Keywords
PCNL, Spinal Anesthesia, General Anesthesia, Complication, Stone-free Rate
This article is included in the Global Public Health gateway.
Background: Percutaneous nephrolithotomy (PCNL) is the preferred treatment for the removal of large kidney stones, sized >20 mm. However, there is still an ongoing debate concerning the best anesthesia for PCNL. This study aimed to compare the efficacy and safety between general and spinal anesthesia for PCNL.
Methods: A systematic review and meta-analysis study. A systematic, electronic literature search was performed in several databases, including PubMed, Scopus, and Google Scholar until July 1st, 2022. The quality of the articles was examined using Crombie's Items (for non-randomized controlled trials (RCTs)) and Jadad Scale (for RCTs). The outcomes assessed were operation time, fluoroscopy time, length of stay, stone-free rate, overall complication rate, specific postoperative complications, cost, pain score, and postoperative analgesic requirement. The article selection was reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. We assessed four RCTs and eight retrospective studies. Meta-analysis of selected studies was performed using the Review Manager 5.3.
Results: General anesthesia resulted in fewer Clavien–Dindo grade II (OR: 0.68; 95% CI: 0.49 – 0.94; p=0.02), major complications (OR: 0.65; 95% CI: 0.45 – 0.94; p=0.02, and lower transfusion rates (OR: 0.70; 95% CI: 0.53 – 0.94; p=0.02). Whereas spinal anesthesia resulted in faster operation time (Mean Difference: -12.98; 95% CI: -20.56 – -5.41; p<0.001, fluoroscopy time (MD: -26.15; 95% CI: -42.79 – -9.50; p=0.002), reduced length of stay (MD: -0.47; 95% CI: -0.75 – 0.20; p<0.001), and lower postoperative analgesic requirement and cost. No significant difference in stone-free rate (OR: 1.08; 95% CI: 0.92 – 1.26; p=0.37). PCNL performed using either general anesthesia or spinal anesthesia is equally safe and effective.
Conclusions: Each method of anesthesia has its own advantages and disadvantages. The final choice between general and spinal anesthesia should be based on the patient's condition and surgical team preference.
PCNL, Spinal Anesthesia, General Anesthesia, Complication, Stone-free Rate
We have adjusted several statements according the the reviewer's kind comments. VAS and cost is now moved under the discussion section from the results section. As there are not sufficient studies to generate a forest plot report for these 2 variables. Table 4 was updated with the information regarding patient's mean age. The literature searching section is now moved under the methods from the results section
See the authors' detailed response to the review by Noor Buchholz
See the authors' detailed response to the review by M. Hammad Ather
Nephrolithiasis remains a common health problem around the globe. Its prevalence is 7–13% in North America, 5–9% in Europe, and 1–5% in Asia. According to the European Association of Urology (EAU) and American Urological Association (AUA), percutaneous nephrolithotomy (PCNL) is the first line treatment modality for renal calculi sized >20 mm.1,2 PCNL is also useful for treating multiple stones, staghorn stones, and stones that are resistant to extracorporeal shockwave lithotripsy.1,2 There are variations to PCNL, including position, imaging modality, dilation method, and anesthesia method.3,4
There is conflicting evidence between the appropriate use of general anesthesia (GA) versus spinal anesthesia (SA) for PCNL. GA was associated with a longer duration of surgery and postoperative length of hospital stay in most studies.3,5–8 GA allows greater flexibility for the anesthesiologist to extend the duration of anesthesia, whereas in SA, this would be more problematic. SA is associated with better postoperative pain control, thereby reducing the need for analgesic drugs.5,8 Some studies have also shown that GA costs more than SA and has a higher rate of complications. The complications usually occur when the patient's position is altered from supine to prone. These complications include brachial plexus injury, spinal cord injury, and lung injury.8,9
The aim of this study was to evaluate the safety and efficacy profile, in terms of stone free rate, of GA and SA in PCNL.
This is a systematic review and meta-analysis study evaluating the efficacy and safety of SA compared to GA in PCNL. The aim of the study is to evaluate the efficacy of safety of GA compared to SA in PCNL. We included studies that involved patients with renal calculi sized >20 mm who underwent PCNL. The intervention group included patients who were administered SA, whereas the control group were administered GA. The safety profile was determined using the complication rate (classified by the Clavien–Dindo scoring system) and degree of specific complications (headache, urinary tract infection (UTI), urosepsis, and transfusion rate). Clavien-dindo class I is any deviation from the normal postoperative course with the allowed therapeutic regimens of antiemetics, antipyretics, analgesics, diuretics, electrolytes, and physiotherapy. Class II is any deviationo requiring pharmacolocigal treatment other than those mentioned in class I. Class IIIa requires any surgical/endoscopic/radiological intervention, Class IIIb requires intervention under general anesthesia. Class IVa is life threatening complication requiring intensive care unit (ICU) management, Class IVb is multiorgan dysfunction, and Class V is death.
The efficacy profile was determined using values of stone free rate, operation duration, and length of stay. Any studies that included patients with any renal anomaly, such as horseshoe kidney, malrotated kidney, or ectopic kidney were excluded. Furthermore, this study also excluded studies involving patients with contraindications for SA and GA, such as spinal deformity, severe cardiac and respiratory failure, or severe renal failure.
A systematic search of the literature was performed until July 1st, 2022, using PubMed (RRID:SCR_004846), Scopus (RRID:SCR_022559), and Google Scholar (RRID:SCR_008878) databases. The keywords used were “Spinal, General, Percutaneous Nephrolithotomy, PCNL, PNL” in PubMed, “spinal, general, anesthesia, percutaneous nephrolithotomy” in Scopus, and “spinal, spine, general, PCNL, PNL, percutaneous, nephrolithotomy” in Google Scholar. All keywords were combined using the Boolean logic. The articles identified were then screened for duplicates, which were then removed. The article selection was reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.29
Two reviewers (RIR and PB) examined the articles independently. In case of any disagreement, a discussion was conducted to resolve the matter. The articles were screened for their relevance through the titles and abstracts. The inclusion criteria were comparative studies or randomized controlled trials (RCTs) concerning the use of SA and GA in PCNL. All included articles were written in English. The exclusion criteria were non-comparative studies; studies that combined SA with another method of anesthesia, such as epidural anesthesia; studies with irrelevant outcomes; and studies that included patients with renal anomalies, such as malrotated kidney, horseshoe kidney, or ectopic kidney. The quality of the articles was examined using the Crombie's Items scale (for non-RCTs) and Jadad Scale (for RCTs).10,11
The process of data extraction of the articles was independently conducted by two reviewers (GN and PB), and any disagreement was resolved by consensus. The variables extracted from the articles were article title, author's name, year of publication, stone free rate, length of stay, operation duration, fluoroscopy time, complication rate (classified using Clavien–Dindo scores), and specific complication rate (headache, UTI, urosepsis, and transfusion rate). Major complication rate was defined by a Clavien–Dindo score 3A or higher. There was no missing data in the data extraction process.
Two reviewers (RIR and GN) performed data analysis independently. Meta-analysis of selected studies was performed using the Review Manager (RevMan) (RRID:SCR_003581) 5.3 application. Alternatively, meta-analysis of selected studies can be performed using STATA (RRID:SCR_012763). For dichotomous variables, the results were presented as odds ratio (OR) with 95% confidence interval (CI). Whereas for continuous variables, the results were presented as the mean difference with 95% CI. Heterogenicity was analyzed using the chi-squared and I2 test, as appropriate. The data were analyzed using the random-effect model when I2>50% and fixed-effect model when I2<50%. Statistical significance was set at p<0.05. Missing data were analyzed in the outcome. For studies that provided minimum and maximum values instead of standard deviation (SD) for the mean difference analysis, estimated SD was then calculated by the formula provided by Walter and Yao (2007).10 Additionally, for studies that provided 95% CI values instead of SD, SD was then calculated using the formula described in the Cochrane Handbook.12
After screening the articles and applying the inclusion and exclusion criteria, we found 127 articles from three databases. After removing duplicates, we included 113 studies. Among these, we excluded 90 studies as they were irrelevant based on their titles and abstracts. Subsequently, after assessing the full text, we included 11 studies in the final qualitative and quantitative (meta-analysis) analyses (Figure 1).
A total of four RCTs and seven retrospective studies were assessed. The retrospective studies were assessed using Crombie's items (Table 1) and RCTs were assessed using the Jadad Scale (Table 2).12,13 There were three grade B and four grade C retrospective studies. All the RCTs had a score of less than three. Anesthesia and PCNL methods for each study are presented in Table 3. Furthermore, study characteristics, such as number of patients, stone burden, mean age, stone free rate, follow-up period, and complication rate are presented in Table 4.
| Author, year | Design | Data | Response rates | Representativeness | Reliable and valid measurements | Statistical significance | Statistic methods | Crombie’s score | Grading* |
|---|---|---|---|---|---|---|---|---|---|
| Gonen et al.(2014)5 | 1 | 0.5 | 0 | 0 | 0.5 | 1 | 0.5 | 3.5 | C |
| Karatag T et al. (2015)6 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 4 | B |
| Mehrabi S et al. (2013)8 | 1 | 0.5 | 0 | 0 | 0 | 1 | 0.5 | 3 | C |
| Cicek T et al. (2014)13 | 1 | 1 | 1 | 0 | 0.5 | 1 | 0.5 | 5 | B |
| Solakhan M et al. (2019)14 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 4 | B |
| Astram A et al. (2015)15 | 1 | 0 | 0 | 0 | 0.5 | 1 | 0.5 | 3 | C |
| Buldu I et al. (2016)16 | 1 | 1 | 0 | 0 | 0 | 1 | 0.5 | 3.5 | C |
| Author, year | Randomized | Double-blind | Withdrawals | Randomization method | Double-blinding described | Score* |
|---|---|---|---|---|---|---|
| Nouralizadeh A et al. (2013)17 | 1 | 0 | 0 | 0 | 0 | 1 |
| Movasseghi G et al. (2014)18 | 1 | 0 | 0 | 1 | 0 | 2 |
| Shah T et al. (2016)7 | 1 | 0 | 0 | 1 | 0 | 2 |
| Bhattarai R et al. (2016)19 | 1 | 0 | 0 | 0 | 0 | 1 |
| Author | Country | Study Design | PCNL Technique | Anesthesia | ||||
|---|---|---|---|---|---|---|---|---|
| Position | Imaging | Dilation | Fragmentation | Spinal | GA | |||
| Mehrabi S et al. (2013)8 | Iran | Retrospective study | Supine | Fluoroscopy | One-shot technique using Amplatz dilator 28F/30F | Pneumatic & shockwave lithotripter | Bupivacaine 0.5% 2–2.5 ml + 0.5 mL fentanyl at L4 & L5 intervertebral space | Midazolam, thiopental, atracurium (dose not specified) |
| Nouralizadeh A et al. (2013)17 | Iran | RCT | Prone | Fluoroscopy | Single-stage technique | Pneumatic lithotripter & holmium laser | Bupivacaine 0.5% 0.25 mg/kg (up to 40 mg) at L3-L4 at intervertebral space | Fentanyl 2 mg/kg, midazolam 0.03 mg/kg, propofol 2 mg/kg, atracurium 0.5 mg/kg |
| Cicek T et al. (2014)13 | Turkey | Retrospective study | Prone | Fluoroscopy | Amplatz dilation | Pneumatic lithotripter | Levobupivacaine 15–20 mg + midazolam 2.5 mg at L2-L3 intervertebral space | Propofol 2.5 mg/kg, 1 mg/kg fentanyl, 0.5 mg/kg atracurium intravenously |
| Movasseghi G et al. (2014)18 | Iran | RCT | Prone | Fluoroscopy | N/A | N/A | Bupivacaine 0.5% 15–20 mg + 0.01–0.02 mg/kg midazolam | Fentanyl 1–2 mg/kg, midazolam 0.01–0.02 mg/kg, thiopental-Na 3-5 mg/kg, atracurium 0.5 mg/kg, propofol 100 mcg/kg/min |
| Gonen et al. (2014)5 | Turkey | Retrospective study | Prone | Fluoroscopy | Amplatz dilation | Pneumatic Lithotripter | Bupivacaine 8–15 mg at L2-L3 intervertebral space | Propofol 2 mg/kg, fentanyl 1 mg/kg, rocuronium bromide 0.6 mg/kg |
| Karatag T et al. (2015)6 | Turkey | Retrospective study | Prone | Fluoroscopy | 4.8Fr “all-seeing needle” microperc system | Holmium: YAG laser | Levobupivacaine 15–20 mg at L3-L4/L4-L5 intervertebral space | Thiopental 5 mg/kg, fentanyl 2 mcg/kg, atracurium 0.5 mg/kg |
| Astram A et al. (2015)15 | Indonesia | Retrospective study | N/A | N/A | N/A | N/A | N/A | N/A |
| Buldu I et al. (2016)16 | Turkey | Retrospective study | Prone | Fluoroscopy | Amplatz dilation | Pneumatic lithotripter | Bupivacaine 15–20 mg + midazolam 2 mg sedation at L3-L4 intervertebral space | Propofol 2 mg/kg, fentanyl 1 mg/kg, unspecified neuromuscular block |
| Shah R et al. (2016)7 | Nepal | RCT | Prone | Fluoroscopy | Serial dilators 26-30 F | Pneumatic Lithotripter | Bupivacaine 0.5 % + 0.5 ml fentanyl (25 mcg) at L3-L4 intervertebral space | Midazolam 40 mcg/kg, fentanyl 2 mcg/kg, propofol 2 mg/kg, vecuronium 0.1 mg/kg |
| Bhattarai R et al. (2016)19 | Nepal | RCT | N/A | N/A | N/A | N/A | 0.5% bupivacaine (hyperbaric) at L3-L4 intervertebral space | Midazolam 0.04 mg/kg, fentanyl 1 mcg/kg, propofol 2.5 mg/kg & Inj. atracurium 0.5 mg/kg |
| Solakhan M et al. (2019)14 | India | Retrospective study | Prone | Fluoroscopy | Amplatz dilation | N/A | Bupivacaine 20 mg 0.5% + midazolam 2 mg at intervertebral space | Propofol 2 mg/kg, fentanyl 1 mg/kg, 0.5 rocuronium bromide mg/kg, midazolam 2 mg/kg |
| Author | Study design | Case | Mean age | Stone size | Mean stone number | Stone free rate (%) | Complications (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spinal | GA | Spinal | GA | Spinal | GA | Spinal | GA | Spinal | GA | Definition | Follow-up time (days) | Spinal | GA | Definition | ||
| Mehrabi S et al. (2013)8 | Retrospective Study | 58 | 52 | 47.4±7.6 | 43.7±8.2 | 31.3±7.9 mm | 34.2±9.8 mm | N/A | N/A | 79.3 | 80 | Stone ≤4 mm | 1 | 15.5 | 11.5 | N/A |
| Nouralizadeh A et al. (2013)17 | RCT | 50 | 50 | 41.16±11.2 | 42.66±13.61 | 5.51±2.87 cm2 | 5.56±2.95 cm2 | N/A | N/A | 78 | 84 | Stone ≤4 mm | 1 | 30 | 40 | Clavien–Dindo I-V |
| Cicek T et al. (2014)13 | Retrospective Study | 440 | 564 | 48.8±14.03 | 47.2±13.83 | 533.9±480.94 mm2 | 529.5±324.12 mm2 | N/A | N/A | 73.6 | 73.9 | Stone ≤4 mm | Immediate postoperative | 18.6 | 20.6 | Clavien–Dindo I-V |
| Movasseghi G et al. (2014)18 | RCT | 30 | 29 | 39.6±9.7 | 46.9±13.6 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Gonen et al. (2014)5 | Retrospective Study | 20 | 26 | 45.6±13.6 | 40.8±12.9 | 558.6±297.2 mm2 | 630.7±486.2 mm2 | N/A | N/A | 96.2 | 95 | Absence of residual stone | 1 | 7.70 | 5 | Clavien–Dindo I-V |
| Karatag T et al. (2015)6 | Retrospective Study | 63 | 53 | 45.8±14.6 | 30.3±22.1 | 155.08±84.9 mm2 | 151.00±75.5 mm2 | 1.4±0.69 | 1.3±0.59 | 93.6 | 90.5 | Absence of residual stone | 1 | 9.40 | 9.3 | Clavien–Dindo I-IIIa |
| Astram A et al. (2015)15 | Retrospective Study | 540 | 220 | 51.09±11.33 | 48.63±11.77 | 36.76±17.66 mm | 40.93±22.87 mm | N/A | N/A | 73.0 | 71.4 | N/A | N/A | 5.18 | 9.5 | N/A |
| Buldu I et al. (2016)16 | Retrospective Study | 47 | 53 | 48.5±13.8 | 46.1±16.6 | 52.9±15.4 mm | 50.6±24.6 mm | N/A | N/A | 61.7 | 52.8 | Absence of residual stone | N/A | 19.1 | 13.2 | Clavien–Dindo I-V |
| Shah R et al. (2016)7 | RCT | 30 | 30 | 36.10±14.18 | 39.13±12.45 | 3.23±1.36 cm | 3.75±1.27 cm | Single or multiple | Single of multiple | 93.3 | 83.3 | Absence of residual stone | 1 | 13.3 | 16.7 | Clavien–Dindo I-V |
| Bhattarai R et al. (2016)19 | RCT | 30 | 30 | 38.03±12.1 | 34.87±9.95 | 27.6±5.8 mm | 26.3±6.6 mm | 3.8±3.1 | 3.4±3.6 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Solakhan M et al. (2019)14 | Retrospective Study | 1,085 | 572 | 34.3±11.1 | 32.7±13.1 | 635.2±304.1 mm2 | 644.5±301.8 mm2 | N/A | N/A | 85.1 | 83.4 | Stone ≤4 mm | N/A | 24.4 | 23.8 | Clavien–Dindo I-V |
A total of 4,072 patients were quantitatively analyzed for operation time in the included studies, with 2,393 patients in the SA and 1,679 in the GA group (Figure 2A). High heterogenicity was detected in these studies (I2=94%), and therefore a random-effects model analysis was performed. Pooled data showed that SA had a significantly faster operation time, as compared to GA, using the random-effect model analysis, and the mean difference for SA versus GA was -12.98 minutes (95% CI, 20.56 to -5.41; p=0.0008).
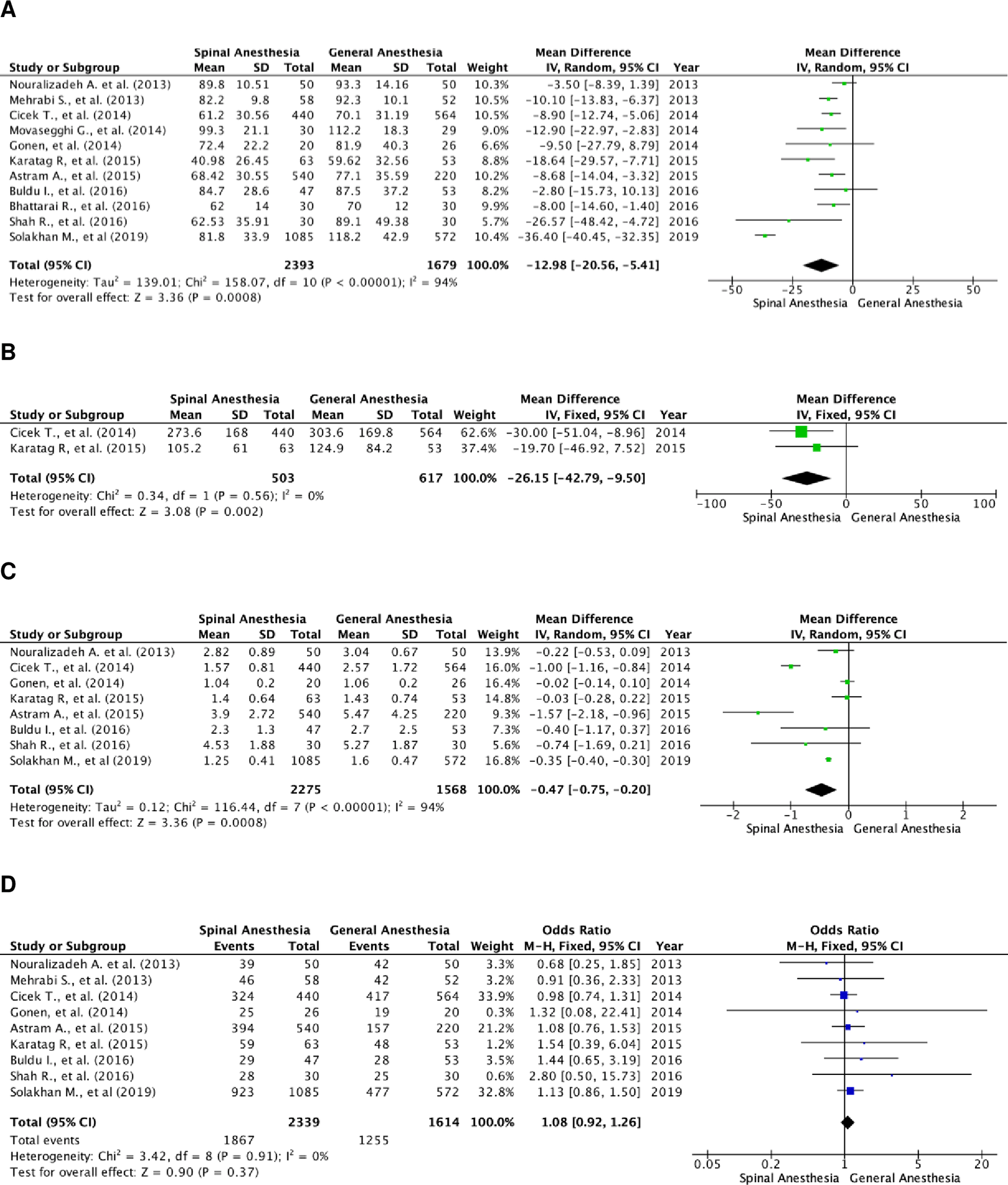
(A) Pooled estimate of operation time using random-effect model; (B) pooled estimate of fluoroscopy time using fixed-effect model; (C) pooled estimate of length of stay using random-effect model; (D) pooled estimate of stone-free rate using fixed-effect model.
There were two studies that compared fluoroscopy time between the two groups (Figure 2B). A total of 1,120 subjects were included, with 503 in the SA group and 617 in the GA group. The studies were homogenous (I2=0%). The SA group had a significantly faster fluoroscopy time as compared to the GA group, with a fixed effect mean difference of -26.15 minutes (95% CI, -42.79 to -9.50; p=0.002).
There were eight studies that assessed the length of hospital stay of the patients. A total of 3,843 patients were included, with 2,275 in the SA group and 1,568 in the GA group (Figure 2C). These studies were heterogenous with I2=94%. Patients in the SA group were discharged sooner as compared to those in the GA group. The result of the random-effects model was statistically significant with a mean difference of -0.47 day (95% CI, -0.75 to -0.20; p=0.0008).
There was a total of nine studies that reported the stone-free rate of patients in both groups. There were 3,953 patients in total, and 2,339 patients belonged to the SA group and 1,614 to the GA group (Figure 2D). Heterogenicity was not found in these studies with I2=0%. Hence, a fixed-effect analysis was performed. There was no significant difference in the stone-free rate between the two groups, with a fixed-effect odds ratio of 1.08 (95% CI, 0.92 to 1.26; p=0.37).
There were nine studies that reported the overall complication rate (Figure 3A).5,18,20 A total of 3,953 patients were included, of which 2,339 patients were in the SA group and 1,614 patients in the GA group. The studies were homogenous (I2=0%). There was no statistically significant difference in the overall complication rates between the two groups. The fixed-effect model’s odds ratio was 0.93 (95% CI, 0.79 to 1.10; p=0.43).
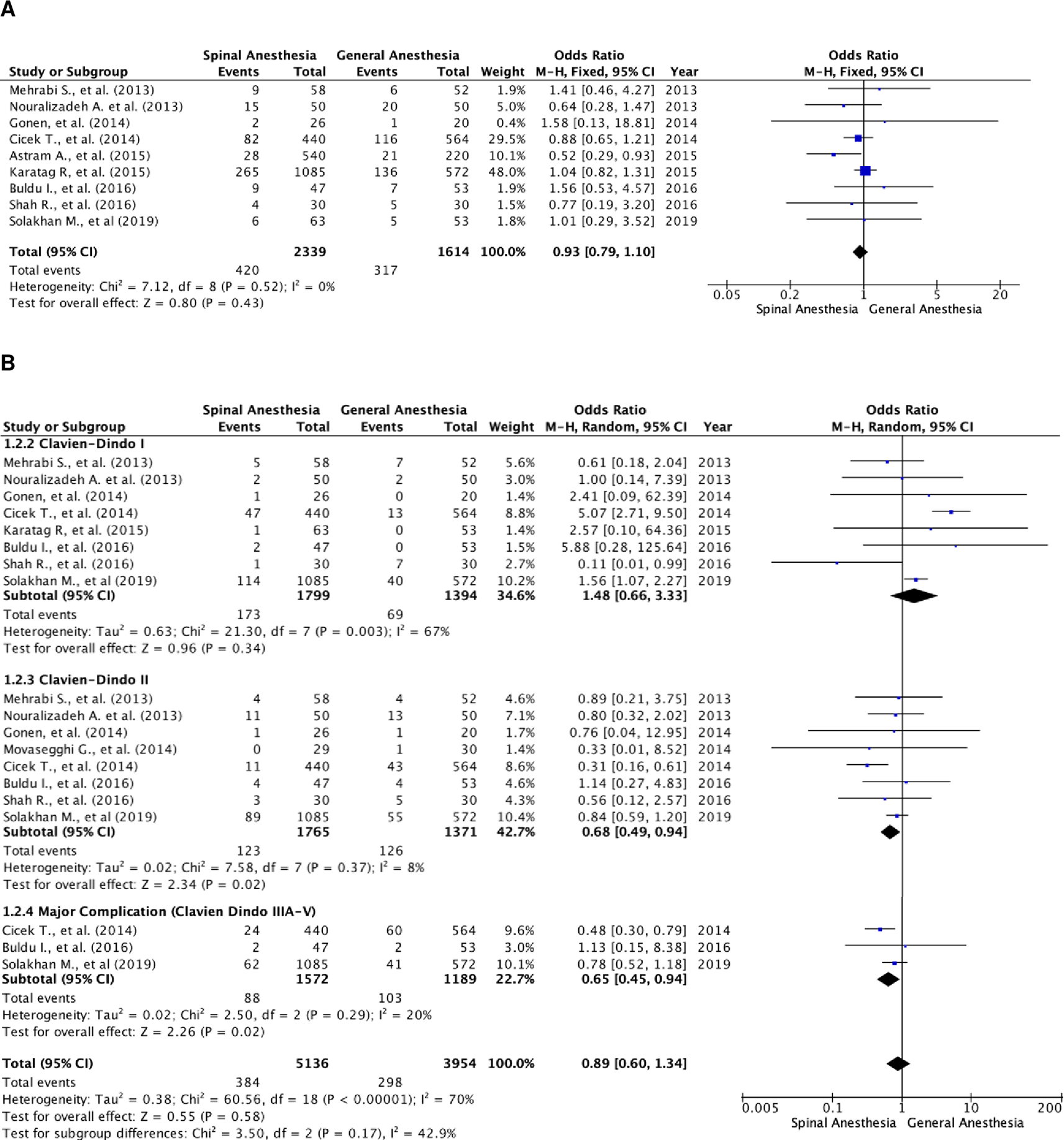
(A) Pooled estimate of overall complication rate using fixed-effect model; (B) pooled estimate of every Clavien–Dindo classification complication.
Further subgroup analysis was performed in relation to every Clavien–Dindo classification (Figure 3B). There were 9,090 events of complications noted, of which 5,136 were in the SA group and 3,954 in the GA group. In these studies, one patient could experience more than one complication, resulting in a higher number of events compared to the total number of patients with complications (Figure 3A). Heterogenicity was noted in patients with Clavien–Dindo grade I complications (I2=67%) and major complication rate (I2=70%). There were notably more patients with Clavien–Dindo grade II and major complications in the SA group as compared to the GA group. The result was statistically significant with a fixed-effect model odds ratio of 0.68 (95% CI, 0.49 to 0.94; p=0.02) and random-effect model odds ratio of 0.65 (95% CI, 0.45 to 0.94; p=0.29). However, there were no differences in Clavien–Dindo grade I complication rate with a random-effect model odds ratio of 1.48 (95% CI, 0.66 to 3.33; p=0.34).
Further analysis of postoperative complications showed that patients in the SA group had higher transfusion rates (Figure 4A). The odds ratio of the fixed-effects model was 0.70 (95% CI, 0.53 to 0.94; p=0.02). There were 11 studies that included transfusion rate as a parameter, with a total of 4,072 subjects, of which 2,398 were in the SA group and 1,674 in the GA group. The studies were not heterogenous (I2=40%).
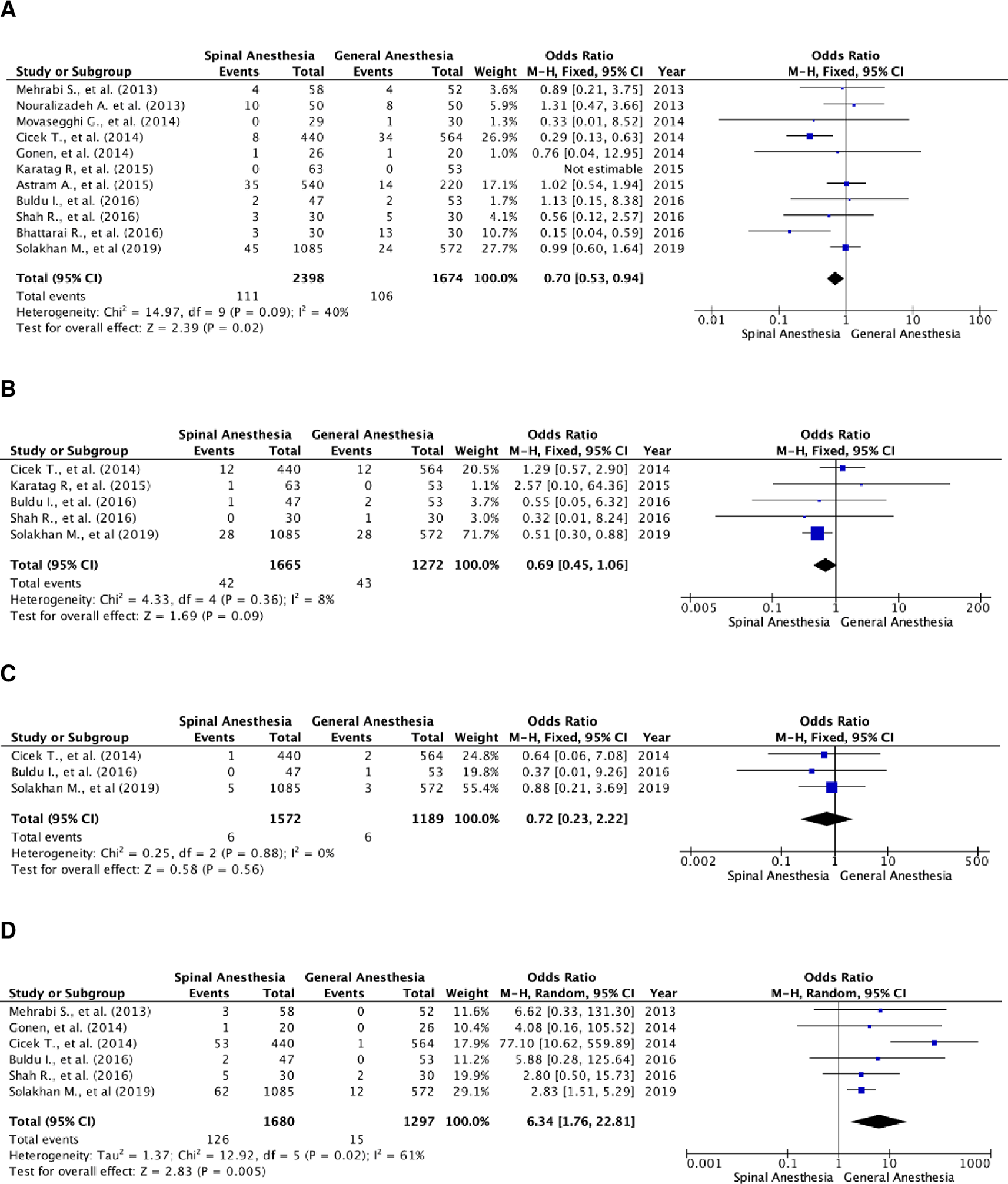
(A) Pooled estimate of transfusion rate using fixed-effect model; (B) pooled estimate of UTI using fixed-effect model; (C) pooled estimate of urosepsis using fixed-effect model; (D) pooled estimate of postoperative headache using random-effect model. UTI, urinary tract infection.
There was no significant difference in UTI and urosepsis between the two groups (Figure 4B and C). Meanwhile, patients in the GA group experienced more postoperative headaches (Figure 4D). The odds ratio of the random-effect model was 6.34 (95% CI, 1.76 to 22.81; p=0.005). There were six studies that reported postoperative headache as a complication, with a total of 2,977 patients, of which, 1,680 patients were in the SA group and 1,297 patients were in the GA group. Heterogenicity was noted with I2=61%.
Postoperative analgesic requirement was described in three studies. Overall, these studies stated that patients in the SA group required significantly less postoperative analgesic drugs, as compared to those in the GA group. Gonen et al., found that patients administered SA (53.8±39.8 mg) require significantly less postoperative intravenous tramadol, as compared to those administered GA (111.5±46.3 mg; p<0.001).5 In addition, Mehrabi et al., also stated that patients in the SA group require significantly less postoperative intravenous opioid drugs (unspecified), as compared to those in the GA group on the first (SA: 7.8±2.3 mg, GA: 12.4±3.1 mg; p=0.03) and second (SA: 11.1±2.1 mg, GA: 13.2±2.1 mg; p=0.06) postoperative days.8 However, the difference in opioid drug requirement on the second postoperative day was not significant. Moreover, Bhattarai et al., stated that patients in the SA group (8.2±1.2 mg) require significantly less postoperative analgesic drugs (unspecified) compared to those in the GA group (14.6±2.4 mg; p=0.0001).19
This systematic review and meta-analysis investigated the efficacy and safety profile of SA compared to GA in PCNL. The best study design to evaluate such type of study is RCT. This study included four RCTs. This study finds that SA and GA are both equally safe and effective for PCNL. Key differences are that GA resulted in fewer Clavien-Dindo grade II complications, major complications, and lower transfusion rates. SA resulted in faster operation time, fluoroscopy time, reduced length of stay, and lower postoperative analgesic requirement and cost.
PCNL is the first-line treatment for large nephrolithiasis, sized >20 mm.1,2 This procedure is traditionally performed after administering GA.20 GA may result in fluid absorption and electrolyte imbalance, therefore performing GA in patients with comorbidities could be challenging. For patients with chronic cardio-pulmonary conditions, such as chronic obstructive pulmonary disease or chronic heart failure, SA may be the method of choice for anesthesia.8,21 Moreover, GA has potential adverse effects such as allergic drug reactions, cardiopulmonary compromise, and aspiration of gastric contents. There are several studies in which SA was performed for PCNL candidates who were critically-ill and morbidly-obese to avoid cardiorespiratory compromise during the procedure.9,22,23
To date, there is no consensus concerning the best mode of anesthesia for PCNL. To address this matter, in 2015, Pu et al., published a meta-analysis comparing GA to regional anesthesia (SA, epidural anesthesia, and spinal-epidural anesthesia).24 To the best of our knowledge, this is the first meta-analysis directly comparing the efficacy and safety of GA and SA in patients who underwent PCNL.
In a majority of the studies in this review, PCNL was performed in the prone position5–7,13,14,16–18; except for one study by Mehrabi et al., in which PCNL was performed in the supine position.8 PCNL performed in the prone position results in a higher stone-free rate than that performed in the supine position. In regard to the safety profile, performing PCNL in the supine position yields superior results than in the prone position.4 The supine position also makes it easier for anesthesiologists to handle cardiorespiratory emergencies intraoperatively, as compared to the prone position.4
The authors chose stone-free rate as the study's primary outcome to compare both the methods of anesthesia from a urologist’s perspective; the secondary outcomes are operation time, fluoroscopy time, length of stay, and complications. In this study, we found that GA is superior in terms of lower Clavien–Dindo grade >II complications, and lower transfusion rate and UTIs, whereas SA is superior in terms of shorter operation time, fluoroscopy time, length of stay, and significantly fewer cases of postoperative headache. Both methods are similar in terms of stone-free rate and overall complication rate. Therefore, surgeons can freely choose between GA or SA for PCNL without having to worry about the efficacy of the anesthesia method.
Every study included in this review reported faster operation time in the SA group as compared to the GA group. The operation and fluoroscopy time was faster in the SA group. In most studies included in this review PCNL was performed in the prone position. When administering GA for PCNL, the patient must be positioned twice. The patient initially must lie in the supine position to be intubated. Thereafter, the patient is pronated for PCNL. This two-stage nature of the procedure may attribute to a longer operation and fluoroscopy time in the GA group.3
Length of hospital stay is shorter in the SA group. This may be attributed to the reduced risk of systemic complications with SA as compared to GA. The method of anesthesia can affect early postoperative recovery for patients. SA is typically performed by administering bupivacaine. A study has demonstrated sensory and motor blockade for 133.16 ± 42.21 minutes after the use of bupivacaine.25 These findings correlate well with the reduced requirement of postoperative analgesics for patients in the SA group as compared to those in the GA group because SA has a lingering effect that lasts post-surgery. A study by Mehrabi et al., showed that on the first postoperative day, patients in the SA group required significantly lower doses of postoperative intravenous opioid drugs, as compared to the GA group.8
Overall complications did not differ between the two groups. In terms of specific complications, patients in the GA group experienced more postoperative headaches as compared to those in the SA group. In patients who were administered GA, postoperative headache, nausea, and vomiting were a common finding.26 A prospective study assessing the postoperative complications of GA in oral and maxillofacial surgery reported that 41% of the subjects experienced postoperative headaches. On the contrary, SA has fewer systemic effects, resulting in less frequently reported postoperative headaches.26
Bleeding is a well-known complication in PCNL. In our meta-analysis we found that patients in the SA group had a higher transfusion rate. This result contradicts previous meta-analyses, where SA is associated with a significant decrease in blood loss compared to GA in surgeries within the pelvic, abdominal, and thoracic cavities and lower extremities.27
Mehrabi et al., reported that GA is more expensive than SA. The cost of drugs and materials are USD 5.4±3.1 (SA) and USD 23±7.3 (GA). Previous studies on orthopedic surgeries have also demonstrated that SA is relatively less expensive as compared to GA.28 Possible heterogeneity in this study may be caused by differences in sample size, anesthesia drug, and stone fragmentation method. In terms of postoperative VAS score, Karatag et al., showed that there was no significant difference (p=0.365) in VAS in both groups. The VAS of the SA and GA group were 3.0±1.3 and 2.9±1.7, respectively.6 Spinal anesthesia is generally preferred than general anesthesia, as SA yields minimal systemic effect compared to GA. Using GA increases the risk of anaphylaxis because of the drug used in GA. However GA eases the procedure by controlling the breathing and renal movement of the patient.5
The limitation of this study is that there are few articles that have reported fluoroscopy time in both the groups. Postoperative analgesic consumption was also difficult to compare between the studies because there was no uniform term for the definition of postoperative analgesic consumption. Few studies have reported major complication rates using the Clavien–Dindo classification, including the incidence of urosepsis. There are only four RCTs included, and the rest of the studies are retrospective studies. Therefore, there could be an overestimation of the result due to selection bias. The RCTs assessed had low scores due to lack of blinding. However, double blinding is no applicable in the studies. Patient must provide consent to be included in the study and anesthesiologists performing anesthesia must be prepared for the upcoming procedure to ensure patient safety. Therefore, the anesthesia modality must be specified prior to the procedure. In this case, a third observer must be involved to assess the outcomes of the subjects without knowing what group they are in to ensure objectivity. There is a limitation in the review process, which is that there were only two reviewers of the articles in this study.
However, this study addresses the advantages and disadvantages of both GA and SA in PCNL, with an aim to provide valuable insights for surgeons to choose the most appropriate method of anesthesia for PCNL.
In terms of efficacy marked by stone-free rate, there are no significant differences between GA and SA. GA is superior in terms of lower Clavien–Dindo grade II complications, transfusion rate, and UTI occurrence, whereas SA is superior in terms of shorter operation time, fluoroscopy time, length of stay, and a significantly lower frequency of postoperative headaches. Both methods are similar in terms of the overall complication rate. Therefore, the decision to choose between GA and SA should be based on the patient's clinical parameters and the surgical team’s preferences.
All data underlying the results are available as part of the article and no additional source data are required.
Open Science Framework: PRISMA checklist for article 'General versus spinal anesthesia in percutaneous nephrolithotomy: A systematic review and meta-analysis', https://doi.org/10.17605/OSF.IO/7KR58.29
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
References
1. Bejrananda T, Pakpirom J: Successful Percutaneous Nephrolithotomy Using Thoracic Paravertebral Block as the Sole Surgical Anesthesia: Cases Report. Journal of Health Science and Medical Research. 2021. Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: uro-oncology, endourology, kidney transplantation
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Endourology and Uro oncology
Are the rationale for, and objectives of, the Systematic Review clearly stated?
No
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
References
1. Sankar K, Anand K, Ramani S, Gayathri B: A Randomized Control Trial to Compare Hemodynamic Parameters of Patients Undergoing Percutaneous Nephrolithotomy Under Combined Spinal-Epidural and General Anesthesia in a Tertiary Hospital.Local Reg Anesth. 2023; 16: 41-49 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Endourology and Uro oncology
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
No
Is the statistical analysis and its interpretation appropriate?
Not applicable
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 01 Nov 23 |
read | read | |
|
Version 1 14 Mar 23 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)