Keywords
Epistaxis, Chromolaena odorata, antithrombotic, antioxidant, angiogenesis
Epistaxis, Chromolaena odorata, antithrombotic, antioxidant, angiogenesis
Epistaxis (nasal bleeding) is a symptom of several diseases such as nose trauma and cardiovascular diseases. It has been reported in 60% of the general population, most commonly in individuals younger than 10 or older than 50.1 Neovascularization (organic formation of blood vessels) and angiogenesis (growth of blood vessels from the pre-existing vasculature) are core mechanisms in epistaxis healing by involving cluster of differentiation 34 (CD34) as a marker of hematopoietic progenitor cells, assisted by CD68, which is able to clear cellular waste and facilitate the attraction and activation of macrophages.2
Herbal medicine for epistaxis management has been intensively studied recently since it could be safer. Chromolaena odorata L. (C. odorata) is a prairie weed found in almost all parts of Indonesia and other tropical countries. The leaves contain compounds such as flavonoids, saponisn, tannins and terpenoids.3,4 All of which are important in the coagulation underlying wound healing, due to their antioxidant activities that inhibit oxidative damage.4 A previous study reported that the 70% ethanolic extract of C. odorata was highly effective for stopping bleeding in Wistar rats5 and its compounds, like stigmasterol and scutellarin tetramethyl ether, have hemostatic6 and anti-inflammatory properties.7 Another study also found that C. odorata leaves in a cream formulation stimulated granulation tissue formation and wound re-epithelialization by increasing fibroblasts and skin cell proliferation, which have been clinically and histologically proven.8
Epistaxis can be treated with a variety of topical vasoconstrictor agents such as in a gel formulation. Gel is less greasy and easily washed off, so it leaves the treated wound with no unpleasant smell.9 This study aimed to assess the hemostatic and angiogenesis effects of C. odorata leaf extract gel in an animal epistaxis model.
An experimental study was conducted to assess the hemostatic and angiogenesis activities of C. odorata leaf extract gel in an animal epistaxis model. A total of 30 healthy male rabbits (Oryctolagus cuniculus) were divided into five groups (one group for each negative and positive control and three groups for different concentrations of C. odorata extract). After epistaxis was created in all animals, the C. odorata extract gel was applied twice a day and the animals were followed until 21 days. The hemostatic activity was evaluated by measuring the clotting time required after the first gel was applied. The angiogenesis activity was evaluated by measuring C. odorata extract’s effect on serum inflammatory cell profiles, serum CD34 and CD68 expression and the histopathology of angiogenesis and fibroblast on days 7, 14 and 21 post-epistaxis.
The leaves of C. odorata were collected from Darussalam, Banda Aceh, Indonesia and herbarium identification was conducted at the Department of Biology in the Faculty of Mathematics and Natural Sciences, Universitas Syiah Kuala, Banda Aceh, Indonesia. The extraction procedure was conducted at the Pharmacology Laboratory, Faculty of Mathematics and Natural Sciences, Universitas Syiah Kuala. In brief, 5 kg of leaves were cleaned with distilled water, sliced into pieces, and dried in the shade for 72hrs. The dried leaves were mechanically powdered with a blender. A total of 1,500 g of powder was divided into 10 bottles of dark macerator, 70% ethanol was added and they were left for 18hrs at room temperature. The mixture was separated by filtration using flannel twice, using the 70% ethanol. The filtrate was concentrated with a rotary evaporator (Buchi R300, Wellington, New Zealand) until a pure extract was obtained.10
The putative compounds were determined using quantitative analysis with gas chromatography and mass spectroscopy (GC-MS) (QP2010 SE, Shimadzu, Kyoto, Japan), controlled with LabSolutions DB/CS software (Shimadzu, Kyoto, Japan). It was started by methylating C. odorata extract in which15 ml of methanol was added. The mixture was then left for 2hrs at room temperature and evaporated until dried. The sample obtained was then added with 10 ml of methanol containing 2 ml of HCl, heated for 1.5hrs and it was separated using column chromatography with silica gel for the stationary phase and hexane-chloroform (9:1) for the mobile phase. The injector temperature was 250°C. The oven temperature was set at 140°C for 10 mins and increased by 7°C/min to 250°C for 10 mins.
The bioactivity score of C. odorata extract was calculated using web-based software (Molinspiration Cheminformatics 2018 on the Web, Bratislava, Slovak Republic). The result was based on the compounds activity in response to G-protein coupled receptor (GPCR) ligand, ion channel modulator, kinase inhibitor, nuclear receptor ligand, protease inhibitor and enzyme inhibitor. The bioactivity was assessed using a score scale: greater than 0.00 indicated significant biological activity, between -0.50 and 0.00 indicated moderate activity and less than -0.50 indicated inactivity.11
Four gel formulations were each made using different concentrations of C. odorata extract. The ingredients are presented in Table 1. A total of 5, 10 and 15 g of C. odorata extract was used to formulate the gel-II (5% w/w, called Co5% group), gel-III (10% w/w, called Co10% group) and gel-IV (15% w/w, called Co15% group), respectively, and then dissolved in a heated water bath, at approximately45°C. To create a homogenous gel, glycerin, propylene glycol and water were added to the mixture while continuously stirring with sodium carboxymethyl cellulose (Na-CMC). The gel was kept overnight at 10-15°C in a dark room. The final weight was adjusted with aquadest to yield a total weight of 100 g.
Evaluation of gel preparations included organoleptic qualities, pH, homogeneity, viscosity and spreadability. An organoleptic test was conducted by assessing the gel visually including the shape, color and odor of each gel preparation. The pH of gel was determined using a universal pH indicator stick. To assess the homogeneity, the gel was applied to a piece of glass to check whether it showed any coarse grains. A digital Rion viscometer (Thermo Scientific, Brookfield, WI, US) was used to assess gel viscosity. The spreadability, the rate (in seconds) at which two slides separate from gel placed between them, was measured using a 7.5 cm glass slide and the specified weight tied to the upper plate was 20 g.
A total of 30 healthy male rabbits (Oryctolagus cuniculus) were used in this study. The animals were maintained separately in sterile cages with 12hr light/dark cycles at 22°C ± 4°C. Food and water were provided ad libitum. The animal adaptation was carried out for one week prior to study. Ill and underweight rabbits were excluded.
All rabbits were sedated with 5 mg/kg intramuscular xylocaine hydrochloride (23.32 mg/mL) and 50 mg/kg ketamine chloride (Parker Davis and Company, Detroit, Michigan, US). A standard mucosal wound was made with a 3mm surgical punch to the rabbit nasal septum. The surgical punch was applied to the nasal mucosa over the right and left front of the septum using slight pressure and was rotated 90° clockwise, followed by 90° counterclockwise to assemble a full-thickness mucosal injury without damaging the cartilage of the nose septum. A total of 0.2 ml of the gel was applied soon after the surgical punch and repeated twice a day. All procedures were conducted by a registered veterinarian with animal care certification.
To measure the homeostasis effect of C. odorata extract, the time required for the blood to stop after the first gel was applied was measured. The times were expressed in minutes.
To measure the blood electrolytes, blood samples were collected on days 7, 14 and 21. Electrolytes in serum samples were examined using an electrolyte analyzer with the ion-selective electrode method (Guangzhou Happycare Electronics, Guangdong, China). Briefly, the suction needle of the electrolyte analyzer was inserted into the serum cup and left to collect the serum for ± 2 seconds. The results of the serum electrolyte levels were recorded as per the manufacturer’s protocol (Guangzhou Happycare Electronics, Guangdong, China).
The titers of serum CD34 and CD68 were measured using Rabbit Cluster of Differentiation 34 (CD34) and CD68 ELISA Kits from ELK Biotechnology (ELK Biotechnology, Wuhan, China). Venous blood samples were collected from the lateral ear vein on days 7, 14 and 21, and serum was prepared by centrifuging the blood at 4000 rpm for 3 mins. A total of 10 μL of serum was used to measure the level of CD34 and CD68 following the manufacture’s protocol (ELK Biotechnology, Wuhan, China). The sensitivity limit of both kits is 0.065 ng/mL while the detection range is between 0.16–10 ng/mL.
To provide the histopathology of angiogenesis, the animals were sacrificed using chloroform at day 21. The nasal septal mucosa was preserved using formaldehyde, and a tissue dehydration process was conducted using serial concentrations of alcohol based on standard protocol.12 The histopathology slices were prepared as thin as 4 μm each based on the protocol.12 Angiogenesis was measured by counting the number of fibroblasts and the new blood vessels through histopathology examination. They were assessed and counted using a BX51M light microscope (Olympus, Tokyo, Japan) with 400x magnification. Each observation was repeated in 5 fields of view.
Bleeding time, the concentration of serum electrolytes, inflammatory cell number, titers of CD34 and CD68 were compared between groups using one-way analysis of variance (Anova) or Kruskal-Wallis analysis as appropriated. All analyses were conducted using SPSS software ver. 24 (SPSS Inc., Chicago, IL, USA) (RRID:SCR_019096).
The protocol for this study and its use of rabbits was approved by the Research Ethics Committee at the Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh (no. 127/KEPH/XII/2021). To minimize the distress to the animals, the appropriate housing with ad libitum feeding was provided during the study while to minimize the pain, anesthesia was used before the rabbits were sacrificed. The procedures were conducted by a veterinarian with animal care certification.
The putative compounds found in the ethanol extract of C. odorata were antithrombotic, antioxidant and anti-inflammatory, indicated as nos. 9, 12, 19, 20, 21, 27, 33, and 35 in Table 2. The eight compounds have effects that increase immune responses and angiogenesis. Figure 1 depicts the quantity and the tendency of peak values of related compounds.
The bioactivity scores for each putative compound from C. odorata extract are presented in Table 3. Compound 19 (neo-menthol) was the most inactive while compound 35 (octacosanol) was the most active against all six molecule groups.
Among the eight antithrombotic, antioxidant and anti-inflammatory compounds, our findings enabled the following observations:
a. GPCR ligand: compounds 9, 12, 21, 27, 33 and 35 were highly active (> 0), compound 20 was moderately active (≤ 0) and compound 19 was inactive (< -0.50).
b. Ion channel modulator: compounds 12, 20, 21, 27 and 35 were highly active (> 0), and compounds 9, 19 and 33 were moderately active (≤ 0).
c. Kinase inhibitor: compound 35 was highly active (> 0), compounds 9, 20, 21, 27, and 33 were moderately active (≤ 0) and compounds 12 and 19 were inactive (< -0.50).
d. Nuclear receptor ligand: compounds 12, 21, 27, 33, 35 were highly active (> 0), compounds 9 and 20 was moderately active (≤ 0) and compound 19 was inactive (< -0.50).
e. Protease inhibitor: compounds 21, 33, and 35 were highly active (> 0), compound 9, 12, 20, and 27 were moderately active (≤ 0) and compound 19 was inactive (< -0.50).
f. Enzyme inhibitor: compounds 9, 12, 20 21, 27, 33, and 35 were highly active (≥ 0) and only compound 19 was moderately active.
In epistaxis rabbits, each concentration of C. odorata ethanol extract gel had a different ability to stop the bleeding (Table 4). The one-way Anova revealed that there was no significant difference in bleeding time between the groups (p=0.928).
The levels of the blood electrolyte of rabbits in the treatment and control groups on days 7, 14 and 21 are presented in Table 5. In rabbits, the normal range values of the electrolytes are sodium (130-155 mmol/L), potassium (4.0-6.5 mmol/dL) and chloride (92-120 mmol/L).13 Almost all the animals from all groups had normal electrolyte levels on days 7, 14 and 21 post-epistaxis. Our data suggested that there were no significantly different levels of Na, K and Cl among groups in each time series (Table 5).
After 7 days of treatment, CD34 concentrations were relatively higher in the positive control and the treatment groups compared to the negative control group and this was maintained until day 21. At days 7, 14 and 21 the highest CD34 concentration was demonstrated in C. odorata 5%, C. odorata 15%, and positive control, respectively (Table 6). Negative control had the lowest CD34 level at each time point. However, our data suggested that there was no significant difference in the mean levels of CD34 among groups on days 7, 14 and 21 with p=0.072, p=0.093 and p=0.093, respectively. The CD34 levels also showed no significant difference between days 7, 14 and 21 (p=0.443).
In comparison to negative controls, C. odorata 10% and 15% groups had consistent higher CD68 levels compared to the negative control group at all time points (i.e., days 7, 14 and 21) (Table 7). On day 14, the 15% C. odorata was able to induce CD68 expression more effectively. However, there was no significant difference in the mean levels of CD68 among groups on days 7, 14 and 21 with p=0.075, p=0.078 and p=0.188, respectively. There were no significant CD68 levels between days 7, 14 and 21 (p>0.050).
At 7 days post-epistaxis, animals in the C. odorata 15% and 10% groups had a higher percentage of fibroblasts compared to the C. odorata 5% group or positive control. At 14 days of recovery, Co10% group had a better ability to induce an increase in fibroblast cells, while positive control was the best on day 21 after recovery (Figure 2). One-way Anova analysis revealed that the percentage of the fibroblasts was no different between days 7, 14 and 21 (p=0.765).
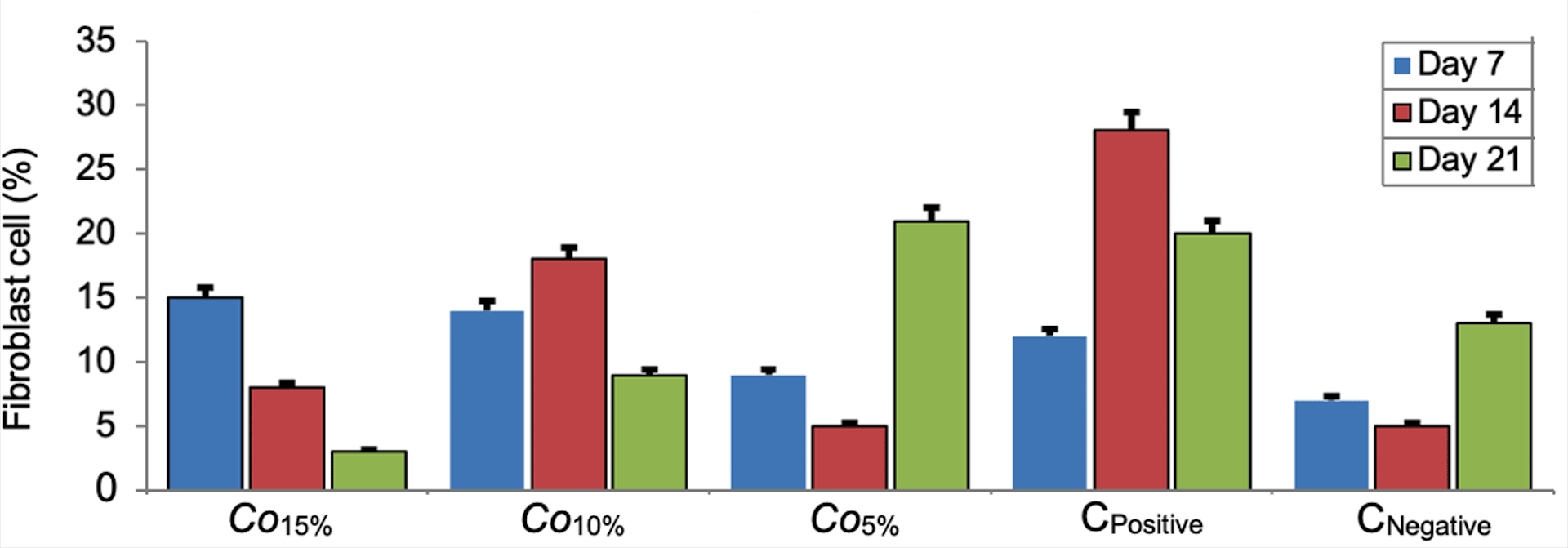
Bar errors indicates percentage of error.
Our data suggested that the highest concentration of C. odorata (15%) demonstrated the ability to initiate angiogenesis, which was greater than the Co10% and Co5% groups. In all groups, the longer the recovery time, the higher percentage of angiogenesis. In general, the positive control group played a lesser role in angiogenesis than the treatment groups (Figure 3). There were no significant differences in angiogenesis between time series (7, 14 and 21 days of receiving C. odorata extract gel) (p=0.478). However, the Kruskal-Wallis test suggested that there was a significant difference in percentage of angiogenesis between concentrations of C. odorata (p=0.018). The profile of fibroblasts and new blood vessels in epistaxis at day 7 from all groups is presented in Figure 4.
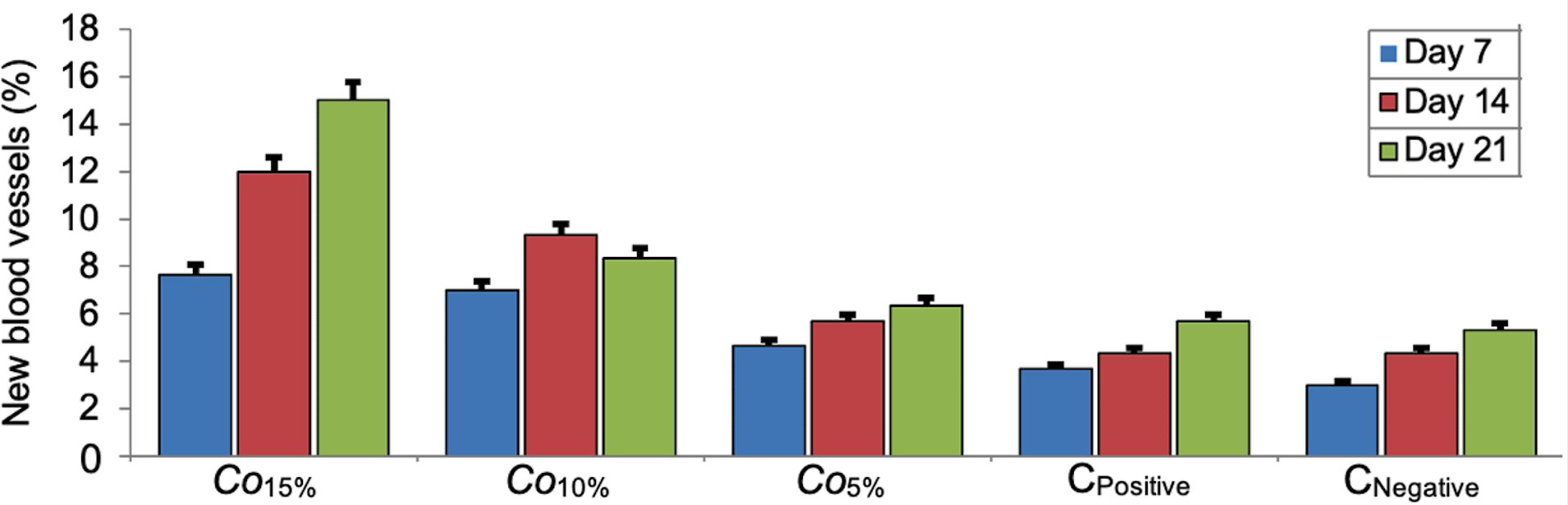
Bar errors indicates percentage of error.
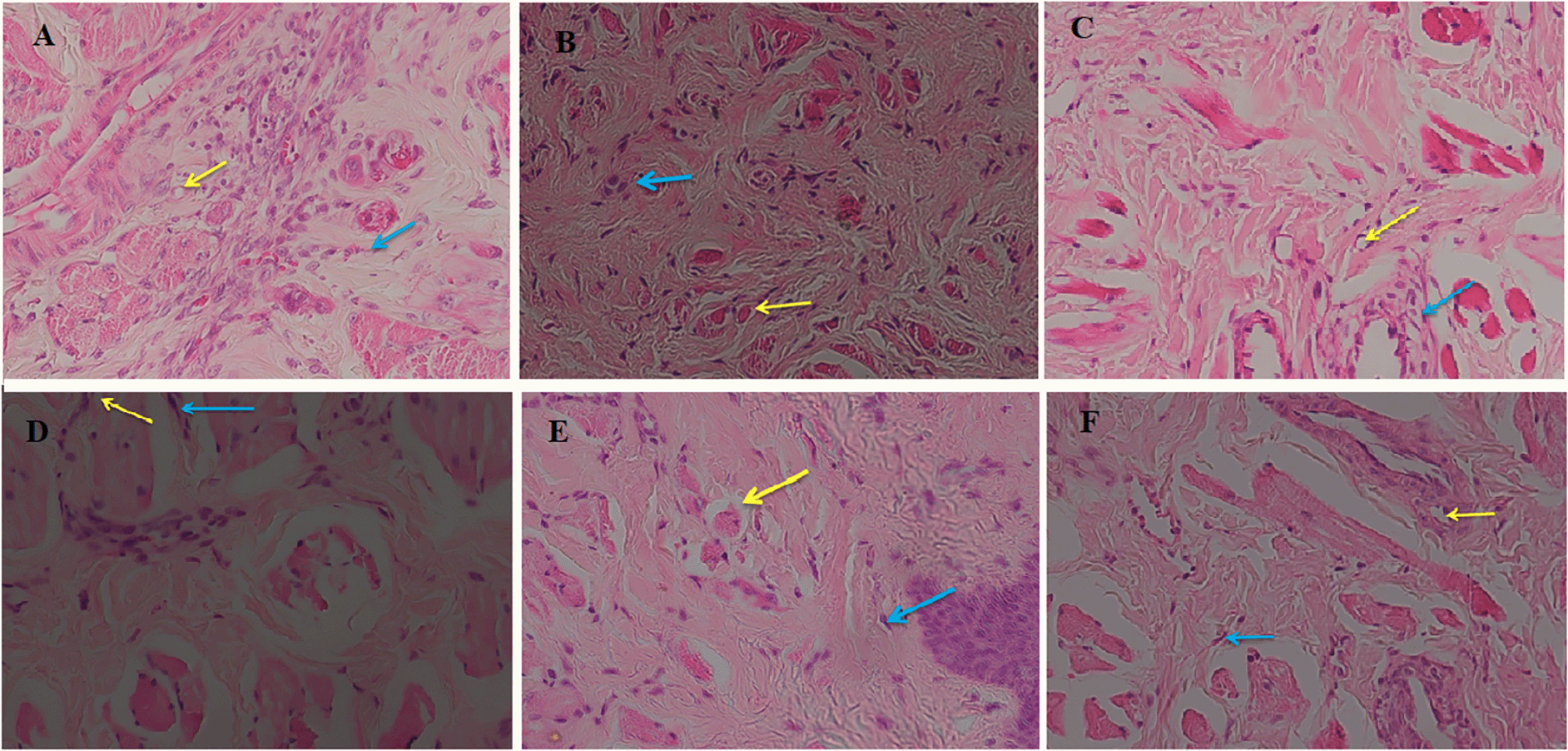
(A) Negative control, (B) Positive control, (C) C. odorata 5%, (D) C. odorata 10%, (E and F) C. odorata 15%. Yellow arrow (new blood vessels), blue arrow (fibroblast).
Our data indicated that C. odorata leaf extract gel contained antioxidant, anti-inflammatory and antithrombotic compounds that might increase the homeostatic and angiogenesis properties observed in this study. These putative compounds are longiverbenone (vulgaron B) (0.58%), phytol, acetate (3.23%), neo-menthol (5.25%), pentadecadien-1-ol (4.02%), octadecanoic acid (1.02%), squalene (22.96%), beta-tocopherol (2.26%) and octacosanol (0.64%). The synergism between C. odorata compounds and blood clotting factors in extrinsic and intrinsic pathways could accelerate the blood clotting, including the conversion of prothrombin to thrombin. Thrombin is an enzyme converting fibrinogen into fibrin that covers the wound and subsequently blood clotting.14 A number of these compounds are also involved in the wound healing phases, including tissue proliferation and remodeling.15
Our study also assessed the bioactivities of C. odorata compounds as GPCR ligand, ion channel modulator, kinase inhibitor, nuclear receptor ligand, protease inhibitor and enzyme inhibitor. The biological activities of those six molecules might contribute in increasing the angiogenesis during epistaxis recovery. Through the ligand mechanism of GPCR, the activation of proteolytic enzymes involved in wound healing is possible.16 These enzymes degrade necrotic cells originating from injured cells, regulate the cell maturation and multiplication, synthesize collagens and regulate perivascular fibrin.17
The ion channel owned by the active compounds of C. odorata could bind to the intracellular domain of the sodium channel with a voltage that blocks the entry of sodium into the cell to prevent depolarization. Depolarization is responsible for initiating a response or signals of pain conduction in peripheral nerves that act on wound healing.18 Modulator channel ions open pores in cell membranes, causing the generation of electric currents. Activation of the receptor will open ion channels so that the charge across the plasma membrane will be distributed effectively. This increased activation will help the cells to proliferate in the process of tissue repair.19
In terms of wound healing, enzyme inhibitors interfere with the response of inflammatory cells such as neutrophils. In contrast, the presence of active compounds from C. odorata provides an inflammatory cell response to release pyruvate kinase M2 (PKM2), an enzyme that acts in the last step of the glycolysis pathway and helps wound healing by promoting angiogenesis at the wound site.20,21
Some compounds from C. odorata exhibit high bioactivity such as octacosanol, which means that this compound might have an impact on expression of the genes involved in wound healing.22 This process occurs through the mechanism of ligand interaction with the receptor. Binding ligands activate nuclear receptors including lipophilic substances such as endogenous hormones, vitamin A, vitamin D and xenobiotic hormones. Since the expression of a large number of genes is regulated by nuclear receptors, ligands that activate these receptors can have important effects on any cell of an organism undergoing repair or other response to the environment.23
C. odorata compounds also have bioactivity against protease inhibitors. In the wound healing process, proteases and their inhibitors contribute to a balance between degradation and deposition of the extracellular matrix (ECM), which is important for the timely and coordinated healing of wounds. However, when the balance is disturbed, the wound develops chronicity, characterized by an increase of proteases and a decrease of protease inhibitors.24 Excessive proteases will degrade ECM, interfering with wound healing.25 This mechanism allows wound healing to also occur in epistaxis with C. odorata binding to excess proteases. In the inflammatory phase, proteases remove the damaged ECM, followed by a proliferation phase where proteases help increase the degradation of capillary basement membrane for angiogenesis, assisting migration and cell release. In the remodeling phase, various cells are mobilized to the injured location to reconstruct the tissue, regulated by the ECM.26
C. odorata increased the expression of CD34 and CD68 level but had no significant effect compared to control groups. CD34 is a marker of increased fibroblast cells, which are critical in angiogenesis during wound healing. They break down fibrin clots, build new collagen and ECM structures to support other cells needed for efficient wound healing.27–29 The increase in CD68, which is expressed by monocytes and macrophages, is in line with a decrease in inflammatory cells and stimulation of fibroblast activity which has an impact on angiogenesis.30,31 Nevertheless, slight fibroblast cell proliferation still occurred in the wound area in the present study. In addition, our study clearly demonstrated that the number of new blood vessels was significantly frequent at wounds from animals given the highest concentration of C. odorata (15%), followed by the 10% and 5% concentrations. This suggests that C. odorata extract could induce the angiogenesis, the process that is crucial during wound healing.
This study has some limitations that need to be discussed. The number of animals used in this study was very small and a study with bigger sample size is required. The present study assessed the level of CD34 and CD68 from the blood and such a source might not be the best indicator. Further study assessing the expression of CD34 and CD68 directly from the wound histopathology using immunohistochemistry is important. It is recommended for further studies be undertaken such as docking analysis32 to predict further interactions between the putative compounds of the C. odorata extract and critical molecules in the angiogenesis pathway.
We identified eight putative compounds with antithrombotic, antioxidant and anti-inflammatory activities from C. odorata extract: longiverbenon (Vulgaron B), phytol (acetate), neo-Menthol, pentadecadien-1-ol, octadecanoic acid, squalene, beta.-tocopherol and octacosanol. These putative compounds have a range of bioactivities as GPCR ligand, ion channel modulator, kinase inhibitor, nuclear receptor ligand, protease inhibitor and enzyme inhibitor. Those putative compounds with their bioactivities might contribute in inducing the angiogenesis during the wound healing processes in epistaxis observed in the present study.
Figshare: Bioactive phytoconstituents and hemostatic and angiogenetic activities of Chromolaena odorata L. leaf extract gel on animal epistaxis model. https://doi.org/10.6084/m9.figshare.20676942. 33
The project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We would like to thank Narra Studio Jurnal Indonesia for their assistance during the preparation of this manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Kandhwal M, Behl T, Singh S, Sharma N, et al.: Role of matrix metalloproteinase in wound healing.Am J Transl Res. 2022; 14 (7): 4391-4405 PubMed AbstractCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Natural products, wound healing, Pharmaceutics, Bioinformatics, Network Pharmacology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Lee K, Seo PJ: Wound-Induced Systemic Responses and Their Coordination by Electrical Signals.Front Plant Sci. 2022; 13: 880680 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Phytochemistry, wound healing, histology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 06 Mar 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)