Keywords
oxygen therapy, cerebral autoregulation, cerebral ischemia, stroke, cerebral blood flow
oxygen therapy, cerebral autoregulation, cerebral ischemia, stroke, cerebral blood flow
The human brain accounts for only 2% of total body weight, yet receives about 15% of the entire cardiac output: with minimal capacity to store energy. It demands a constant supply of oxygen (O2) and nutrients to meet metabolic needs and maintain cerebral function. Even a short interruption in cerebral blood flow (CBF) can initiate a cascade of pathological events.1,2
In order to maintain this equilibrium, cerebral autoregulation (CA) and hyperemia are responsible for regulating CBF: while hyperemia maintains localized microscopic blood flow by regulating global CA via constriction and dilation and maintaining CBF between 40 and 60 mL/100 g/min over a wide range of mean arterial pressure (MAP; 50–150 mm Hg).3,4 Outside this range and in the absence of other pathophysiological occurrences, hypotension can technically cause cerebral ischemia, whereas hypertension could lead to a hemorrhagic stroke: both can ultimately initiate a sequence of neuropathological events such as neuroinflammation, synaptic dysfunction, and neuronal death (excitotoxicity, apoptosis, necrosis, etc., also known as programmed or not-programmed cell-death mechanisms).5 In the present narrative review we will summarize some of the general physiological and pathophysiological mechanisms underscoring the role of CA in regulating CBF in various scenarios.
In a healthy brain, CA—alone and by far—prevents the development of either ischemia or hyperemia caused by MAP changes; however, CA capabilities decline with aging and/or may become impaired, for instance after cerebrovascular accidents. The brain, during these physiological and clinical scenarios is more vulnerable, and it is even further exposed to more damage than it would have been if exposed to the same situations when younger or with better reserves, we could say (hypothetical model in Figure 1).
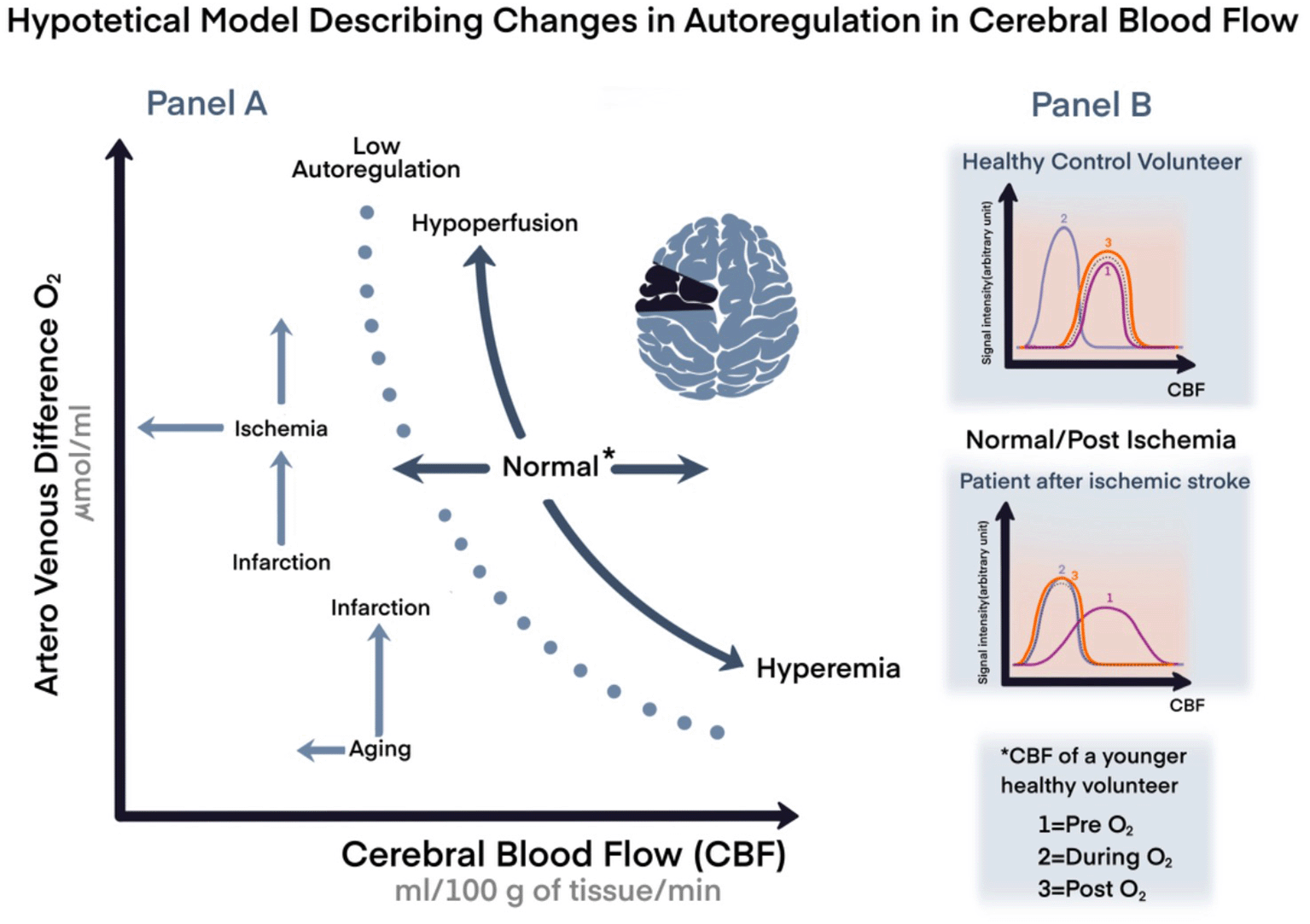
Panel A. A representation of cardiovascular coupling during normal or stressful events with variation in age, and compensation mechanisms. Panel B. Hypothetical model comparing changes in cerebral blood flow (CBF) in healthy volunteer compared to a patient with chronic ischemic stroke by magnetic resonance imaging.
*Arbitrary unit=signal intensity; CBF of a young volunteer; CBF: cerebral blood flow; O2: oxygen; 1: pre O2; 2: during O2; 1: post O2.
The CA can be assessed by measuring CBF response to changes in cerebral perfusion pressure (CPP),6–8 which is a difference between MAP and mean intracranial pressure (ICP).9 However, CBF can also be influenced by combinations of O2 and carbon dioxide (CO2) tensions in the blood (Figure 2).10,11 CBF variations are measured after a ‘steady state’ change in MAP (such as before and after starting a vasopressor infusion) and dynamic CA, which is defined as a response to rapid changes in MAP (such as deflation of a blood pressure cuff).12 Rather than measuring two distinct physiological mechanisms,13 (static and dynamic), the CA expresses the relationship between MAP and CBF velocity over different timeframes.14 In this review, using CA and CBF preservation, we reappraise the risks and benefits of therapeutic O2 administration in patients with brain ischemia and other clinical situations that require supplemental O2 as part of routine clinical care.
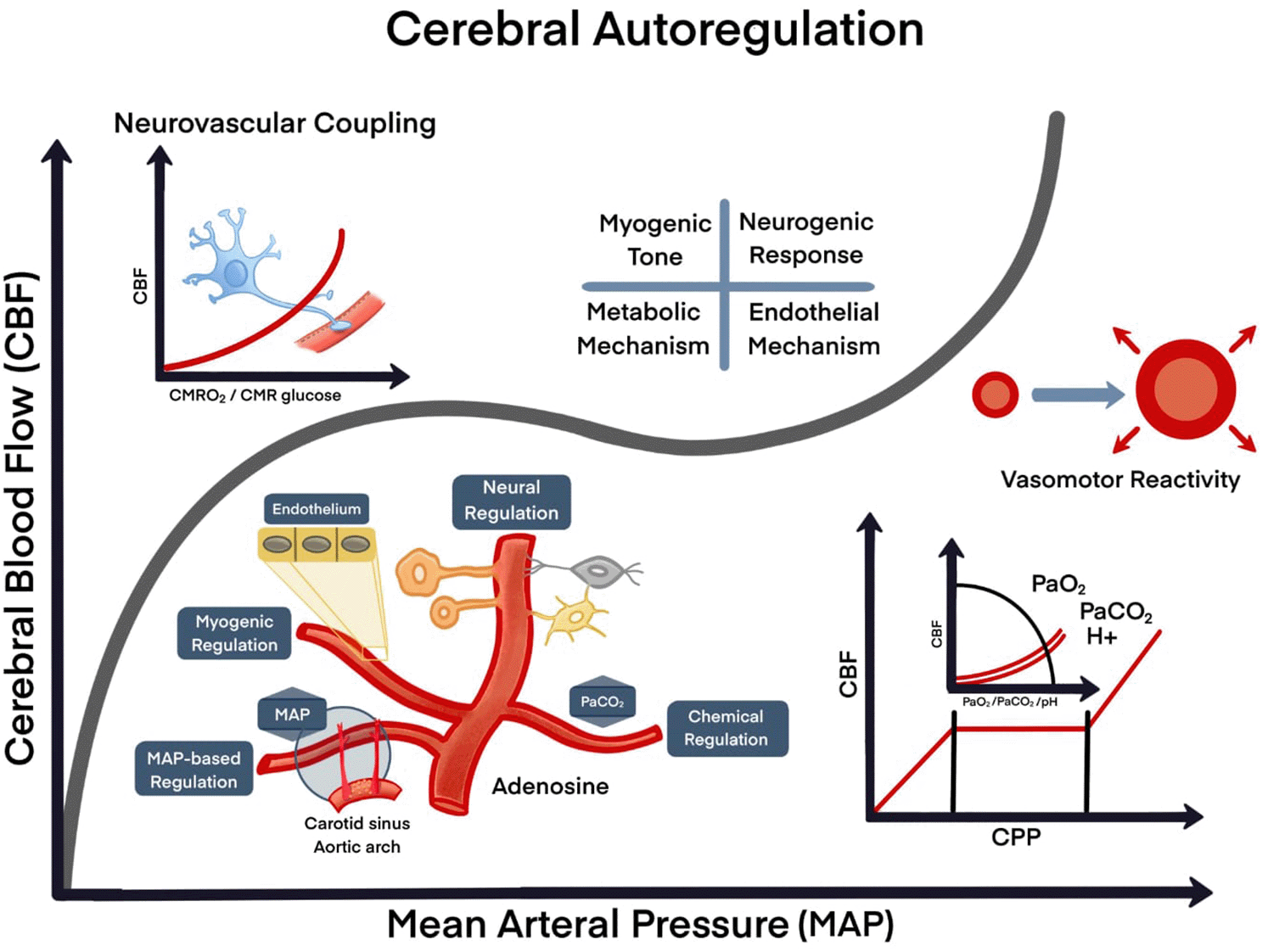
A. Cellular interaction (neurons, endothelial cells and glial cells); B. Nutrients/energy (glucose/oxygen); C. Activity/changes of demand, physiological; D. Pathological (seizures, stroke, trauma). Four mechanisms control mechanisms: myogenic, neurogenic, metabolic and endothelial.
CBF: cerebral blood flow; MAP: mean arterial pressure; CMRO2: cerebral metabolic rate of oxygen; CMR: the cerebral metabolic rate; PaO2: O2 partial pressure in arterial blood; PaCO2: CO2 partial pressure in arterial blood; H+: hydrogen ion; pH: potential of hydrogen.
We searched PubMed for English language abstracts, using searching terms such as “oxygen therapy” AND “cerebral autoregulation” OR “ischemic preconditioning” OR “anesthetic neurotoxicity” OR “ischemic stroke” OR “traumatic brain injury” OR “anesthesia”, from 1975 to October 2021. We chose open and blinded studies, reviews and meta-analyses, and available commentaries and editorials related to MESH terms. Because of the nature of the review (narrative, clinical-experience oriented, translational research motivated), an international criteria/PRISMA is not included, and studies are not graded formally by the level of evidence.
Carl Wilhelm Scheele was the first scientist to discover O2 in 1771 by heating mercuric oxide, silver carbonate, and magnesium nitrate, but he did not publish his finding till 1777. Meanwhile, in 1774, Joseph Priestley reported that O2 allowed a candle to burn more brightly and has been given credit for O2 discovery.15 The application of O2 in medicine was first reported by Antoine Lavoisier, who described the role of O2 in human respiration. In 1798, the Pneumatic Institute (Bristol, England), started O2 distribution for treating patients with asthma and congestive heart failure.15 In 1880 with the development of O2 cylinders for storage and transport, and in the 1900s with the invention of nasal cannulas and masks, O2 therapy for respiratory pathology became a routine clinical practice.16–18
The application of hyperbaric O2 utilization is one of the most advanced applications of O2 therapy; it began in 1662 before O2 discovery when a British physician Henshaw compressed air in a closed chamber.19 In the late 1800s, specially designed hyperbaric O2 chambers were built (the first one in North America around 1860).19 In 1972, Takuo Aoyagi invented pulse oximetry, allowing clinicians to measure continuous peripheral O2 saturation and better guide O2 therapy in a clinical context.20
Under physiological conditions, 98% of inspired O2 is transported in the blood bound to hemoglobin, while the remaining 2% is freely dissolved in the plasma. The bonded O2 to hemoglobin known as oxyhemoglobin increases the partial pressure (PO2) and oxyhemoglobin saturation in the blood.21 Oxygen in the blood comes from lung inhaled air which is transported to organs and cells utilized for energy production essential for organ function.22 Nutrients such as carbohydrates, proteins, and fats are initially broken down into substrates which entered the tricarboxylic acid (TCA) cycle23 and are converted into nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2). The NADH and FADH2 entered the electron transport chain (ETC), which is composed of several protein complexes located in the inner membrane of the mitochondria. Both NADH and FADH2 donate electrons to the ETC, which carries them down while allowing protons to be pumped into the inner membrane space.23 After electrons reach complex IV, the O2 accepts the electrons and is reduced to water.24
The protons pumped in the intermembrane space create a mitochondrial membrane potential to convert adenosine diphosphate (ADP) to adenosine triphosphate (ATP) via a synthase enzyme, the main energy molecule in the body.23 Without O2, the ETC would not have a final electron acceptor and the production of ATP would become much less efficient.
Oxygen is usually administered under hypoxemia which occurred as a result of a decrease in arterial O2 tension.25 Oxygen therapy is beneficial in pathologies that increased O2 utilization and/or decreased O2 delivery to tissues.24 In patients with acute respiratory failure, supplemental O2 remains an essential treatment component, which has significantly improved with advanced O2 delivery systems.26
General anesthesia (GA) is a typical clinical situation when O2 is commonly administered. GA can indeed decrease O2 partial pressure in arterial blood (PaO2) via multiple mechanisms: functional residual capacity (FRC) decreases after induction of anesthesia due to the diaphragm being cranially displaced in supine position; the decrease in FRC reduces lung compliance and increases airway closure at end-expiration, predisposing to atelectasis and hypoxemia27; moreover, apnea periods during airway manoeuvres can lead to rapid arterial hemoglobin desaturation and hypoxia; therefore, anesthesiologists commonly administer a high inspired O2 fraction (FiO2) before tracheal intubation and extubation.23,24,28
Despite some benefits that O2 supplementation could serve in a variety of clinical scenarios (simple hypoxia, hypoventilation, organ ischemia events, increased O2 demand, toxic hypoxia, to cite few), an indiscriminate administration of therapeutic O2 at high concentrations and/or for a prolonged time may lead to adverse effects: of those, formation of reactive O2 species (ROS) and increased systemic vascular resistance29 could further play a role in generating or contributing for instance to brain injury or other organ injuries. Even a physiological vascular territory where possibly O2 may exert by common sense a salubrious effect, like the pulmonary, may indeed negatively affect hypoxia-induced pulmonary vasoconstriction HIPV, therefore causing ventilation/perfusion (V/Q) mismatching. Overall what we have described as the “oxygen paradox”, is also “The Tale of Two Cities”.30
a. Reactive oxygen species (ROS)
Physiologically, when O2 is utilized in the mitochondrial ETC, only a small amount of ROS is formed by the partial reduction of O2.31 Although ROS such as superoxide anion, hydrogen peroxide, and hydroxyl radical can lead to cell damage, their action is usually counteracted by various intra- and extracellular antioxidants such as superoxide dismutase, glutathione, cytochrome c oxidase.32
With an increase in O2 supplementation, ROS production is also increased via outstrips the available antioxidant levels. ROS causes damage to nucleic acids, proteins, and lipids leading to cell damage and ultimately cell death.31 An excess of O2 can also lead to inflammation and possible lung damage.29 In lungs, hyperoxia and ROS stimulate nuclear factor kappa B, which releases plasminogen activator inhibitor-1 and tissue factor. The release of these factors causes activation of the coagulation cascade, which may induce further cell damage.21 Importantly, ROS have been shown to play a role in both neuronal death and neurovascular recovery after cerebral ischemia. ROS, interleukin-1β and hydrogen peroxide (H2O2) activate p38-mitogen-activated protein kinase, which is associated with protein oxidation and damage in post-ischemic rodent brains.33 Intriguingly, while ROS mediates neuronal damage during the early phase of ischemia, in later phases it mediates vascular endothelial growth factor (VEGF), angiogenesis, and recovery in the post-ischemic brain.34
b. Oxygen-mediated vasoconstriction and other vaso-mediated mechanisms
ROS production linked to excess O2 inhibits cyclooxygenase, decreasing prostaglandins, and thereby lead to vasodilation.35 The ROS superoxide anion inactivates nitrous oxide (NO), a vasodilator, through various mechanisms.36,37 A mechanism by which hyperoxia causes vasoconstriction is by converting arachidonic acid to 20-hydroxy-eicosatetraenoic acid (20-HETE).38 These mechanisms have been described causing systemic vasoconstriction and decreased perfusion to almost all organs,36 supposedly with the exception of the lungs and the placenta: yet HIPV may be disrupted by O2 and similar concerns have been raised for adequate placental flow and the autoregulation of optimal fetal perfusion.39 Hyperoxia-mediated vasoconstriction may affect more the microvasculature rather than larger vessels: for example, the large coronary arteries do not constrict under hyperoxic conditions40; also, a decrease in prostaglandin levels (affected by ROS production) normally cause vasodilation mainly in the microvasculature.41
c. Oxygen therapy post-cardiac arrest and myocardial infarction
Supplemental O2 administration after myocardial infarction (MI) was originally adopted to attenuate tissue ischemia. However, it has been postulated that ROS can in fact cause tissue injury and excess O2 can lead to decreased peripheral coronary perfusion, which in turn may adversely affect patient outcomes.40 A Cochrane review and meta-analysis showed that patients with ST-elevation myocardial infarction (STEMI) or non-STEMI (NSTEMI) treated one hour of inhaled O2 within 24 hours of symptom onset, exhibited an increase in infarct size [measured as increased creatine kinase (CK)] as compared to patients treated with air.42 However, a randomized control trial was unable to show that O2 therapy after MI caused any difference in mortality.43
The effect of O2 therapy on post-ventricular fibrillation arrest and subsequent return of spontaneous circulation (ROSC) has also been reported.44 In a 28 patients, randomized to receive either 0.3 FiO2 or 1.0 FiO2, their serum samples collected at 24 and 48 hours were analysed for neuron specific enolase (NSE) and protein S-100, showed signs of neuronal injury. Although statistically there was no difference in the NSE and protein S-100 levels between the two groups, a higher NSE levels were reported in patients who received 1.0 FiO2 and did not underwent therapeutic hypothermia.44 There is some evidence suggesting that O2 may not improve outcomes after MI and even worsen the outcome, increasing the risk of neurological injury after cardiac arrest and return of spontaneous circulation (ROSC).
d. Oxygen therapy after ischemic stroke
The role of hyperoxia in patients with a previous stroke has not been entirely and clearly elucidated. In acute ischemic stroke, the occluded blood vessels disrupt brain O2 delivery causing hypoxia/ischemia and a main infarction area, as well as another area called penumbra, surrounded by healthier tissues amenable of being rescued if cerebral perfusion and oxygenation are restored in a timely fashion. Besides the direct vascular mechanisms of vascular patency though, there are many others which have been discussed so far: those include ROS production and nutrients supply, as well as mechanisms related to the vascular integrity per se or “barriers” integrity, i.e. the blood brain barrier being one of them and discussed later in the paragraph Changes in the Neurovascular Unit after Ischemic Stroke.
Animal studies reported that an early administration of supplemental O2 after stroke may improve clinical outcomes10,45: however, hyperoxia may physiologically induce cerebral vasoconstriction, which is the other main topic of our narrative review.46 In a similar clinical scenario, the role of O2 and neuronal survival/remodeling has been questioned in other clinical scenarios such as neonatal asphyxia.47
The neurovascular unit (NVU) includes multiple cell types (neurons, interneurons, astrocytes, smooth muscle cells, pericytes, endothelial cells) and extracellular matrix which are closely associated with cerebral vasculature and their interaction with each other to maintain the blood-brain barrier (BBB) and regulate CBF.48,49 A tight junction between endothelial cells of the blood vessels and BBB’s selective permeability control via NVU pericytes proteins is crucial. The NVU is also involved in vascular tone regulation and an increased in neuronal activity leading to increased CBF and thereby increased oxygenation.50
Cerebral autoregulation is also involved in vasoconstriction and vasodilation of cerebral arterioles via myogenic, neurogenic, and metabolic regulation (Figure 2).51,52 Myogenic regulation acts via smooth muscle cell contraction in the wall of blood vessels,53 is the key to maintaining CBF when MAP is outside the autoregulation range, whereas within the autoregulation range neurogenic regulation by the NVU plays a major role.52 As part of the NVU, neurons respond to decreased glucose and hypoxia by generating molecular signals, such as glutamate, to communicate with interneurons and astrocytes.54 Astrocytes on their part can alter vascular tone via prostaglandins, ATP, NO, and lactate.48,54
Cerebral blood flow is primarily affected by blood gas concentrations, including O2, CO2, and nitric oxide (NO).55–57 Hypoventilation causes an increase in arterial CO2 tension (hypercapnia) and leads to vasodilation resulting an increase in CBF, whereas inhalation of 100% O2 reduces CBF by 10–15%.58,59 In essence, CBF is directly influenced by blood oxygenation levels and vice versa. Under hypoxic conditions (or hypercapnia), autoregulation initiates activation of mechanisms leading to vasodilation, whereas hyperoxia (or hypocapnia) increases O2 bioavailability as CBF decreases due to vasoconstriction (Figure 2).10,11
The partial pressure of O2 (PaO2) and partial pressure of carbon dioxide (PaCO2) can also have a combined effect, depending on their respective partial pressures. Ogoh et al. studied the effect of O2 therapy on dynamic CA by exposing nine healthy volunteers to four respiratory interventions: normoxia (0.21 FiO2), isocapnic hyperoxia (0.4 FiO2), isocapnic hypoxia (0.14 FiO2), and hypocapnic hypoxia (0.14 FiO2).60 He used transcranial Doppler to measure middle cerebral artery blood velocity to determine CA. The CA was impaired with normocapnic hypoxia; however, it improved with mild hypocapnic hypoxia. He concluded that hypocapnia-caused vasoconstriction led to improved CA, thus outweighing the negative effect of hypoxia on CA.60
In the event of CPP reduction such as an acute ischemic stroke, a compensatory mechanism starts to preserve O2 and nutrient supply to the brain. Based on a human study (MRI was used to assess O2 extraction fraction (OEF) in patients with various degrees of middle cerebral artery (MCA) stenosis or acute stroke),61 it was found that the increase in OEF from baseline was higher in the severe MCA stenosis group compared to the mild stenosis group, where collateral circulation allows for some perfusion distal to the lesion. Patients with severe MCA stenosis have a greater reduction in CBF and little collateral circulation, necessitating other mechanisms such as improving OEF to increase brain oxygenation.61
In animal studies, occluding the MCA caused an increase in CBF and OEF one hour after occlusion; however, at 2–3 hours post-occlusion CBF and OEF had both decreased.62 These findings suggest that soon after MCA occlusion compensatory mechanisms attempt to maintain cerebral perfusion and oxygenation. However, at later stages of MCA occlusion these compensatory mechanisms, and presumably cerebral oxygenation, decrease.62
To summarize, to maintain O2 and nutrient delivery to the brain during acute stroke, CA causes arterioles to dilate, therefore increasing CBF. Once this compensatory mechanism has been used up, O2 extraction fraction (OEF) can increase significantly to keep O2 metabolism running, but at maximal OEF, continuing or deteriorating CBF reduction may eventually lead to cell death.62
Ischemic stroke and subsequent cerebral hypoxia can cause disruption of the NVU and damage to the BBB via the hypoxia-inducible factor 1 (HIF1) transcription factor.63 Additionally, HIF1a and cytokines such as TNF-alpha and IL-1B lead to the activation of matrix metalloproteinases (MMPs) which break down the BBB and increase its permeability.64 A decrease in ATP production and failure of enzymes to maintain normal ion gradients cause endothelial cell swelling and BBB dysfunction.65 Ischemic stroke leads to degradation of extracellular proteins, detachment of pericyte, astrocyte, and microglia activation.49 These changes in the BBB and the NVU allow for peripheral immune cells to enter the brain causing inflammatory stress.66 Loss of BBB integrity also leads to vasogenic edema and increases the risk of hemorrhagic transformation, which can worsen brain damage after stroke.67
General anesthesia (GA) can directly impact cerebral autoregulation, therefore potentially inducing a direct organ damage, in particular brain damage. Besides that, GA could also be cause of neurotoxicity due cell-toxicity, yet anesthesia may also be responsible for molecular mechanisms of neuroprotection therefore modulating neurotoxicity: these mechanisms are encompassed for instance in the same processes responsible for cell and organ protection after ischemic precondition and postconditioning.
A further consideration pertains the use of O2 not simply for the preparation prior to general anesthesia in otherwise healthy subjects, yet in patients during or after cardiac arrest or ischemic stroke that require therapeutic use of O2, and in particular when they require procedures involving GA: at a time when CBF and oxygenation are compromised, GA may pose another threat to organs health or possibly be a reason of organ protection. Here we briefly review the available evidence around some of the potential effects of GA on brain tissue and CA.
Volatile anesthetics can cause neurotoxicity, especially in neonatal rats and non-human primates, although the exact mechanism remains under investigation. They can lead to neuroapoptosis, neurodegeneration, and long-term neurocognitive deficits in animal models through N-methyl-D-aspartate (NMDA) receptor antagonism and GABA receptor activation.68 Ultimately, they can also create oxidative stress in the mitochondria, leading to ROS production which triggers a chain of events causing apoptosis,69 and contribute to neurotoxicity.69–71
Both in vitro and in vivo studies have shown evidence of propofol-induced cell death.72,73 The mechanism of propofol-induced neurotoxicity is thought to be mediated by multiple pathways including apoptosis, decreased neurogenesis, disruption of dendrite formation, neuroinflammation, Ca2+ signalling, microRNAs, and activation of p75 neurotrophic receptor.72,73
Volatile anesthetics also, seem to play a role in organ protection through ischemic preconditioning and postconditioning, shown by cardiac animal studies a similar mechanism has been found in brain cells.74 Sevoflurane is involved in neuroprotection when administered at specific times before ischemia–reperfusion. Sevoflurane-induced energy preservation decreases both focal and global ischemia and improves outcomes.75 Additionally, sevoflurane and other volatiles inhibit glutamate receptors which are normally stimulated to cause cell injury during ischemia.75
Propofol also induces neuroprotection through ischemic preconditioning and postconditioning via a different mechanism from volatile anesthetics.75 Propofol regulates cytochrome c, Cx43, UCP2, and mitochondrial DNA (mtDNA) transcription, which all play a role in neuroprotection after ischemia. It protects the integrity of the mitochondrial membrane during ischemia, preventing cytochrome c detachment and activation of the apoptosis pathway, thereby preventing neuronal cell death.75
While the hemodynamic effects of commonly used anesthetics such as sevoflurane, propofol, and dexmedetomidine are well known, their effect on CA is less extensively studied. Usually the administration of sevoflurane at 1 minimal alveolar concentration (MAC), while it somewhat decreases MAP, overall it maintains CBF: CA is overall preserved as shown by Juhász et al. in 29 patients cohort who underwent GA.76 Using dexmedetomidine with sevoflurane and nitrous oxide anesthesia would also appear to not affect CA.77 McPherson et al. reported fentanyl has no known impact on CA.78
General anesthesia using propofol with target-controlled infusion also maintains CA and CO2 reactivity through a balance between vasodilation and vasoconstriction.73,79 CA was unchanged in patients undergoing GA with propofol-remifentanil infusion but decreased in those receiving high-dose sevoflurane. Additionally, higher CO2 levels did not affect CA in the propofol-remifentanil group, while the high-dose sevoflurane group experienced a further reduction in CA.80 These studies suggest that CA is maintained in patients receiving propofol at doses required for GA.
Currently, CA can be assessed by raising BP by ~10 mmHg via a pharmaceutical agent while measuring intracranial pressure (ICP), or by continuous monitoring of brain tissue PO2 using an intraparenchymal probe: a decrease, or no change, in ICP indicates an intact CA, while an increase in ICP suggests compromised CA.81 Non-invasive techniques such as transcranial Doppler (TCD) and near-infrared spectroscopy (NIRS) can be used to assess cerebral reactivity, autoregulation, and neurovascular coupling; however, TCD failed to provide more global CBF, whereas NIRS is limited by the infrared penetration power in the deepest parts of the brain. Both methods have accuracy, sensitivity, and reproducibility issues, and lack a complete cerebral hemisphere coverage.82,83 Other methods, such as positron emission tomography (PET), single-photon emission computed tomography (SPECT), and computed tomography (CT) can compute CBF, cerebral blood volume (CBV), mean transit time (MTT), and O2 extraction fraction (OEF); however, they require a radioactive tracer with a short half-life.84–86 Gas-challenge magnetic resonance imaging (MRI) is emerging as a viable option to probe CA by measuring the change in CBF, the cerebral metabolic rate of oxygen (CMRO2), and OEF.87–89
Inhalation of pure O2 or carbogen (a mixture of 3–5% CO2 and 95–97% O2) changes blood oxygenation in capillaries and veins by altering oxyhemoglobin versus deoxyhemoglobin content. This manifests as a change in T2*-weighted MRI signal, which is the principal blood oxygenation level-dependent (BOLD) imaging contrast (see Figure 1, panel B as a hypothetical model of O2 inhalational effect assessed by MRI).90,91
Gas challenge images have been used to compute vessel size.92 Under physiological conditions, the arterial blood is 98% oxygenated resulting in a small difference in magnetic susceptibility between arteries and tissue. With O2 inhalation, an increase in FiO2 from 0.21 to 1.0 leads to a significant change in BOLD contrast. In ischemic stroke patients, Donahue et al. applied carbogen challenge MRI to evaluate cerebrovascular reserve capacity in patients with intracranial stenosis.93 Carbogen increases the fractional concentration of inspired CO2 (FiCO2) and NO-mediated vasodilation, which in turn increase MRI signal intensity. In contrast to O2, carbogen challenge MRI has been extensively applied to study several pathologies such as malignancy, hypoxia, Alzheimer’s, retinopathy, type 2 diabetes, hepatic fibrosis, and chronic kidney disease.94–97
Gas-challenging MRI is emerging as a non-invasive mainstream method to study neurophysiology. In a healthy brain, a hypercapnia-induced increase in CBF affects the CMRO2 and OEF. Several studies reported both CMRO2 and OEF are significantly altered in patients with stroke, tumor, and cerebrovascular degenerative disorder.98,99 Both hypercapnia and hyperoxia induce CBF changes leading to BOLD MRI signal by affecting venous blood deoxyhemoglobin concentration has also been applied to compute these two critcal functions.100–102
A localized or global change in oxy- and deoxyhemoglobin concentrations in the brain can alter magnetic susceptibility and blood transverse relaxation time (T2 and T2* relaxation time); this has been recently applied to compute OEF. A change in blood gases can alter magnetic susceptibilities: these changes can be measured by quantitative mapping (QSM), an advanced MRI signal processing method that uses both the magnitude and phase component of a gradient echo MRI signal.
A magnetic susceptibility difference between the veins and the surrounding tissue has been applied to estimate venous O2 saturation. Uwano et al. and Zaitsu et al. applied QSM to measure OEF and validated it with PET imaging, a gold standard to measure OEF.103,104 Previously, Zhang et al. used caffeine and hyperventilation as stimuli to alter susceptibility via a change in oxy- versus deoxyhemoglobin ratio and measured OEF and CMRO2 using QSM.105,106 However, because both caffeine and hyperventilation induce vasoconstriction, the CBF reduction results in a smaller magnetic susceptibility difference between the oxy- versus deoxyhemoglobin creating weaker MRI contrast.105,106 To obviate those limitations, Ma et al. recently applied a carbogen inhalation vasodilator challenge: it increased CBF and resulted in a larger magnetic susceptibility difference between the oxy- versus deoxyhemoglobin, creating stronger MRI contrast.107
To summarize, CA can be assessed by various methods, all of which have specific limitations. Emerging non-invasive imaging techniques relying on the gas challenge look promising for the study of cerebral pathophysiology.
While exogenous O2 administration is still part of routine clinical management and therapeutic algorithms, it remains unclear at present whether this practice should continue indiscriminately, considering its potential toxic effects. Specifically, despite the clear dependence of the human brain on a constant O2 and nutrient supply, a compelling clinical and experimental body of evidence questions the utility of excess O2 in acute and post-recovery brain ischemia. Many of these findings have been the object of translational research, particularly in the field of neurophysiology imaging. It may be prudent and possibly best practice to limit exogenous O2 administration in the clinical setting to the shortest period required, and consider reducing inspired O2 fractions in patients more predisposed to CA disruption, such as post-stroke recovered patients.
PG: designed the work, contributed to literature search, collected data, interpreted data and wrote the manuscript
NF: designed the work, contributed to literature search, collected data, interpreted data, wrote the manuscript
DS: designed the work, literature search, collected data, interpreted data, edited the manuscript and figures
MH: contributed to literature search, wrote the manuscript, interpreted data, edited the manuscript and figures
DC: initiated this report, designed the work, collected data, interpreted data, edited the manuscript and figures
All authors read and approved the final manuscript.
The authors acknowledge Ilija Ilic who graphically designed figures presented in the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Preventive, therapeutical and ethical approach to the preclinical and clinical research of genes and modulators of redox cell signalization in immune, inflammatory and proliferatory cell response
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neuroscience, Obstetric Anesthesiology, Cardiovascular Physiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 09 May 23 |
read | |
|
Version 1 06 Mar 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)