDC. If a person is qualified, the booster dosages are also included.11 The primary objectives of this study were to identify the most common symptoms associated with COVID-19 infection during pregnancy and assess the pre-existing risk of hospitalization, preterm delivery, and other complications related to COVID-19 illness, taking into account the severity of the disease.
The institutional scientific and ethical committees of the Faculty of Pharmacy, Mustansiriyah University, and the Baghdad Health Directorate, Al-Karkh, formally approved the research protocol on November 10, 2021 (Ethics Board approval code: 2021099).
Since the data has been anonymized, and because it is impracticable to obtain consent. The ethical committee waived the need for consent to participate. There is sufficient protection of participants’ privacy and an adequate plan to protect the confidentiality of data.
This observational retrospective study was carried out in the obstetrics and gynecology wards of Al-Yarmouk Teaching Hospital and Karkh Hospital for childbirth between November 15, 2021, and August 27, 2022, where pregnant women with COVID-19 were admitted to the hospital for treatment or to give birth.
The sample size was determined and computed using the computer application G*Power 3.1.9.7 (RRID: SCR 013726). The smallest total sample size was 314 patients, with an effect size of 0.20 and 95% power at a two-tailed alpha of 0.05 and a 95% confidence interval (f). After reviewing the hospital’s medical records or files, the biostatistics department provided 450 patient records; only 359 were selected for the research, while the rest fulfilled the exclusion criteria Figure 1.
Information was gathered using the research team’s specially created data collection form to match the study’s objectives. Information on the patient’s age, gestational age, comorbidities (diabetes, hypertension, and anemia), and gynecological and obstetrical history (parity, gravidity, abortion, and the number of previous C-sections). Pregnancy outcomes and birth status (abortion, preterm birth, stillbirth, and requirement for NICU).
The severity of COVID-19 infection was categorized according to the following12–14:
Asymptomatic: No symptoms.
Mild disease: Fever, cough, sore throat, nausea, vomiting, diarrhea, loss of taste or smell but no dyspnea; normal O2 saturation and standard chest X-ray.
Moderate disease: Symptoms of mild disease plus evidence of lower respiratory tract infection (exam and imaging), O2 saturation ≥ 94% on room air.
Severe disease: Symptoms of moderate disease but O2 saturation < 94%, PaO2/FiO2 < 300 mmHg, respiratory frequency > 30 breaths per minute, or lung infiltrates > 50%.
Critical disease: Symptoms of severe disease but intubated with respiratory failure, septic shock, and multiorgan dysfunction.
Since research operations, data collection, data entry, and data quality assurance are unplanned, retrospective cohort studies are frequently thought to have a higher bias. Using old data may affect any of these categories. But the authors of this study were keen to ensure that every eligible individual has an equal probability of selection. The research developed for settings considered, data completeness and quality assessment were conducted to ensure less bias during sample selection and study procedure.
The collected data were analyzed using SPSS version 25 statistical program for Windows (RRID: SCR 016479). The data are presented as mean ± standard deviation, number, and frequency. The chi-squared test was used to determine the significance of the associations between related categorical variables. Odds ratios were used to quantify the relationship between exposure and disease. Binary logistic regression was used to predict the relationship between independent and predicted variables. A two-sample t-test was used to determine the significance of differences between the means of the numerical data. A P-value less than 0.05 were considered a discrimination point for significance.
The mean age of COVID-19-infected pregnant women was 26.78-year ± 6.694 standard deviation (between 15-46 years), gestational age was 35.77-week ± 6.133 standard deviation (between 6-42 weeks), and birth weight at delivery was 2937.10 gram ± 607.457 standard deviation. Eight women were in the first trimester (2.2%), 15 in the second trimester (4.2%), and the majority of the cases (336) were in the third trimester (93.6%) as presented in Table 1.
Data present that 43.5% of the cases were asymptomatic, 35.4% were mild, 13.9% were moderate, and 7.2% were severe COVID-19 cases (Figure 2).
Study findings revealed that COVID-19-infected pregnant women with severe infection or aged ≥40 years had more extended hospital stays (≥3 days) than those with non-severe illness or aged less than 40 years (P-value < 0.05) (Table 2).
| Severity | Hospital stay | P-value | Odd Ratio | 95%CI | |||
|---|---|---|---|---|---|---|---|
| ≥3 days | <3 days | ||||||
| N | % | N | % | ||||
| Severe (n = 26) | 8 | 30.8% | 18 | 69.2% | 0.004** | 3.450 | 1.41-8.47 |
| Not severe (n = 333) | 38 | 11.4% | 295 | 88.6% | |||
| Age ≥ 40 | 6 | 35.3 | 11 | 64.7 | 0.004** | 4.118 | 1.44-11.74 |
| Age < 40 | 40 | 11.7 | 302 | 88.3 | |||
Table 3 shows those COVID-19-infected pregnant women who suffered from fever, cough, shortness of breath, and dyspnea had significantly more severe COVID-19 infection than those who did not suffer from the above clinical manifestations. P value = 0.001 for all conditions. Other symptoms didn’t differ among severity P-value > 0.05.
| Symptoms | Severe (n = 26) | Not severe (n = 333) | P-value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Fever | 24 | 12.8% | 163 | 87.2% | 0.001** |
| Cough | 14 | 18.4% | 62 | 81.6% | 0.001** |
| SOB/dyspnea | 20 | 44.4 | 25 | 55.6% | 0.001** |
| Diarrhea | 1 | 6.7% | 14 | 93.3% | 0.930 |
| Vomiting | 3 | 14.3% | 18 | 85.7% | 0.199 |
| Nausea | 1 | 12.5% | 7 | 87.5% | 0.495 |
| Musculoskeletal pain | 1 | 7.7% | 12 | 92.3% | 0.949 |
| Headache | 4 | 13.3% | 26 | 86.7% | 0.179 |
| Weakness | 4 | 14.3% | 24 | 85.7% | 0.134 |
| Loss of smell or taste | 1 | 6.3% | 15 | 93.8% | 0.875 |
| Sore throat | 2 | 12.8% | 13 | 87.2% | 0.352 |
Table 4 showed that fever and shortness of breath were the most predictable signs and symptoms that may result in severe COVID-19 after adjustment for other symptoms that showed a significant association with the severity of COVID-19 condition according to the chi-square test presented in Table 3.
| Sign/symptoms | B | S.E. | Wald | df | P-value | Exp(B) |
|---|---|---|---|---|---|---|
| Fever | 1.749 | 0.817 | 4.58 | 1 | 0.032* | 5.747 |
| Cough | -0.713 | 0.619 | 1.329 | 1 | 0.249 | 0.490 |
| SOB/dyspnea | 3.676 | 0.632 | 33.83 | 1 | 0.001** | 39.51 |
| Constant | -4.446 | 1.092 | 16.59 | 1 | 0.001 | 0.012 |
Distribution of COVID-19 medications
Table 5 shows that COVID-19-infected pregnant women who received multiple antibiotics, oxygen therapy, steroids, enoxaparin, and CPAP had significantly more severe COVID-19 infection than those who did not need the above treatments. P-value = 0.001 for all conditions.
| Variables | Severe (n = 26) | Not severe (n = 333) | P-value | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Antibiotics | No antibiotics | 1 | 1.2% | 81 | 98.8% | 0.001** |
| One antibiotic | 10 | 5.7% | 165 | 94.3% | ||
| Multiple antibiotics | 15 | 14.7% | 87 | 85.3% | ||
| Antiviral | No anti-viral | 23 | 7.5% | 285 | 92.5% | 0.686 |
| Antiviral | 3 | 5.9% | 48 | 94.1% | ||
| O2 | 22 | 95.7% | 1 | 4.3% | 0.001** | |
| Steroids | 17 | 20.7% | 65 | 79.3% | 0.001** | |
| Supplement | 4 | 6.6% | 57 | 93.4% | 0.821 | |
| Enoxaparin | 18 | 15.9% | 95 | 84.1% | 0.001** | |
| CPAP | 4 | 100.0% | 0 | 0.0% | 0.001** | |
Table 6 presents the distribution of the recorded risk factors according to COVID-19 severity. Pregnant women with a history of diabetes, hypertension, and previous cesarean sections presented with a significant predominance of severe cases. The mean age of severe COVID-19-infected pregnant women was significantly higher than that of not severe cases (P-value = 0.001), while gestational age did not reveal any difference between severity types (P-value > 0.05).
| Risk factors | Severe (n = 26) | Not severe (n = 333) | P-value | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||
| Diabetes | 4 | 36.4% | 7 | 63.6% | 0.001** | ||
| Hypertension | 14 | 31.1% | 31 | 68.9% | 0.001** | ||
| Anemia | 15 | 10.1% | 134 | 89.9% | 0.082 | ||
| Previous cesarean | 15 | 10.9% | 123 | 89.1% | 0.036* | ||
| Risk factors | Severe | Not severe | PV | ||||
|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||
| Age | 26 | 32.19 | 7.451 | 333 | 26.35 | 6.455 | 0.001** |
| Gestational age | 26 | 35.23 | 4.752 | 333 | 35.81 | 6.232 | 0.658 |
After adjustment for other risk factors, hypertension was found to be the most predictive risk factor for severe COVID-19 infection among pregnant women with a positive medical history (Table 7), according to the chi-square test presented in Table 6.
| Risk factors | B | S.E. | Wald | df | P-value | Exp(B) |
|---|---|---|---|---|---|---|
| DM | 1.458 | 0.768 | 3.604 | 1 | 0.058 | 4.297 |
| HT | 2.216 | 0.462 | 23.057 | 1 | 0.001** | 9.173 |
| Previous C/S | -0.305 | 0.459 | 0.442 | 1 | 0.506 | 0.737 |
| Constant | -3.950 | 1.654 | 5.707 | 1 | 0.017 | 0.019 |
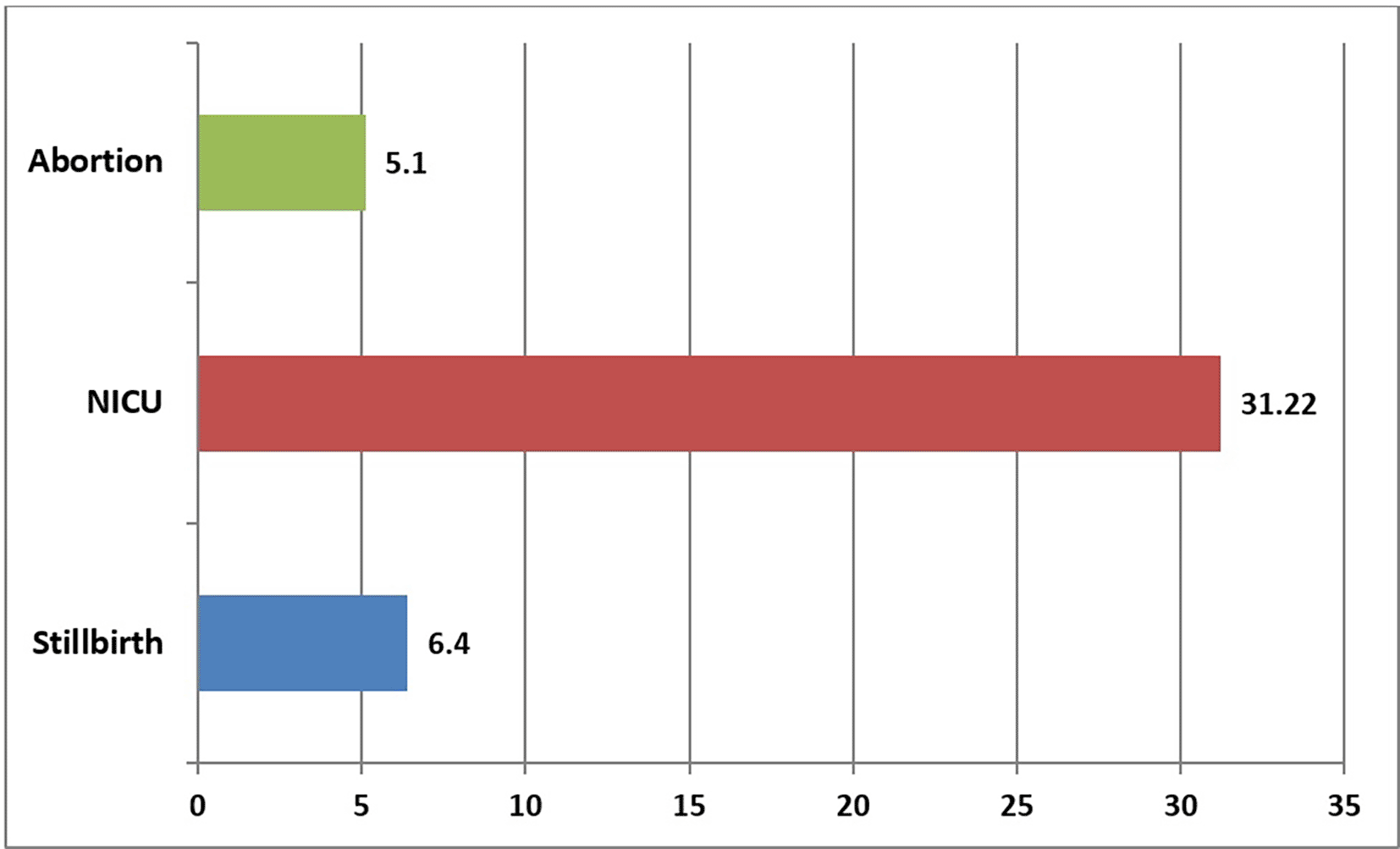
Figure 3 shows that 5.1% of pregnancies ended with abortion, and 31.22% needed NICU and 6.4% ended with stillbirths among all the studied cases. Pregnancy outcomes according to the severity of covid-19 infection, as presented in Table 5, showed that 35% (7 out of 20) of stillbirth deliveries were in mothers with severe COVID-19 infection, which is significantly higher than stillbirth in non-severe cases, 65% (13 out of 294). (P-value = 0.001), (OR = 8.8), (95%CI = 3.1-24.8). Severe COVID-19-infected pregnant women delivered 13.9% (11 out of 79) of babies requiring NICU admission, significantly higher than mothers with non-severe infection (28%; 68 out of 239) (P-value = 0.014), (OR = 2.8), (95%CI = 1.2-6.4). Severe COVID-19-infected pregnant women delivered 19.4% (14 of 72) of preterm babies, significantly higher than mothers with non-severe infections (24.6%, 58/235) (P-value = 0.001), (OR = 5.16), (95%CI = 2.23-11.95) (see Table 8).
In the current study, the mean age of the 359 pregnant women was approximately 26 years, and most of them were admitted to the hospital in the third trimester (gestational age of 35.77 weeks). These data agree with others, where the higher hospitalization rate of pregnant women with COVID-19 was in the third trimester.15,16
Recently, human health has been severely threatened by COVID-19 infections that can range in severity from asymptomatic to severe (O2 saturation < 94%, hypoxia, lung involvement, and increased respiratory rate) or critical (multiorgan dysfunction and respiratory failure).17 According to recent research, pregnant women are more likely to experience severe illnesses, including the need for hospitalization, ICU admission, and mechanical ventilation.18 Asymptomatic and mild pregnancy cases (43.5%, 35.4%) in the current study represented the most hospitalized cases, while severe cases represented the least (7.2%) of the total cases. These results agree with previous studies, which revealed that most COVID-19-infected pregnant women were asymptomatic and had mild cases.19,20
The length of hospital stay (LOHS) was defined as the duration between the patient’s admission to the hospital and discharge. Data on duration of stay provide insights into the effectiveness of care over time, including associations between length of stay and hospital-acquired conditions (HACs). For example, extended hospital stays have been linked to high mortality rates for specific diseases and an increased risk of hospital-acquired infections. The current study showed that patients who experienced significantly extended LOHS (equal to or more than three days) were those with severe COVID-19 infection and those aged ≥ 40 years. This result agrees with Wang et al. in 2022, who reported similar findings concerning the age and severity of illness with hospital stay.9
The most common symptoms of COVID-19 are fever, cough, dyspnea, and myalgia.5,21 COVID-19-infected pregnant women in this study had a fever, cough, shortness of breath, and other signs and symptoms such as nausea, vomiting, diarrhea, headache, weakness, and loss of smell or taste, which occurred in a small percentage of the included cases. These results agree with Gillian et al. in 2020, who reported that fever, cough, and SOB were the most common symptoms in pregnant women with COVID-19 infection.22 The common symptoms were more pronounced among severe COVID-19 infected pregnant women (P-value = 0.001) than those with mild or moderate cases. After adjusting for other signs and symptoms, fever and shortness of breath were the most predictable indicators of severity using binary logistic regression. Although Lian et al., in 2021, stated similar results regarding fever but argued by considering cough as a predictor of developing severe illness,23 the reason behind this argument may be due to pregnancy itself, as the shortness of breath may occur in normal pregnancy by excluding the pathological causes. It is a challenge for clinicians to differentiate.24
The data obtained in this study documented the use of multiple antibiotics in severe cases, representing 14.7% of total medications. Cephalosporins were the most commonly used group in 73% of the cases, followed by macrolides, doxycycline, and aminoglycosides. However, the use of carbapenems, quinolones, and penicillins was much lower. The use of multiple antibiotics among COVID-19-infected pregnant women, despite several guidelines that recommend antibiotics, except in the presence of secondary bacterial infection.25,26
Medical oxygen is an essential medicine in treating COVID-19 and is used mainly to alleviate the severity of the infection.27 According to the Society for Maternal-Fetal Medicine, the target SpO2 for pregnant women should be higher than that recommended for the general population (SpO2 92%) owing to the increased O2 demand during pregnancy.28 Oxygen therapy was used in 23 patients in this study; of them, 22 patients had severe COVID-19 infection, all of whom showed improvement in clinical status and alleviation of the severity of the disease. According to numerous randomized trials, systemic corticosteroid therapy enhances clinical outcomes and lowers mortality in COVID-19 hospitalized patients who require supplementary oxygen.29–31 In contrast, systemic corticosteroids have not been proven beneficial and may even be harmful in COVID-19 hospitalized patients who do not need additional oxygen.32,33 In Pregnant women at risk of premature delivery, a brief course of betamethasone or Dexamethasone, which is known to cross the placenta, is frequently used to reduce neonatal complications of prematurity and decrease maternal mortality.34,35 In 22.8% of the cases included in this study, corticosteroids were used for both maternal and fetal purposes; however, pregnant women with COVID-19 infection who received corticosteroids had a more severe COVID-19 disease than those who did not (P = 0.001), so the more severe infection results in greater corticosteroid use, which is in line with national guidelines.
Inflammation and prothrombotic conditions have been linked to COVID-19, and fibrin breakdown products, fibrinogen, and D-dimer levels have increased.36 Some studies have linked the elevation of these markers to worse clinical outcomes.37,38 In addition, pregnant women have a higher risk of thromboembolism than non-pregnant women because pregnancy is a hypercoagulable state.39 Although there are no data for or against the use of anticoagulant therapy in the context of COVID-19 during pregnancy, the American College of Obstetricians and Gynecologists (ACOG) advises that VTE prophylaxis can be considered for pregnant women hospitalized with COVID-19, especially for those with severe disease. In the current study, enoxaparin was used in 31.5% of the cases, and its use was significantly associated with severe COVID-19 in pregnant women compared to those with non-severe disease (P = 0.001). Moreover, patients with COVID-19 successfully used CPAP to avoid endotracheal intubation. However, there is little information regarding the use of CPAP in expecting mothers with acute respiratory failure (ARF) caused by SARS-CoV-2 pneumonia.40 In this study, CPAP use was significantly associated with severe COVID-19 in pregnant women (P = 0.001), and there was no need for it in the non-severe cases.
Numerous risk factors have been linked to the progression of COVID-19 into a severe and critical stage, including advanced age, male sex, and underlying comorbidities such as hypertension, diabetes, obesity, chronic lung diseases, heart, liver, and kidney diseases, tumors, and pregnancy.41,42 In the current study, COVID-19-infected pregnant women with a history of diabetes, hypertension, and previous cesarean section presented with a significant predominance of severe cases. In addition, the mean age of severe COVID-19-infected pregnant women was significantly higher (32.19 years) than that of non-severe patients. Allotey et al. 2020 mentioned that pregnant women with diabetes, hypertension, BMI > 30, and age > 35 years tend to have severe COVID-19 infection.5 Pregnant women with previous C-sections tend to have a severe infection, which may be due to the increased number of C-sections accompanied by additional complications.43 Also, individuals with COVID-19 with high blood pressure are related to higher mortality; the severity of COVID-19 may be exacerbated by inflammation and dysfunction of the immune system, gastrointestinal tract, and renin-angiotensin-aldosterone system; in addition, changes in innate cellular immunity contribute to high glucose environment, besides to more virulent growth of microbes.44,45
After adjusting for risk factors that showed a significant association with the severity of infection (hypertension, diabetes, and previous C-section), predictions of risk factors were made using binary logistic regression to determine the most predictable risk factor. The test revealed that the incidence of COVID-19-infected pregnant women presenting with hypertension and diabetes developing severe infection was 9.173 and 4.297, respectively. These results are in agreement. In addition, Lian et al., in 2021, stated that patients with advanced age, H.T., and hypertension tend to have more severe COVID-19 infection.23
Because COVID-19 affects both the mother and the fetus and negatively impacts pregnancy outcomes, pregnant women with the infection need particular care and attention.46 This study explored the association between the severity of COVID-19 and adverse pregnancy outcomes. Among the entire study population, 6.3% (n = 20) of pregnant women had a stillbirth, 25% (n = 79) were admitted to the NICU, 4.8% (n = 16) had an abortion, and 22.6% (n = 72) had preterm delivery. There were 26 cases of COVID-19-infected pregnant women who presented with severe infection (delivery occurred in 25 patients) and had a higher rate of stillbirth deliveries (7 out of 25). In addition, admission to the NICU was higher among severe COVID-19-infected pregnant women (11 out of 24) and had preterm delivery (14 out of 25) than among non-severe cases. These results agree with those of Dileep et al. in 2022, who reported a strong association between the severity of COVID-19 infection and preterm birth, NICU admission, and low birth weight.46 Another study reported severe COVID-19 disease in pregnant women was associated with preterm and stillbirth deliveries.47
The collected data were retrospectively limited to two hospitals during one year. As a result, some of the collected data (particularly laboratory investigations) were not generally distributed in the patient’s records. In addition, the medical staff did not document important information, such as weight and body mass index (BMI), which strongly contributes to severity.
Most COVID-19-infected pregnant women tend to have asymptomatic or mild cases of infection. However, severe COVID-19 illness is linked to pregnancy and birth complications, mainly among pregnant women with a positive medical history.
Zenodo: Underlying data for the impact of COVID-19 severity on pregnancy outcomes among Iraqi women: a retrospective observational study, https://doi.org/10.5281/zenodo.7607351. 48
This project contains the following underlying data:
Zenodo: STROBE checklist for: The impact of COVID-19 severity on pregnancy outcomes among Iraqi women: a retrospective observational study, https://doi.org/10.5281/zenodo.7607382. 49
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The author would like to thank Mustansiriyah University in Baghdad, Iraq, for its support in the present work, and special thanks to all field participants.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neurology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Systematic review; meta-analysis; maternal health
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmaceutical science, clinical pharmacy, pharmacogenetics, medicine, and clinical trials.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 27 Feb 23 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
My comments toward improvement of this manuscript:
(A) Major Concern:
1) The Title: is not fully informative as it reflects only a single ... Continue reading Thank you for this important research.
My comments toward improvement of this manuscript:
(A) Major Concern:
1) The Title: is not fully informative as it reflects only a single part of the (about three) objectives described in the Introduction and Abstract of this article.
(B) Moderate and Minor Concerns:
*1) ‘Introduction’:
Authors may wish to present epidemiological data of the global and local burden of the disease in general and in this group. The rationale/significance of the study should be clearly explained in this section
*2) Sentence in introduction:”is the source of the coronavirus disease“
Suggestion: Change ‘source’ to a more appropriate expression (e.g. ‘causative agent’)
*3) Sentence in introduction:”global pandemic a significant loss"
-"global pandemic and significant loss”
*4) Sentence in introduction:”Severe acute respiratory syndrome coronavirus infection in pregnant women can cause...”
Substitute with the acronym ("SARS-CoV-2 infection in pregnant...etc")
*5) Sentence in introduction:”…than non-pregnant people"
Substitute 'people' with 'women' or 'ones'
*6) Sentence in introduction:”Compared to non-pregnant women, pregnant women with COVID-19 are less likely to experience symptoms”
To remove any misunderstanding it is better to complete the idea by linking it together with the other more important and relevant fact about being more likely to require ICU admission and mechanical ventilation. Remember to cite.
*7) Sentence in introduction:”according to recommendations from the Society for Maternal- Fetal Medicine, American College of Obstetricians and Gynecologists, and CDC"
The mentioned bodies may be operating in USA only. The population under study is in Iraq. So unless the Iraqi authority had adopted them, authors may wish to change to “according to international protocols and recommendations”
*8) Sentence in introduction:”The primary objectives of this study were to identify the most common symptoms associated with COVID-19 infection during pregnancy”
Should mention “Iraqi women”
*9) Methods::
Authors may wish to succinctly introduce data about the study area.
*10) Study design
It is preferable to bring this part first before the ethical considerations and consent paragraphs and move the latter two to the end of the section.
*11) Inclusion criteria, item 1 “Pregnant women with COVID-19 positive PCR … without symptoms”:
Unless authors mean OPD patients, this broad inclusion criteria already covers the next two (2 and 3)
*12) Figure1. in the ‘Excluded’ label:
Since data were secondary from patients’ files and no consent was sought, how come “13 declined to participate”?
*13) Exclusion criteria:
Authors may wish to briefly mention reasons for exclusion in items 2 and 3
*14) Sentence in ‘Bias’: “But the authors of this study were keen to ensure that every eligible individual has an equal probability of selection…etc”
Authors may either show clearly what they exactly did to avoid bias or they may rephrase and summarize the whole paragraph and move down to be included within the limitations.
*15) Figure 3:
Live healthy birth should also be added as an outcome, otherwise label the Figure as: “unfavorable (or abnormal) outcomes of pregnancy …etc”
*16) Table 8:
Same as above
*17a) Conclusion:
Too brief. Should be extended a bit to reiterate all the objectives, summarize the important findings and include some recommendations.
*17b) Conclusion; sentence “…pregnant women with a positive medical history.
“Authors may wish to clearly name these medical conditions here.
*18) Other observations:
I feel as if an important risk factor was overlooked: 'twin/multiple pregnancy' ..??
Thank you
Yasir E. A. Elsanousi, MBBCh, DTM&H, MeHM, EMDM
Infectious Disease Specialist, Disaster Medicine Consultant
My comments toward improvement of this manuscript:
(A) Major Concern:
1) The Title: is not fully informative as it reflects only a single part of the (about three) objectives described in the Introduction and Abstract of this article.
(B) Moderate and Minor Concerns:
*1) ‘Introduction’:
Authors may wish to present epidemiological data of the global and local burden of the disease in general and in this group. The rationale/significance of the study should be clearly explained in this section
*2) Sentence in introduction:”is the source of the coronavirus disease“
Suggestion: Change ‘source’ to a more appropriate expression (e.g. ‘causative agent’)
*3) Sentence in introduction:”global pandemic a significant loss"
-"global pandemic and significant loss”
*4) Sentence in introduction:”Severe acute respiratory syndrome coronavirus infection in pregnant women can cause...”
Substitute with the acronym ("SARS-CoV-2 infection in pregnant...etc")
*5) Sentence in introduction:”…than non-pregnant people"
Substitute 'people' with 'women' or 'ones'
*6) Sentence in introduction:”Compared to non-pregnant women, pregnant women with COVID-19 are less likely to experience symptoms”
To remove any misunderstanding it is better to complete the idea by linking it together with the other more important and relevant fact about being more likely to require ICU admission and mechanical ventilation. Remember to cite.
*7) Sentence in introduction:”according to recommendations from the Society for Maternal- Fetal Medicine, American College of Obstetricians and Gynecologists, and CDC"
The mentioned bodies may be operating in USA only. The population under study is in Iraq. So unless the Iraqi authority had adopted them, authors may wish to change to “according to international protocols and recommendations”
*8) Sentence in introduction:”The primary objectives of this study were to identify the most common symptoms associated with COVID-19 infection during pregnancy”
Should mention “Iraqi women”
*9) Methods::
Authors may wish to succinctly introduce data about the study area.
*10) Study design
It is preferable to bring this part first before the ethical considerations and consent paragraphs and move the latter two to the end of the section.
*11) Inclusion criteria, item 1 “Pregnant women with COVID-19 positive PCR … without symptoms”:
Unless authors mean OPD patients, this broad inclusion criteria already covers the next two (2 and 3)
*12) Figure1. in the ‘Excluded’ label:
Since data were secondary from patients’ files and no consent was sought, how come “13 declined to participate”?
*13) Exclusion criteria:
Authors may wish to briefly mention reasons for exclusion in items 2 and 3
*14) Sentence in ‘Bias’: “But the authors of this study were keen to ensure that every eligible individual has an equal probability of selection…etc”
Authors may either show clearly what they exactly did to avoid bias or they may rephrase and summarize the whole paragraph and move down to be included within the limitations.
*15) Figure 3:
Live healthy birth should also be added as an outcome, otherwise label the Figure as: “unfavorable (or abnormal) outcomes of pregnancy …etc”
*16) Table 8:
Same as above
*17a) Conclusion:
Too brief. Should be extended a bit to reiterate all the objectives, summarize the important findings and include some recommendations.
*17b) Conclusion; sentence “…pregnant women with a positive medical history.
“Authors may wish to clearly name these medical conditions here.
*18) Other observations:
I feel as if an important risk factor was overlooked: 'twin/multiple pregnancy' ..??
Thank you
Yasir E. A. Elsanousi, MBBCh, DTM&H, MeHM, EMDM
Infectious Disease Specialist, Disaster Medicine Consultant