Keywords
open field assay, Trpm8, Temperature
The open field assay is used to study anxiety-related traits and anxiolytic drugs in rodents. This assay entails measuring locomotor activity and time spent in the center of a chamber that is maintained at ambient room temperature. However, the ambient temperature in most laboratories varies daily and seasonally and can differ between buildings. We sought to evaluate how varying ambient temperature and core body temperature (CBT) affected open field locomotor activity and center time of male wild-type (WT, C57BL/6) and Transient Receptor Potential Subfamily M Member 8 (Trpm8) knock-out (Trpm8-/- ) mice. TRPM8 is an ion channel that detects cool temperatures and is activated by icilin.
Mice were placed in the open field at 4°C and 23°C for 1 hour. Distance traveled and time spent in the center were measured. Mice were injected with icilin, M8-B, diazepam, or saline, and changes in activity level were recorded.
The cooling agent icilin increased CBT and profoundly reduced distance traveled and center time of WT mice relative to controls. Likewise, cooling the ambient temperature to 4°C reduced distance traveled and center time of WT mice relative to Trpm8-/- mice. Conversely, the TRPM8 antagonist (M8-B) reduced CBT and increased distance traveled and center time of WT mice when tested at 4°C. The TRPM8 antagonist (M8-B) had no effect on CBT or open field behavior of Trpm8-/- mice. The anxiolytic diazepam reduced CBT in WT and Trpm8-/- mice. When tested at 4°C, diazepam increased distance traveled and center time in WT mice but did not alter open field behavior of Trpm8-/- mice.
Environmental temperature and drugs that affect CBT can influence locomotor behavior and center time in the open field assay, highlighting temperature (ambient and core) as sources of environmental and physiologic variability in this commonly used behavioral assay.
open field assay, Trpm8, Temperature
To address the Reviewers comments, the Abstract Methods were corrected to state an open-field duration of 1 hour. The use of male mice only was re-emphasized and stated as a limitation in the discussion. The rationale for doses tested was elaborated as part of the previous revision and an additional reference was included. It was highlighted that skin temperature was not tested, rather core body temperature was measured with a rectal thermometer. An additional reference was included and described to demonstrate that previous studies have shown a rapid change in core body temperature post-icilin injection using thermal telemetry and at doses lower than the one used in the current study. This is to highlight that our results may be due to the use of a less-sensitive device and lower sample size. Finally, our response emphasizes that the behavior of Diazepam and M8-B injected mice are compared with saline-injected controls in Figure 5. The Reviewer is also guided to a new Figure 3, which was part of the previous revision and better represents the experiment.
See the authors' detailed response to the review by Fiona Hollis
See the authors' detailed response to the review by Spyridon Sideromenos
Drugs used to treat neurological and neuropsychiatric disorders are typically evaluated in rodent models for safety and efficacy prior to use in humans.1 Characterizing animal models of neuropsychiatric disorders often relies on behavioral traits such as motor function, social interactions, anxiety-like and depressive-like behavior, substance dependence, and various forms of cognitive function.2 Due to the complexity of most behavior tests, researchers must carefully consider the sources of variability introduced by experimenters, testing environments, and intraspecies differences.
Rodent physiology and behavior are influenced by environmental temperature.3,4 For example, an innocuous cold stimulation at 15°C altered sleeping, rearing, climbing, and eating behavior in wild-type (WT) mice.5 This cold stimulation did not alter these behaviors in mutant mice lacking the Transient Receptor Potential Subfamily M Member 8 (TRPM8) cation channel, which is a receptor for menthol and icilin (mint-derived and synthetic cooling compounds, respectively) and plays an important role in thermosensation.5,6 Additionally, mice deficient in uncoupling protein 1 (UCP-1), a key metabolic regulator highly expressed in brown adipose tissue, were reported to display selective enhancement of anxiety-related behavior exclusively under thermogenic conditions (23°C), but not at thermoneutrality (29°C).7
Environmental temperature sensation and perception is also influenced by core body temperature (CBT). Alterations to CBT can be a consequence of physiological changes associated with disease state, exercise, metabolic function, and hormonal changes. Further, numerous drugs can affect body temperature including barbiturates, cyclic antidepressants, hypoglycemic agents, opioids, antihistamines, and anticholinergic drugs.8–11 It is currently unclear if drugs such as these, which are used to treat neurological and neuropsychiatric disorders, affect CBT directly or indirectly, and if the behavioral tasks that are commonly used to study these drugs are influenced by changes in CBT.
Here we sought to evaluate how ambient temperature and changes in CBT influence locomotor activity and center time in the open field assay—an assay that is commonly used to study anxiolytic drugs and animal models of anxiety. In addition to using WT mice, we also used Trpm8-/- mice, which lack the primary receptor for cool temperature sensation in mammals, as well as drugs that activate (icilin) or antagonize (M8-B) this receptor. Diazepam was also evaluated as a model anxiolytic drug. By precisely controlling environmental temperature and TRPM8 activity (genetically and pharmacologically), we found that commonly used measures associated with the open field assay are profoundly sensitive to ambient temperature and CBT. To enhance rigor and reproducibility, we recommend that ambient and core temperature be precisely controlled when performing the open field assay. Moreover, drugs that increase activity and center time in the open field test may do so via thermoregulatory mechanisms, independent of effects on anxiety.
Animal protocols in this study were approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill and were performed in accordance with these guidelines and regulations at the University of North Carolina at Chapel Hill (NIH/PHS Animal Welfare Assurance Number D16-00256 A3410-01, expiration April 30, 2025; USDA Animal Research Facility Registration Number 55-R-0004; AAALAC Institutional Number #329, re-accreditation November 2020). All data presented in this study are from male mice obtained from crossing Trpm8+/- male with Trpm8+/- female mice. Trpm8 mutant mice were obtained from Jackson Laboratories (B6.129P2-Trpm8tm1Jul/J; stock #008198). Mice were raised in a facility with a 12 h:12 h light:dark cycle with ad libitum access to food (Teklad 2020X, Envigo, Huntingdon, UK) and water. All mice were tested at 8-12 weeks of age. Mice were excluded if they showed signs of distress or lethargy. Genomic DNA was isolated from tail clips using Proteinase K. Genotyping was performed by polymerase chain reaction (PCR) amplification of genomic DNA with primers: WT Forward 5′-CCT TGG CTG CTG GAT TCA CAC AGC-3′, Mutant Reverse 5′-CAG GCT GAG CGA TGA AAT GCT GAT CTG-3′, WT Reverse 5′-GCT TGC TGG CCC CCA AGG CT-3′. Premade buffers along with the platinum Taq were used for amplification (Invitrogen) to amplify DNA in a BioRad DNA Engine (PTC-200). Nucleotides were obtained from Qiagen. Amplification cycle was as follows: 94°C for 3 min, 36 cycles of 94°C for 30 s, 68°C for 60 s and 72°C for 60 s. A final incubation was performed at 72°C for 2 min. The same mice were used for tests in Figures 3-6. All tests were performed 1 week apart.
Drugs or control (saline or DMSO) were administered intraperitoneally (i.p.). Icilin (I9532-50MG, Sigma) was dissolved in DMSO and administered at 50 mg/kg body weight (bw). Diazepam (RXDIAZEP5-10, Shop Med Vet) was diluted in saline to 1 mg/mL administered at 2 mg/kg bw. M8-B hydrochloride (SML0893-25MG, Sigma) was dissolved in DMSO to 6 mg/ml and administered at 12 mg/kg bw.
CBT was assessed using a Digi-Sense Thermocouple Meter (Fisher 13-245-293) to measure rectal temperature. Male mice were acclimated to the procedure 2x each day for one week prior to testing. Temperature was measured 30, 60 and 90 minutes post drug administration for diazepam and M8-B. To assess the effects of icilin on CBT, measures were taken every 15 minutes.
Exploratory activity in a novel environment was assessed by a 1 h trial in an open-field chamber (45 cm × 45 cm × 40 cm) 30 minutes post icilin or diazepam administration, and 1 h following M8-B administration. The total distance moved by each mouse in the open arena, and time spent in the center region of the open-field, were recorded by camera (Sony) connected to the EthoVision software (Noldus Wageningen). Testing was performed at room temperature (23°C, lighting: 660 lux) or in the cold room (4°C, lighting: 400 lux).
Data were graphed using GraphPad Prism (v9.5.0) and analyzed with a paired t-test approach. Open field comparing genotype and temperature was analyzed using mixed effect analysis with multiple comparisons, Sidak (Figure 3). All studies were randomized, double blind, and vehicle-controlled consisting of 6-14 mice. All animals were group housed.
An earlier version of this article can be found on bioRxiv (doi: https://doi.org/10.1101/2022.10.17.512128).
We found that the ambient temperature varied throughout the day (data not shown, can be found as Underlying data20), week, and season in a room that we previously used for behavioral studies (Figure 1A-C, Building 1).20 Temperature fluctuations are presumably common in laboratory settings because building heating, ventilation, and air conditioning are set to maximize human comfort during the work day and minimize energy use during off-peak hours, like evenings and on weekends. Moreover, room temperature was over 4°C warmer in the winter months and 2°C warmer in the summer months in a different laboratory located in a different building (Building 2; Figure 1B and C). Temperature differences over days, seasons, and buildings represent a major source of variability, especially for behavioral experiments that are carried out at “room temperature” and that could be influenced by temperature. To address this source of uncontrolled variability, we worked with the university to custom engineer the heating, ventilation, and air conditioning within our behavioral room so that the temperature could be precisely maintained at a set temperature (we chose 23°C) without fluctuations over the course of the day and seasons (Figure 1B and C). This temperature-controlled room, and a cold room set at 4°C, were used for all subsequent behavioral studies.

(A) The average temperature in a semi-temperature controlled room for each month of one year (n=11-14). Temperature during the (B) winter and (C) summer measured every 20 minutes for one week within lab space, located in two different buildings and in a room that was specifically engineered to maintain temperature with minimal fluctuations over hours, days, weeks, and years (n=3-4). Temperature monitored with a La Crosse Technologies weather station.
TRPM8 is a principal sensor of cold temperatures in mammalian primary sensory neurons.12,13 To explore the impact of TRPM8 stimulation on open field behavior, we administered (i.p.) 50 mg/kg bw icilin, a TRPM8 agonist, or DMSO control to WT mice. Previous studies have shown a rapid change in core body temperature post-icilin injection using thermal telemetry and at doses lower than the one used in the current study, and these significant changes in CBT lasted at least 90 minutes.14 Using a less sensitive device to measure CBT, we did detect an Icilin-induced significant increase in CBT beginning at 90 minutes post injection, consistent with these studies (Figure 2A). We likely did not see an earlier change in CBT due to our low sample size and less sensitive temperature detection method. To assess the impact of increased CBT on open field behavior, we administered 50 mg/kg bw icilin, waited 10 minutes, and then measured distance traveled and time spent in the center (Figure 2B and C). Icilin administration significantly reduced activity in the open field, suggesting that an increase in CBT led to a reduction in open field behavior.

(A) CBT was measured every 15 minutes following 50 mg/kg bw i.p. administration of icilin (n=14) or vehicle (n=11). (B) Distance traveled and (C) time spend in the center in the open field at 23°C was measured 10 minutes post-icilin administration for one hour. Data represent means ± SEM. CBT, core body temperature; WT, wild-type; bw, body weight; i.p., intraperitoneally.
Stimulation of TRPM8 channels with icilin impacts the behavior of WT mice in the open field (Figure 2). Thus, we hypothesized that cold stimulation of TRPM8 channels would similarly impact open field behavior and that mice lacking TRPM8 channels would resist the effect of cold stimulation on open field behavior. We found that WT and Trpm8-/- mice display similar distance traveled and time spent in the center when tested at 23°C (Figure 3A and B). When mice were tested at 4°C, an effect of genotype was revealed, in which the WT mice display reduced distance traveled and center time compared to Trpm8-/- mice (Figure 3A and B).

(A) Distance traveled and (B) center time were assessed in WT and Trpm8-/- mice at room temperature (23°C) and in the cold room (4°C). Data represent means ± SEM and compares genotype and temperature using mixed effect analysis with multiple comparisons (Sidak). n=8-15 mice. WT, wild-type; Trpm8, Transient Receptor Potential Subfamily M Member 8. *p<0.05, **p<0.01, ****p<0.0001.
We next used a TRPM8 antagonist (M8-B) to block cold-induced stimulation of TRPM8 channels in WT mice. Administration (i.p.) of M8-B at 12 mg/kg bw decreased the CBT of WT but not Trpm8-/- mice at >1 h post injection at room temperature (Figure 4A). Thus, mice were placed in the open field chamber 1 h following M8-B administration. We found that TRPM8 antagonist administration partially recovered the reduction in open field behavior at 4°C in WT mice (Figure 4B and C, Figure 5A and B). These data suggest that environmental temperature sensation influences open field behavior in mice.

(A) Effect of 12 mg/kg bw M8-B- i.p. administration on CBT of WT (n=8) and Trpm8-/- mice (n=8). Total distance traveled (B) and center time (C) at 4°C 1 h following 12 mg/kg bw M8-B administration. Data represent means ± SEM. n=8-10 mice. Trpm8, Transient Receptor Potential Subfamily M Member 8; WT, wild-type; bw, body weight; CBT, core body temperature; i.p., intraperitoneal.

(A) Distance traveled and (B) center time in WT mice. (C) Distance traveled and (D) center time in Trpm8-/- mice. Data represent means ± SEM. n=6-8 mice. WT, wild-type; Trpm8, Transient Receptor Potential Subfamily M Member 8. Data in this figure are also shown in Figures 4B and 4C and 6B and 6C.
Benzodiazepines, such as diazepam, are commonly prescribed to reduce anxiety in humans. Diazepam functions to increase gamma-aminobutyric acid (GABA) in the brain and is used to treat anxiety. To investigate whether the anxiolytic effect of diazepam was associated with a reduction in CBT in mice, WT and Trpm8-/- mice were administered 2 mg/kg bw of the drug at room temperature. Diazepam exposure led to a reduction in CBT at 30–90 minutes post injection (Figure 6A). This reduction in CBT was associated with a near-significant increase in distance traveled and center time displayed by WT but not Trpm8-/- mice, when tested at 4°C (Figure 6B and C, Figure 5).
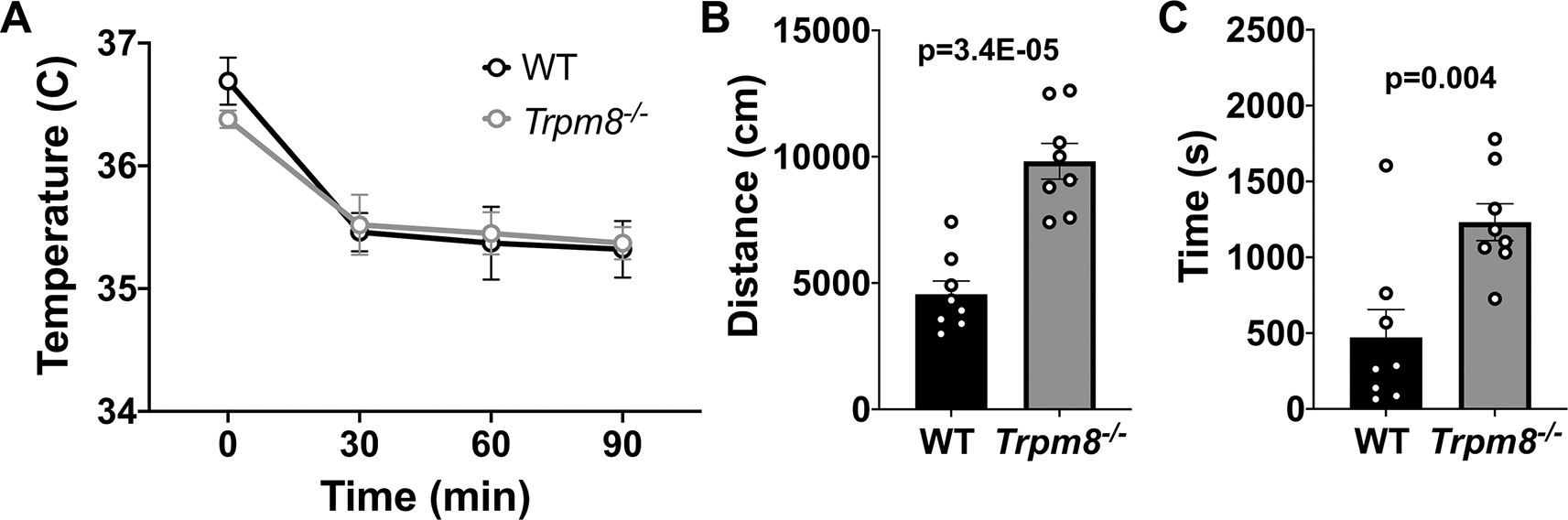
(A) CBT measured at 30, 60 and 90 minutes post 2 mg/kg bw diazepam in WT (n=8) and Trpm8-/- mice (n=8). (B) Total distance traveled and (C) time spent in center at 4°C, 30 minutes post 2 mg/kg bw diazepam administration. Data represent means ± SEM. N=8-10 mice. CBT, core body temperature; bw, body weight; WT, wild-type; Trpm8, Transient Receptor Potential Subfamily M Member 8.
In this study we compared the behavioral effects of the anxiolytic drug diazepam, with the effects of other drugs that alter cool temperature sensation and CBT, including icilin and M8-B in male WT and Trpm8-/- mice. We observed CBT and open field behavioral effects of icilin and M8-B in WT mice, but not Trpm8-/- mice.
The effect of drugs on core body temperature may be mediated by acting on any component of the thermoregulatory system. These components include heat production, heat conservation, and thermosensing-related pathways within the nervous system that coordinate thermoregulation.8 Clark et al.,8 present a thorough study of drug-induced changes in body temperature and provide a source of information on interactions between certain drugs and the thermoregulatory system. The data present an extensive review of the magnitude of body temperature changes induced by psychoactive compounds while taking into account the species, administration route, dose, and environmental temperature differences. However, with psychoactive drugs, including the drugs used in this study, it is difficult to discern the underlying cause of behavior effects. Here we selected drug doses that are commonly used and well tolerated by mice in the behavior field.15–18 DMSO at the concentration used to dissolve icilin and M8-B have also been reported to be well tolerated by mice.19 Of note, our vehicle controls were saline injected in Figure 5, which may limit the interpretations of this study. Considering the effects of drug-induced changes on body temperature and the impact of CBT on behavior, studies using rodent models of psychological disorders should consider potential alterations to the perception of environmental temperatures.
The spontaneous behavior of animal models is often used to evaluate the efficacy of drugs used to treat neuropsychiatric disorders. One main concern with animal models is the lack of standardization between laboratories, which can lead to results that are not reproducible. We suggest that stricter testing protocols include assessment of room temperature and control for drug-induced alterations to CBT. For consistency, only male mice were used in this study. However, it should be stated that female mice may display different drug-induced behavioral responses and should be explored in future studies. Ideally, the lighting conditions between all testing environments should be identical. In this study, the animals experienced a slight difference in lighting between the 23°C (660 lux) and 4°C (400 lux) conditions. However, both settings provided very bright conditions and the slight variation is not expected to have altered avoidance behavior.
While the testing environments used in this study (23°C and 4°C) represent a wider temperature range than is observed between laboratories, this proof of principle study demonstrates that temperature can have an influence on behavior. More importantly, in this study we demonstrate that the body temperature changes induced following drug administration could lead to similar physiological responses induced by normal room temperature fluctuations.
By providing greater understanding of the relationship between body temperature and behavior in mice, our data highlight the importance of assessing CBT, environmental temperature, and drug-induced changes to thermoregulation. Thus, consideration of ambient and CBT is a straightforward approach to enhance rigor and reproducibility in studies of neuropsychiatric disorders.
Figshare: The open field assay is influenced by room temperature and by drugs that affect core body temperature. https://doi.org/10.6084/m9.figshare.21954539. 20
This project contains the following underlying data:
- csv. files containing the data for each individual graph represented in the paper. Each file has been named according to the experiment performed.
- Completed ARRIVE checklist
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Behavioral neuroscience
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Environmental physiology, Thermoregulation
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neuroscience
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Behavioral neuroscience
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Pádua-Reis M, Nôga DA, Tort ABL, Blunder M: Diazepam causes sedative rather than anxiolytic effects in C57BL/6J mice.Sci Rep. 2021; 11 (1): 9335 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Behavioral neuroscience
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 4 (revision) 03 Jul 24 |
read | read | |
|
Version 3 (revision) 19 Feb 24 |
read | read | |
|
Version 2 (revision) 07 Dec 23 |
read | ||
|
Version 1 02 Mar 23 |
read | ||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)