Keywords
Vitamin D deficiency, 25(OH)D, glycated hemoglobin, hypertension, quality of life, vitamin D supplementation, sociodemographic factors
This article is included in the Sociology of Health gateway.
Vitamin D deficiency, 25(OH)D, glycated hemoglobin, hypertension, quality of life, vitamin D supplementation, sociodemographic factors
Deficiency of vitamin D, an essential nutrient for bone, tooth, and skeletal development,1 has become a global and national health concern.2 Worldwide, prevalence rates of low vitamin D status range between 6% and 40% depending on age, ethnicity, and geography.3 In India as well, the prevalence of vitamin D deficiency or insufficiency is very high and can range from 34%—70%4,5 depending on various sociodemographic factors.6
According to the Endocrine society, vitamin D deficiency and insufficiency are defined as serum 25-hydroxyvitamin D (25[OH]D) levels of <20 ng/mL and 21-29 ng/mL, respectively.7 Vitamin D deficiency is associated with various comorbidities such as cardiovascular diseases including hypertension and congestive heart failure, diabetes mellitus, autoimmune diseases such as rheumatoid arthritis, multiple sclerosis, and Crohn’s disease, various forms of cancers, and mental health disorders such as schizophrenia and depression8 and impaired quality of life (QoL).9
The goal of treatment for vitamin D deficiency/insufficiency is to normalize systemic vitamin D levels via optimal sunlight exposure and dietary and/or supplementary sources. The Endocrine society of India has recommended 1000-2000 IU per day of vitamin D supplements,10 but several studies have shown tolerance of and beneficial outcomes with higher doses depending on season of administration.8,11 Monthly administration of 60,000 IU of cholecalciferol in healthy Indian women with hypovitaminosis D was sufficient to restore 25(OH) D levels in summer months, but higher doses were required during winter months.12 Eight weeks of vitamin D3 (60,000 IU/week) oral granules were found to increase serum 25(OH) D levels to the optimal levels in 78.1% of the vitamin D deficient patients.13 Nevertheless, disparity does exist between the prescription patterns with regard to dose, route and frequency of vitamin D supplementation in Indian clinical practice, thereby increasing the risk of overdosing or vitamin D toxicity.14 Moreover, large-scale studies evaluating sociodemographic factors associated with vitamin D deficiency/insufficiency in India and efficacy with regard to improvement in clinical signs and symptoms, safety outcomes, and impact on QoL following vitamin D supplementation have hitherto not been evaluated. Hence, this noninterventional, observational study was performed to understand the profiles of Indian patients diagnosed with vitamin D deficiency or insufficiency and assess the effect of vitamin D supplementation on clinical signs and symptoms, QoL and safety of such patients.
This was a multicenter, prospective, non-interventional, observational study conducted at nine centers in India between 19 August 2020 and 25 June 2021 and involved adult patients diagnosed with vitamin D deficiency or insufficiency. The study investigators were either orthopedists, cardiologists, diabetologists, or general physicians. The study was conducted in compliance with the principles of the Declaration of Helsinki, International Conference on Harmonization - Good Clinical Practice (GCP) guidelines, and Indian regulatory guidelines (Indian Council of Medical Research and Indian GCP guidelines). The study protocol and protocol amendments, consent process, patient authorization form (PAF), the case report form (CRF), and all study-related documents were approved by the Royal Pune Independent Ethics Committee. All patients provided written informed consent prior to participating in any study-related procedures. The trial was prospectively registered on Clinical Trials Registry – India (CTRI/2020/03/024109) on 20 March 2020.
Each enrolled patient was prescribed vitamin D supplement as per physician’s discretion for 8 (non-diabetic patients) or 12 weeks (diabetic patients). The study consisted of a baseline visit on day 0, a follow-up visit at week 4, end-of study visit at week 8 for non-diabetic patients, and end-of-study visit at week 12 for diabetic patients. The total study duration was approximately 10 months including enrolment period. All patients were followed-up for primary and secondary endpoints.
Male or female patients aged ≥18 years diagnosed with vitamin D deficiency (serum 25 [OH] D level <20 ng/mL) or insufficiency (serum 25 [OH] D level of 21-29 ng/mL) of known or unknown origin who provided written informed consent were included in the study. In addition, patients with hypertension and diabetes were required to be on stable anti-hypertension and anti-diabetes treatment two months prior to enrollment to be eligible for inclusion. Patients who were on treatment for vitamin D deficiency or insufficiency, patients with serum 25(OH) D level >30 ng/mL, and pregnant and lactating women were excluded.
The primary endpoints were 1) to understand the patient profiles in terms of demographic and anthropometric measurements, exposure to sunlight, lifestyle pattern, and socioeconomic status, and 2) to determine the clinical characteristics in terms of clinical signs and symptoms at baseline and weeks 4 and 8. The key secondary endpoints were 1) to assess the type and number of comorbidities including their duration at baseline, 2) to assess the prescription pattern of vitamin D supplements by type of formulation, dose, route, and frequency of administration at weeks 4 and 8, 3) to assess change in 25(OH) D levels from baseline to weeks 4 and 8 and 4) to assess number and proportion of patients with adverse drug reactions (ADRs)/serious ADRs. The key exploratory endpoints were 1) to assess change in QoL score from baseline to week 8, 2) to assess mean change in systolic blood pressure (SBP) and diastolic blood pressure (DBP) from baseline to 8 weeks in hypertensive patients with no modification in antihypertensive medications during the study period and 3) to assess mean change in glycated hemoglobin (HbA1c) levels from baseline to 12 weeks in diabetic patients with no modification in antidiabetic medications during the study period.
Patient profiles
Patients were grouped by gender, age groups (18 to ≤30, 31 to ≤40, 41 to ≤50, or >50 years), exposure to sunlight, time of exposure to sun (morning, afternoon, or evening hours), past sunscreen use, daily frequency of past sunscreen use (once a day, twice a day, or more than thrice a day), current sunscreen use daily frequency of current sunscreen use (once a day, twice a day, or more than thrice a day), skin color (brown, olive, pale, dark brown, or reddish), dietary habits (vegetarian and non-vegetarian, non-vegetarian, or vegetarian), smoking habits (non-smoker, former, or current smoker), alcohol consumption habits (non-drinker, former or current drinker), education level (illiterate, primary school certificate, middle school certificate, high school certificate, intermediate/post-high school diploma, graduate/post-graduate, or professional/honors), occupation (unemployed, unskilled worker, semi-skilled worker, skilled worker, clerical, shop-owner, farmer, semi-professional, or professional), monthly family income in Indian rupees (≥2000, 1000-1999, 750-999, 500-749, 300-499, 101-299, or ≤100), socioeconomic status based on Kuppuswamy scale15 (lower class [score <5], upper-lower class [score 5-10], lower-middle class [score 11-15], upper-middle class [score 16-25], or upper class [score 26-29]), for assessment of association with vitamin D deficiency or insufficiency.
Clinical characteristics
The 100-mm visual analog scale (VAS) was used to assess patients’ clinical signs and symptoms such as increased risk of illness/infections, excessive fatigue and tiredness, bone and lower back pain, depression, impaired/poor wound healing, bone loss or low bone mineral density, hair loss, muscle pain, back pain, dizziness, left knee pain, left sided subtruch, leg cramps, pain in heel, pain in lower limb, tingling in hand and feet, and weakness. VAS is a self-administered, continuous scale anchored by two verbal descriptors on either extreme, i.e., 0 mm representative of no symptoms and 100 mm representative of worst imaginable symptoms. Changes in VAS scores for each sign and symptom from baseline to weeks 4 and 8 were evaluated.
Comorbidities associated with vitamin D deficiency/insufficiency
Proportion of patients by number of comorbidities, their duration, and proportion by system organ class (SOC) and preferred term (PT) were assessed at baseline. SOCs and PTs were coded using the Medical Dictionary for Regulatory Activities MedDRA version 24.0.
Prescription patterns of vitamin D supplements
Patients received vitamin D supplements as per physician discretion. The formulation type, dose, route and frequency of use were assessed at weeks 4 and 8.
Changes in clinical biomarkers
Changes in vitamin D levels from baseline to weeks 4 and 8 were assessed for all patients. In addition, change in SBP and DBP of hypertensive patients from baseline to week 8, and change from in HbA1c levels of diabetic patients from baseline to week 12 were also evaluated.
Assessment of QoL
The 20-item short form survey (SF-20) was used to assess QoL at baseline and week 8.16 This questionnaire consists of 20 questions grouped into six domains including physical functioning (PF: 6 questions), role problem (RP: 2 questions), social functioning (SF: 1 question), mental health (MH: 5 questions), current health perceptions (CH: 5 questions), and body pain (BP: 1 question). For all questions, except questions 1 and 3, the lowest raw score represented worst possible score and highest score represented best possible score. The raw scores for questions 1 and 3 were reversed to align with the scores of the remaining questions. The highest and lowest possible scores were 18 and 6 (range 12) for PF, 6 and 2 (range 4) for RP, 6 and 1 (range 5) for BP, 6 and 1 (range 5) for SF, 30 and 5 (range 25) for MH, and 25 and 5 (range 20) for CH, respectively. The scores were then transformed to the 0% to 100% scale such that transformed score = ([actual raw score – lowest possible raw]/raw score range) × 100. Scores across each of these domains were reported on a 0% to 100% scale, with 0% representing “the worst possible score” in that domain and 100% “the best possible score”. Patient’s QoL assessment was done at baseline and week 8 by using SF-20 questionnaire.16 The transformed QoL scores and change in scores for each domain from baseline to week 8 were determined.
Safety and tolerability assessments
Incidence of adverse drug reactions (ADRs) and serious ADRs and their relationship to study treatment were monitored throughout the study. All ADRs and serious ADRs were classified along with the standards of Medical Dictionary for Regulatory Activities (MedDRA) version 24.0 (RRID:SCR_003751) and grouped by PT and SOC. ADR was defined as a response to a medicinal product that was noxious and unintended, where response meant that a causal relationship between the study drug and the adverse event was at least a reasonable possibility. A serious ADR was defined as an ADR that resulted in the death of a patient, was life threatening, required hospitalization, caused prolongation of hospitalization, caused persistent or significant disability/incapacity, or required medical or surgical intervention to prevent serious outcome.
As this was an observational study, no formal statistical sample size calculation was performed. A total of 201 patients were planned to be enrolled.
Enrolled population comprised of all patients who met the eligibility criteria and were enrolled in the study. The full analysis set (FAS) comprised of patients from the enrolled population with at least one post-baseline visit and had no major protocol violations that could have altered the integrity of the study.
25[OH]D) levels by patient profiles were summarized using descriptive statistics using the FAS population and statistical analysis was performed using analysis of variance (ANOVA) at 5% level of significance. Clinical characteristics as assessed by change in clinical signs and symptoms using VAS scores from baseline to weeks 4 and 8 were summarized as descriptive statistics using the categorical variables summarized as frequency counts (n) and percentages (%). Continuous variables were summarized as mean (standard deviation [SD]) and analyzed using paired t test unless otherwise specified. Data were analyzed using SAS® version 9.4 (SAS Institute Inc., USA; RRID:SCR_008567).
Of 248 screed patients, 201 met the eligibility criteria, were enrolled in the study and comprised the enrolled population (Figure 1). Of these, 23 patients did not have at least one post-baseline visit and were excluded from the FAS. Of the 201 enrolled patients, 45 were lost to follow-up and 2 withdrew consent; thus, 154 patients completed the study. Table 1 summarizes the demographics and baseline characteristics of the enrolled population. The study had a female preponderance (61.7% women), with the mean (SD) age and BMI of the enrolled population being 43.4 (14.52) years and of 27.1 (5.52) kg/m2. Among the enrolled patients, 24.4% patients comprised the diabetes subgroup, 15.4% patients comprised the hypertension subgroup, and 8.9% patients had both diabetes and hypertension. There were 87 patients who had at least one comorbidity and the mean (SD) duration of comorbidities was 1.96 (3.127) years. Types of comorbidities observed in decreasing order of frequency were diabetes mellitus, hypertension, asthenia, arthralgia, back pain, fatigue, hypothyroidism, myalgia, anemia, thyroid disorder, gastritis, gait disturbance, pain, obesity, pain in extremity, peripheral neuropathy, and insomnia.
| Characteristic | Overall (N = 201) |
|---|---|
| Age (years), mean (SD) | 43.4 (14.52) |
| Female | |
| Age categories (years), n (%) | |
| 18-≤30 | 47 (23.4) |
| 31-≤40 | 38 (18.9) |
| 41-≤50 | 52 (25.9) |
| >50 | 64 (31.8) |
| Female gender | 124 (61.7) |
| BMI (kg/m2) | 27.1 (5.52) |
| Patients with diabetes* | 49 (24.4) |
| Patients with hypertension† | 31 (15.4) |
| Patients with diabetes and hypertension | 18 (8.9) |
| Education level | |
| Graduate or post-graduate | 72 (35.8) |
| High school certificate | 46 (22.9) |
| Middle school certificate | 22 (10.9) |
| Professional or honors | 21 (10.4) |
| Intermediate or post-high school diploma | 19 (9.5) |
| Primary school certificate | 13 (6.5) |
| Illiterate | 8 (4.0) |
| Occupation | |
| Skilled worker | 54 (26.9) |
| Unemployed | 53 (26.4) |
| Profession | 23 (11.4) |
| Shop-owner | 22 (10.9) |
| Clerical | 14 (7.0) |
| Semi-skilled worker | 13 (6.5) |
| Unskilled worker | 9 (4.5) |
| Semi-profession | 9 (4.5) |
| Farmer | 4 (2.0) |
| Family income per month (in INR) | |
| ≥2000 | 98 (48.8) |
| 1000-1999 | 36 (17.9) |
| 750-999 | 14 (7.0) |
| 500-749 | 15 (7.5) |
| 300-499 | 29 (14.4) |
| 101-299 | 4 (2.0) |
| ≤100 | 5 (2.5) |
| Socioeconomic status‡ | |
| Lower class (total score <5) | 3 (1.5) |
| Upper lower class (total score 5-10) | 25 (12.4) |
| Lower middle classes (total score 11-15) | 96 (47.8) |
| Upper middle class (total score 16-25) | 58 (28.9) |
| Upper class (total score 26-29) | 19 (9.5) |
| Exposure to sunlight | 132 (65.7) |
| Average daily sun exposure (min) | 34.37 (24.023) [n = 131] |
| Time of exposure to sun | n = 132 |
| Morning hours | 84 (63.6) |
| Afternoon hours | 39 (29.5) |
| Evening hours | 6 (4.5) |
| Missing | 3 (2.3) |
| Past sunscreen use | 21 (10.4) |
| Daily frequency of past sunscreen use | n = 21 |
| Once a day | 15 (71.4) |
| Twice a day | 5 (23.8) |
| More than thrice a day | 1 (4.8) |
| Current sunscreen use | 24 (11.9) |
| Daily frequency of current sunscreen use | n = 24 |
| Once a day | 16 (66.7) |
| Twice a day | 5 (20.8) |
| Thrice a day | 3 (12.5) |
| Skin color | |
| Brown | 98 (48.8) |
| Beige [olive] | 51 (25.4) |
| Pale | 34 (16.9) |
| Dark brown | 10 (5.0) |
| Reddish | 8 (4.0) |
| Dietary habits | |
| Both vegetarian and non-vegetarian | 118 (58.7) |
| Vegetarian | 54 (26.9) |
| Non-vegetarian | 29 (14.4) |
| Smoking habits | |
| Never | 179 (89.1) |
| Former | 13 (6.5) |
| Current | 9 (4.5) |
| Alcohol consumption habits | |
| Never | 181 (90.0) |
| Former | 11 (5.5) |
| Current | 9 (4.5) |
| Family history of vitamin D deficiency or insufficiency | 11 (5.5) |
| Father | 5 (2.5) |
| Mother | 8 (4.0) |
| Sister | 1 (0.5) |
| Other | 2 (1.0) |
Baseline 25(OH) D levels were assessed by various demographic and anthropometric factors, and subgroups within these factors were assessed for significant association with poor vitamin D levels. Most of these factors did not influence vitamin D levels, except for current sunscreen use (P = 0.0297) and socioeconomic status (P = 0.0222; Table 2). Gender, age category, exposure to sunlight, time of exposure to sun, past sunscreen use, daily frequency of past sunscreen use, skin color, dietary habits, smoking habits, alcohol consumption habits, education level, occupation, family income, and socioeconomic status had no significant effect (P > 0.05) on vitamin D status deficiency versus insufficiency. However, an odds ratio of 5.88 for patients in the upper-lower class indicated that these patients had 4.88-fold (P = 0.033) significantly higher odds for vitamin D deficiency versus insufficiency in comparison with patients in the lower class (Table 2).
| Characteristic | Vitamin D level (N = 178) | Vitamin D status (N = 178) | |||||
|---|---|---|---|---|---|---|---|
| n | Mean (SD), ng/mL | P value* | Deficient | Insufficient | OR (95% CI) | P value† | |
| Gender | 0.3184 | 0.941 | |||||
| Female | 113 | 14.18 (6.835) | 89 (50.0) | 24 (13.5) | 0.97 (0.46-2.04) | 0.941 | |
| Male | 65 | 15.25 (6.847) | 51 (28.7) | 14 (7.9) | |||
| Age (years) | 0.1343 | 0.886 | |||||
| 18 to ≤30 | 43 | 13.11 (6.804) | 35 (19.7) | 8 (4.5) | |||
| 31 to ≤40 | 33 | 13.71 (6.664) | 27 (15.2) | 6 (3.4) | 0.99 (0.31-3.12) | 0.686 | |
| 41 to ≤50 | 49 | 14.66 (6.780) | 38 (21.3) | 11 (6.2) | 1.25 (0.46-3.42) | 0.776 | |
| >50 | 53 | 16.22 (6.859) | 40 (22.5) | 13 (7.3) | 1.39 (0.52-3.71) | 0.502 | |
| Exposure to sunlight | 0.3239 | 0.941 | |||||
| Yes | 112 | 14.96 (6.986) | 87 (48.9) | 25 (14.0) | 0.705 | ||
| No | 66 | 13.91 (6.583) | 53 (29.8) | 13 (7.3) | 1.15 (0.55-2.44) | ||
| Time of exposure to sun | 0.1869 | 0.433 | |||||
| Morning hours | 71 | 15.61 (6.871) | 56 (31.5) | 15 (8.4) | |||
| Afternoon hours | 33 | 14.84 (7.409) | 23 (12.9) | 10 (5.6) | 1.63 (0.64-4.13) | 0.250 | |
| Evening hours | 5 | 9.72 (4.734) | 5 (2.8) | 0 | 0.33 (0.01-8.31) | 0.410 | |
| Pat sunscreen use | 0.0957 | 0.496 | |||||
| Yes | 21 | 12.24 (6.936) | 18 (10.1) | 3 (1.7) | 0.65 (0.19-2.23) | 0.496 | |
| No | 157 | 14.89 (6.788) | 122 (68.5) | 35 (19.7) | |||
| Daily frequency of past sunscreen use | 0.4712 | 0.802 | |||||
| Once a day | 15 | 12.88 (7.646) | 12 (6.7) | 3 (1.7) | |||
| Twice a day | 5 | 9.26 (4.080) | 5 (2.8) | 0 (0.0) | 0.32 (0.01-9.72) | 0.543 | |
| More than thrice a day | 1 | 17.4 | 1 (0.6) | 0 (0.0) | 1.22 (0.01-129.2) | 0.757 | |
| Current sunscreen use | 0.0297 | 0.200 | |||||
| Yes | 22 | 11.61 (5.789) | 20 (11.2) | 2 (1.1) | 0.40 (0.10-1.62) | 0.200 | |
| No | 156 | 14.99 (6.889) | 120 (67.4) | 36 (20.2) | |||
| Daily frequency of current sunscreen use | 0.4287 | 0.991 | |||||
| Once a day | 16 | 12.45 (6.048) | 14 (7.9) | 2 (1.1) | |||
| Twice a day | 3 | 11.16 (6.797) | 3 (1.7) | 0 (0.0) | 0.83 (0.02-33.20) | 0.962 | |
| Thrice a day | 3 | 7.60 (1.164) | 3 (1.7) | 0 (0.0) | 0.83 (0.02-33.20) | 0.962 | |
| Skin color | 0.7436 | 0.272 | |||||
| Brown | 85 | 14.39 (6.120) | 70 (39.3) | 15 (8.4) | 3.30 (0.15-73.94) | 0.621 | |
| Beige [olive] | 46 | 14.41 (7.072) | 36 (20.2) | 10 (5.6) | 4.32 (0.19-99.50) | 0.894 | |
| Pale | 31 | 15.20 (8.2932) | 22 (12.4) | 9 (5.1) | 6.33 (0.27-148.7) | 0.347 | |
| Dark brown | 9 | 16.73 (8.5123) | 5 (2.8) | 4 (2.2) | 12.27 (0.44-343.0) | 0.080 | |
| Reddish | 7 | 12.36 (4.9442) | 7 (3.9) | 0 (0.0) | - | - | |
| Dietary habits | 0.3844 | 0.206 | |||||
| Both vegetarian and non-vegetarian | 97 | 13.92 (6.434) | 81 (45.5) | 16 (9.0) | 0.57 (0.25-1.27) | 0.075 | |
| Non-vegetarian | 27 | 15.31 (6.539) | 19 (10.7) | 8 (4.5) | 1.22 (0.44-3.37) | 0.301 | |
| Vegetarian | 54 | 15.37 (7.652) | 40 (22.5) | 14 (7.9) | - | ||
| Smoking habits | 0.5751 | 0.982 | |||||
| Never | 163 | 14.67 (6.816) | 128 (71.9) | 35 (19.7) | |||
| Former | 10 | 12.46 (7.657) | 8 (4.5) | 2 (1.1) | 1.06 (0.23-4.89) | 0.973 | |
| Current | 5 | 15.66 (6.583) | 4 (2.2) | 1 (0.6) | 1.21 (0.15-9.45) | 0.888 | |
| Alcohol consumption habits | 0.5829 | 0.829 | |||||
| Never | 165 | 14.65 (6.810) | 130 (73.0) | 35 (19.7) | |||
| Former | 6 | 11.78 (8.531) | 5 (2.8) | 1 (0.6) | 1.00 (0.14-7.30) | 0.814 | |
| Current | 7 | 15.24 (6.564) | 5 (2.8) | 2 (1.1) | 1.67 (0.32-8.62) | 0.594 | |
| Education level | 0.6918 | 0.280 | |||||
| Illiterate | 8 | 11.93 (5.242) | 8 (4.5) | 0 (0.0) | |||
| Primary school certificate | 12 | 17.38 (8.877) | 7 (3.9) | 5 (2.8) | 12.46 (0.49-316.2) | 0.082 | |
| Middle school certificate | 19 | 15.31 (6.587) | 12 (6.7) | 7 (3.9) | 10.19 (0.43-241.2) | 0.108 | |
| High school certificate | 42 | 14.28 (5.917) | 36 (20.2) | 6 (3.4) | 3.03 (0.13-69.91) | 0.351 | |
| Intermediate/post-high school diploma | 16 | 14.67 (8.325) | 12 (6.7) | 4 (2.2) | 6.12 (0.24-153.4) | 0.620 | |
| Graduate or post-graduate | 61 | 14.63 (6.781) | 49 (27.5) | 12 (6.7) | 4.29 (0.20-94.15) | 0.838 | |
| Profession or honors | 20 | 13.600 (7.3308) | 16 (9.0) | 4 (2.2) | 4.63 (0.19-114.5) | 0.996 | |
| Occupation | 0.4407 | 0.758 | |||||
| Unemployed | 45 | 14.63 (8.183) | 35 (19.7) | 10 (5.6) | |||
| Unskilled worker | 9 | 19.77 (7.954) | 5 (2.8) | 4 (2.2) | 2.77 (0.63-12.23) | 0.080 | |
| Semi-skilled worker | 12 | 14.25 (5.445) | 10 (5.6) | 2 (1.1) | 0.80 (0.16-3.97) | 0.928 | |
| Skilled worker | 52 | 14.26 (6.539) | 40 (22.5) | 12 (6.7) | 1.04 (0.41-2.69) | 0.647 | |
| Clerical | 8 | 13.55 (3.897) | 8 (4.5) | 0 (0.0) | 0.20 (0.01-4.43) | 0.295 | |
| Shop-owner | 19 | 15.26 (6.155) | 14 (7.9) | 5 (2.8) | 1.28 (0.38-4.36) | 0.466 | |
| Farmer | 3 | 12.49 (5.860) | 3 (1.7) | 0 (0.0) | 0.48 (0.01-15.87) | 0.714 | |
| Semi-profession | 7 | 16.35 (9.097) | 5 (2.8) | 2 (1.1) | 1.54 (0.27-8.78) | 0.458 | |
| Profession | 23 | 12.82 (5.195) | 20 (11.2) | 3 (1.7) | 0.58 (0.15-2.23) | 0.513 | |
| Monthly family income (in INR) | 0.9456 | 0.585 | |||||
| ≥2000 | 86 | 14.09 (6.960) | 70 (39.3) | 16 (9.0) | |||
| 1000-1999 | 31 | 15.69 (7.780) | 21 (11.8) | 10 (5.6) | 2.09 (0.83-5.25) | 0.331 | |
| 750-999 | 9 | 13.93 (5.767) | 8 (4.5) | 1 (0.6) | 4.27 (0.56-32.63) | 0.203 | |
| 500-749 | 15 | 15.07 (6.168) | 13 (7.3) | 2 (1.1) | 1.23 (0.44-3.49) | 0.819 | |
| 300-499 | 28 | 14.50 (6.149) | 22 (12.4) | 6 (3.4) | 0.79 (0.18-3.53) | 0.404 | |
| 101-299 | 4 | 16.42 (6.925) | 2 (1.1) | 2 (1.1) | 0.75 (0.11-5.08) | 0.472 | |
| ≤100 | 5 | 14.64 (8.685) | 4 (2.2) | 1 (0.6) | 1.42 (0.18-11.57) | 0.970 | |
| Socioeconomic status | 0.0222 | 0.051 | |||||
| Lower class (score <5) | 3 | 15.20 (5.645) | 3 (1.7) | 0 (0.0) | |||
| Upper-lower class (score 5-10) | 22 | 18.94 (6.810) | 12 (6.7) | 10 (5.6) | 5.88 (0.17-199.6) | 0.033 | |
| Lower-middle class (score 11-15) | 86 | 14.28 (6.761) | 69 (38.8) | 17 (9.6) | 1.76 (0.06-56.29) | 0.786 | |
| Upper-middle class (score 16-25) | 49 | 13.16 (6.535) | 43 (24.2) | 6 (3.4) | 1.05 (0.03-35.45) | 0.207 | |
| Upper class (score 26-29) | 18 | 14.41 (6.775) | 13 (7.3) | 5 (2.8) | 2.85 (0.08-101.5) | 0.517 | |
Vitamin D supplementation and change in vitamin D levels
Enrolled patients were prescribed different vitamin D supplements such as 25-OH cholecalciferol, cholecalciferol, and ergocalciferol. Of 178 patients in the FAS, 140 (78.7%) were receiving cholecalciferol, 4 (2.2%) were receiving ergocalciferol at a dose of 60,000 IU/week orally, and 2 (1.1%) were receiving 25-OH cholecalciferol at a dose of 60000 IU/week orally, at week 4. Among the 140 (78.7%) patients on cholecalciferol, 134 (75.3%) were receiving 60000 IU and 6 (3.4%) were receiving 400 IU. For these patients, the route was oral for 132 (74.2%) patients and intramuscular for 8 (4.5%) patients. Frequency was daily for 6 (3.4%) patients and once a week for 134 (75.3%) patients.
At week 8, 147 (82.6%) patients were receiving cholecalciferol, of which 141 (79.2%) patients were receiving 60,000 IU and 6 (3.4%) patients were receiving 400 IU; of these, the route was oral for 139 (78.1%) patients and intramuscular for 8 (4.5%) patients. Frequency was daily for 6 (3.4%) patients and once a week for 141 (79.2%) patients. Ergocalciferol was prescribed to 11 (6.2%) patients at a dose of 60,000 IU/week.
The mean (SD) serum 25(OH) D levels were increased from baseline value of 14.57 (6.839) ng/mL to 27.86 (16.629) ng/mL at week 4 and 31.94 (28.995) ng/mL at week 8 (Table 3). The change from baseline values was statistically significant at all both-baseline visits (week 4: 13.25 (16.605), P < 0.0001; week 8: 17.57 (28.186), P < 0.0001).
| Visit | Overall (N = 178) | ||
|---|---|---|---|
| 25(OH) D levels, ng/mL | Absolute, mean (SD) | Change from baseline, mean (SD) | P value* |
| Baseline (n = 178) | 14.572 (6.8393) | - | - |
| Week 4 (n = 124) | 27.857 (16.6288) | 13.254 (16.6051) | <0.0001 |
| Week 8 (n = 120) | 31.936 (28.9952) | 17.566 (28.1860) | <0.0001 |
| Visit | Patients with hypertension (n = 28) | ||
|---|---|---|---|
| SBP, mmHg | Absolute, mean (SD) | Change from baseline, mean (SD) | P value* |
| Baseline (n = 28) | 120.0 (15.63) | - | |
| Week 8 (n = 26) | 111.2 (8.16) | −6.9 (14.36) | 0.0212 |
| DBP, mmHg | Absolute, mean (SD) | Change from baseline, mean (SD) | P value* |
|---|---|---|---|
| Baseline (n = 28) | 84.8 (8.64) | - | |
| Week 8 (n = 26) | 79.2 (4.84) | −5.5 (10.28) | 0.0110 |
| Visit | Patients with diabetes (n = 45) | ||
|---|---|---|---|
| HbA1c, % | Absolute, mean (SD) | Change from baseline, mean (SD) | P value* |
| Baseline (n = 45) | 8.11 (2.620) | - | - |
| Week 12 (n = 24) | 6.49 (1.671) | −0.88 (1.625) | 0.0143 |
Change in clinical signs and symptoms
Changes in VAS scores of clinical signs/symptoms from baseline to weeks 4 and 8 are shown in Figure 2. Very few patients experienced back pain, dizziness, left knee pain, left sided subtruch, leg cramps, pain in heel, pain in lower limb, tingling in hand and feet, or weakness at baseline; hence, these symptoms were not analyzed for changes at weeks 4 and 8. Among the remaining symptoms, the mean (SD) VAS scores of bone and lower back pain, bone loss/low bone mineral density, and muscle pain were significantly reduced from baseline to week 4 (P < 0.0001 for all) and week 8 (P < 0.0001 for all, except muscle pain [P = 0.0009]) VAS scores for risk of illness/infections and excessive fatigue and tiredness also tended to decrease, but these changes were not significantly significant (Figure 2).
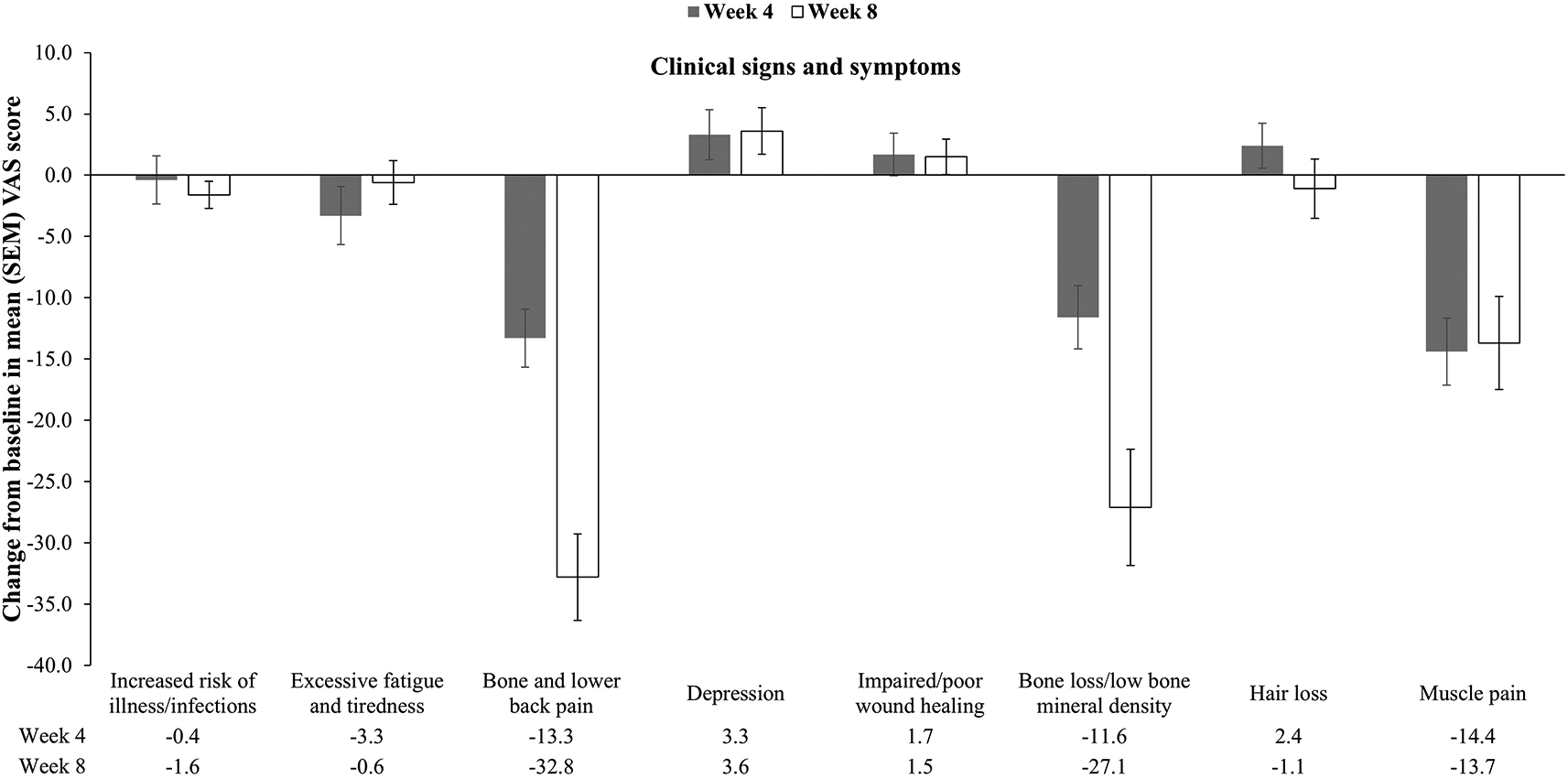
FAS, full analysis set; ns, not significant; SEM, standard error of mean; VAS, visual analog scale.
**P<0.001, ***P<0.001 versus baseline by paired t test.
Effect of vitamin D supplementation on blood pressure of hypertensive patients
Among patients with hypertension, i.e. patients who were on stable antihypertension medication for two months before study enrollment, there was statistically significant decrease in the mean (SD) SBP from 120.0 (15.63) mmHg at baseline to 111.2 (8.16) mmHg at week 8 (P = 0.0212) and in mean DBP from 84.8 mmHg at baseline to 79.2 mmHg at week 8 (P = 0.0110) (Table 3).
Effect of vitamin D supplementation on HbA1c levels of diabetic patients
Among patients with diabetes, i.e., patients who were on stable anti-diabetes medications for two months prior to enrollment, the mean HbA1c decreased significantly from baseline (8.11%) to week 12 (6.49%) (P = 0.0143) (Table 3).
Effect of vitamin D supplementation on quality of life scores
Out of the six domains comprising the SF-20 questionnaire for (QoL) assessment, improvement was observed in four domains after two months of vitamin D supplementation. There was an improvement in mean (SD) score of 10.92 (46.259) points for physical functioning, 21.52 (43.482) points for role functioning, 35.42 (38.299) for body pain, and 9.15 (18.137) points for health perception from baseline to week 8 (Figure 3).
No ADRs, serious ADRs, or OPRIs were reported after two months of vitamin D supplementation. All the patients had normal results for vital parameters (pulse rate, respiratory rate, body temperature and blood pressure) and physical examination after two months of vitamin D supplementation (data not shown). None of the patients discontinued the vitamin D treatment due to any ADR. At the end of two months, 2 patients had 25[OH] D levels >100 ng/mL and 2 patients had levels >150 ng/mL.
Recently, modernization with prolonged indoor working hours, sun-shy nature of Indians, reduced skin exposure to sunlight due to clothing habits, high atmospheric pollution, low dietary calcium intake, and decreased maternal stores of vitamin D in women due to repeated, unplanned and unspaced pregnancies were found to be some of the key factors contributing to high prevalence of vitamin D deficiency in a sun-drenched country like India.1 In this multicenter, prospective, non-interventional, observational study conducted to assess patient profiles and treatment paradigms in adult patients diagnosed with vitamin D deficiency/insufficiency in the real world setting in India, it was observed that patients were primarily prescribed supplements like 25-OH cholecalciferol, cholecalciferol and ergocalciferol with once weekly oral dose of 60,000 IU for 4 to 8 weeks. Clinical symptoms like bone and lower back pain, bone loss or low bone mineral density and muscle pain and certain quality of life domains were improved after vitamin D supplementation for two months. Moreover, significant improvement in SBP and DBP levels among patients with hypertension and HbA1c levels among patients with diabetes was observed following 8 and 12 weeks of vitamin D supplementation, respectively. While no ADRs or serious ADRs were reported, 4 patients had 25(OH) D levels above >100 ng/mL.
In the present study, it was observed that men had slightly higher vitamin D levels than women, and patients aged >50 years had higher vitamin D levels than patients aged 18 to ≤50 years. Similar results were noted in other studies where vitamin D levels were higher in men and participants aged >50 years.17,18 Different studies have shown that aging affects vitamin D production, thereby necessitating vitamin D replacement therapy in older patients, which could explain the relatively higher vitamin D levels in this age group.19,20 In this study, patients who were exposed to sunlight in the morning and at a frequency of more than thrice a day had higher vitamin D levels, consistent with studies that found daytime exposure to sun and longer duration of exposure to increase serum vitamin D3 levels.21 While sunscreen lotions play an important role in protecting against sunburn, they also filter out the radiation that is responsible for vitamin D synthesis in the skin. This fact is supported by our study results where patients who did not use sunscreen had higher vitamin D levels.22 In the present study, patients with dark brown skin had higher vitamin D levels, consistent with results of a study from Southeast Asia where people with dark skin were able to achieve sufficient vitamin D levels by getting adequate sun exposure in terms of time of exposure and duration.23 In our study, vegetarian or non-vegetarian patients had similar vitamin D levels, suggesting absence of a strong association between dietary status and vitamin D levels. A study by Baig et al. found that non-vegetarians were vitamin D deficient compared to vegetarians,24 whereas in a study by Rai et al., it was found that non-vegetarians had higher vitamin D levels.25 These contradictory results indicate that not only the type of diet but other factors may contribute to vitamin D levels in individuals with different dietary status.26 Our study observed that former smokers and alcohol consumers had low vitamin D levels than non-smokers and non-alcohol consumers. These results supported other studies showing negative effects of smoking and alcohol consumption on 25-hydroxyvitamin D levels.27–29 We observed that socioeconomic status had no significant effect on vitamin D deficiency or insufficiency. However, patients belonging to the upper-lower class had significantly higher odds for vitamin D deficiency versus insufficiency in comparison with patients in the lower class.
With regard to clinical signs and symptoms of vitamin D deficiency, patients with bone loss or low bone mineral density (BMD) and muscle pain showed improvement after vitamin D supplementation. These results support those of other studies where supplementation with vitamin D and bisphosphonates increased BMD,30 supplementation with vitamin D and calcium prevented osteoporosis,31 and muscle weakness leading to muscle pain reversed with vitamin D therapy.32
Vitamin D deficiency/insufficiency has been associated with metabolic abnormalities like hypertension and diabetes.33,34 In our study, the mean blood pressure in hypertensive patients receiving vitamin D supplements was decreased significantly from baseline and remained normal. These patients were also on stable anti-hypertensive treatment for two months prior to enrolment. However, studies have shown that vitamin D plays a vital role in regulating blood pressure in hypertensive patients with vitamin D deficiency/insufficiency.33,35 In this study, we also noted significant reduction in HbA1c levels in diabetic patients who received vitamin D supplementation along with their regular anti-diabetic treatment. There is an association between vitamin D levels and glycemic control, which has been reported in studies suggesting that poor vitamin D status is a potential risk modifier for both types of diabetes.34,36–38
We assessed the effect on QoL of patients after vitamin D supplementation using the SF-20 questionnaire where we observed improvement in four QoL domains, namely, physical functioning, role functioning, body pain, and health perception. This modest effect may be due to the relatively short duration of exposure to vitamin D or may be due to other confounding factors.39
In the present study, vitamin D supplementation in patients with vitamin D deficiency or insufficiency was found to have an acceptable safety profile as no ADRs or serious ADRs related to the vitamin D supplements prescribed were observed, and very few patients showed 25(OH) D levels exceeding 100 ng/mL, the generally accepted cut-off for vitamin D sufficiency.
The strengths of the present study include a sample size spanning different geographies and of patient profiles that could allow pan-India generalizability of the findings. Limitations of the study were the relatively short duration of treatment, absence of long-term follow-up, and inadequate representation of patients with prevalent metabolic comorbidities associated with poor vitamin D status.
In conclusion, sunscreen use and socioeconomic status had significant impact on vitamin D status among Indian patients with vitamin D deficiency/insufficiency. Vitamin D supplementation for two months restored 25(OH) D levels in patients with deficiency or insufficiency, improved key clinical symptoms associated with vitamin D deficiency/insufficiency and some QoL domains, improved HbA1c levels in diabetic patients on stable anti-diabetes treatment, and blood pressure in hypertensive patients on stable anti-hypertension treatment, without any adverse safety outcomes. Nevertheless, studies with long-term follow-up and larger sample sizes are required to assess the impact of vitamin D supplementation on QoL and metabolic comorbidities such as hypertension and diabetes.
All authors had full access to all data presented in this study and take responsibility for the accuracy of the data analysis. All authors provided critical feedback on the manuscript draft and revisions to shape the analysis and manuscript.
Figshare: [Tiwaskar M et al._Vitamin D registry_Underlying data]. https://doi.org/10.6084/m9.figshare.21617742.v1. 40
The project contains the following underlying data:
Figshare: STROBE checklist for ‘[Evaluation of patient profiles, treatment paradigms and clinical efficacy, and safety outcomes in adult patients with vitamin D deficiency or insufficiency in India: A multicenter, prospective, non-interventional study]’. https://doi.org/10.6084/m9.figshare.21716441.v1. 41
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors thank Nilesh Inamdar, Innvocept Global Solutions for support with medical writing and editorial assistance.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
At the request of the author(s), this article is no longer under peer review.
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)