Keywords
Citrus, foliar sprays, foliar uptake, laser light
This article is included in the Agriculture, Food and Nutrition gateway.
Citrus, foliar sprays, foliar uptake, laser light
One of the most prevalent methods used in modern-day agriculture to improve crop health, and hence yield, is the foliar application of agrochemicals.1 However, several barriers cause retardation and interfere with the efficient penetration and utilization of these substances. For example, the leaf surface is coated by a waxy cuticle that serves as a barrier for the prevention of water loss and pathogenic entry into the plant body. Due to its water impermeable nature, the cuticle also prevents the entry of externally applied soluble compounds such as most agrochemicals. The movement of substances into the leaf occurs primarily through the stomata,2 located mainly on the less-exposed abaxial side of citrus leaves.3 This property reduces the functional surface area leading to reduced agrochemical penetration through the foliar route. As consequence, considerable quantities of applied chemicals end up in the plant’s natural environment and can generate undesirable ecological impacts.
An example of research focused on foliar application of substances into plant leaves is the study of agrochemicals to treat citrus Huanglongbing (HLB),4,5 a bacterial disease of citrus that has spiraled the Florida citrus industry to the brink of disappearance and actively spreading across other regions of the world on a large scale.6 In the case of HLB infections, therapies considered against the causative bacterium Candidatus liberibacter (CLas)7 include treatment with antimicrobial agents as a promising alternative to enhance the crop’s lifespan.5 However, response to such treatments is diminished due to challenges associated with the application process and the ensuing slow penetration into the phloem, the conductive tissue where bacterial populations aggregate within infected citrus plants.8
A novel alternative was recently identified to enhance penetration of agrochemicals into the leaf. This system involves the generation of leaf perforations of approximately 250 μm in diameter using a CO2 laser directly focused upon the leaf surface. The perforations not only puncture the cuticle but also perforate the epidermis and few layers of underlying palisade parenchyma.7 When applied on treated leaves, penetration of sample substances was increased over 2,000% over untreated leaves.9
The use of laser micro-perforations as a plant pharmacodynamics-enhancing technique has several drawbacks. First, apart from the cuticle, laser-induced pores also affect the underlying leaf epidermis and palisade parenchyma due to the inevitable removal of material from both areas.9,10 Second, this methodology requires highly focalized lasers to achieve maximum efficiency within field conditions, which is technologically complex due to the intrinsic irregularity of leaf surfaces, their random orientation, and depths within a tree.
The goal of this investigation was to devise a lesser invasive mechanism to enhance substance uptake into the leaves using specific laser beams. Our study describes a novel methodology for enhancing the penetration of agrochemicals into citrus leaves without the drastic requirement for physically perforating leaf tissues. This novel technique is based upon epidermal water content-dependent selective absorption of Erbium laser light. Partial separation of the waxy cuticle is successfully obtained across an area of several square centimeters through the application of a single laser beam. Since there is no damage afflicted to the leaf epidermal tissue, the cuticle rapidly regenerates within a brief period, thereby recovering its protective functions.
‘Valencia’ orange leaves from greenhouse-reared plants of approximately one meter were selected. ‘Valencia’ orange is one of the main species affected by Huanglongbing (HLB) disease, and its leaves are covered by a thick cuticle layer that can impede the absorption of foliarly applied treatments, making it an ideal model organism. Laser treatments were applied to attached leaves and these remained on the tree for post-laser application in order to provide the live conditions. Treated leaves were consequently detached and transported to the laboratory for further analysis.
It is well-established that leaves have a high-water content including the epidermis. Since water has a strong absorption band in the 3000 nm wavelength region, an in-house Er:YAG laser (fundamental wavelength = 2940 nm; 200 μs pulse duration) was used for foliage irradiation from a distance of approximately 30 cm. Each laser treatment consisted of a single shot performed without specific focusing of the laser on the leaf surface. In order to discern the varying effect of laser intensity on the leaf wax cuticle three laser energy levels were employed, with a spot area of 0.78 cm2. This spot area was determined by the diameter of the laser crystal used in the experiment (1cm), as no focusing element was employed. This allowed for a direct measurement of the effect of laser intensity on the leaf wax cuticle, without the added variable of a focusing element. No lens was used to focalize the laser and the beam divergence was 0.1 mrad, consequently disregarding the necessity for standardizing irradiation distance in such assays. In all cases, one single pulse sufficed to obtain the effect of wax cuticle exfoliation. The laser energies tested for the removal of wax across the investigated leaf foliage ranged from 0.3-0.76 J/cm2, depending upon the laser energy level.
To visualize the penetration of applied substances into the leaf, a fluorescent analog of glucose solution (NBDG: 2-NBDG = 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxyglucose. λ Ex/Em (nm) 465/540) was applied to the leaf surface. The NBDG solution was prepared and used at a concentration of 30 mM.9
Microscopic observations were performed using a Carl Zeiss™ Axio Scope A-1® microscopy platform equipped with a Canon™ EOS Rebel T3i® camera and a Carl Zeiss™ AxioCam ICc 1®. Low magnification images were obtained using a Zeiss™ Stemi SV11® fluorescent stereoscope (Carl Zeiss Microscopy™+ GmbH, Göttingen, Germany).
For High-magnification observations of the plant samples with fluorescence, a Carl Zeiss AxioScope A1 fluorescent microscope (Carl Zeiss Microscopy GmbH, Göttingen, Germany) equipped with a Zeiss Axio Cam ICc1, with filter Set 43 or Rhodamine filter from Zeiss (Ex: BP 545/25, Em: BP 525/50) for red and green fluorescence, was used.
Low magnification images were observed under a Wild Heerbrugg stereoscope (Wild Heerbrugg Instruments, Ltd., Heerbrugg, Switzerland) using a Green –Only bandpass filter, Stereo Microscope Fluorescence Adapter SFA-LFS-GO (NIGHTSEA, Electron Microscopy Sciences, Hatfield, PA. 19440). Images were captured with a Canon PowerShot S3 IS (Martin Microscope Co., Easley, SC). The procedures and techniques described in this manuscript have been thoroughly described to allow for replication of the results. All relevant materials, steps, and parameters have been included to ensure full reproducibility of the experiment.
The effect of increasing laser energy levels applied to the leaf surface was studied by treating 20 leaves from the same tree with three different energy levels. The results of these pulses on three representative leaves are presented in Figure 1. The yellow areas in Figure 1 represent the modified leaf cuticle according to the level of energy applied. Exfoliation of the leaf cuticle increased proportionally with laser intensity level. For the highest energy density value at 0.76 J/cm2, the exfoliation effect was achieved across (85-90%) the entire laser-spot area (Figure 1c).
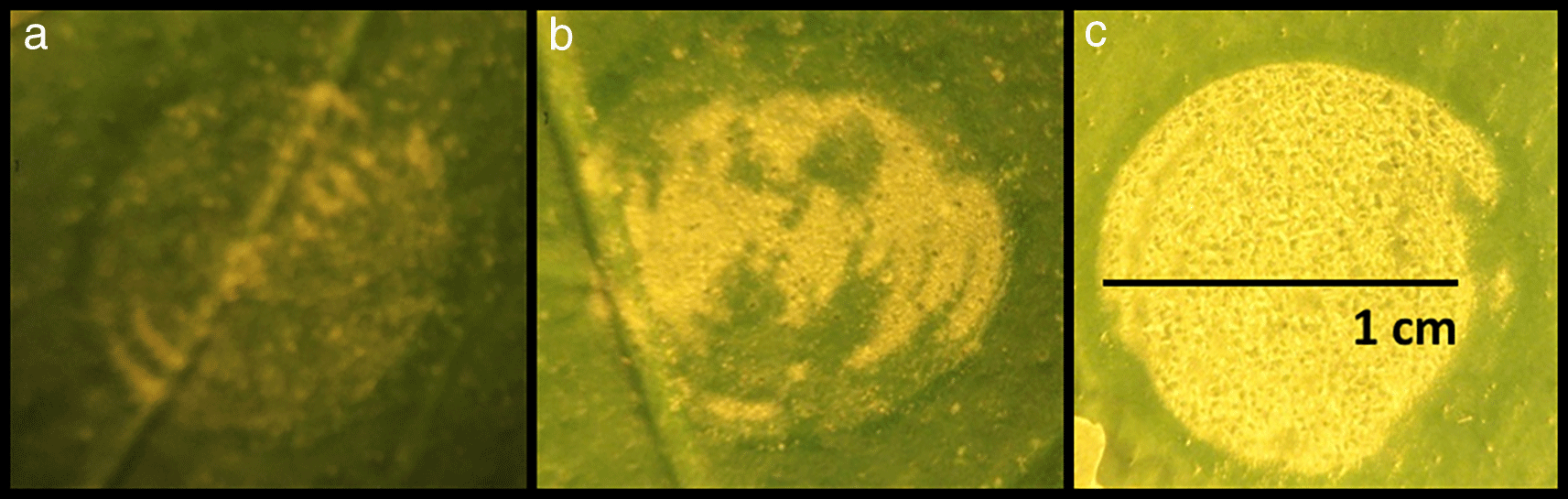
Figure depicts three different levels of laser energy-intensity. a) 0.3 J/cm2, b) 0.5 J/cm2, c) 0.76 J/cm2.
A close-up of a treated portion of a leaf using 0.76 J/cm2 energy density is shown in Figure 2. The waxy material, which appears relatively smooth in untreated leaves (Figure 2a), aggregated and segregated after treatment, forming clean breaches into the epidermis (Figure 2b). Under these conditions, only the cuticle was affected as the green underlying epidermal layer of cells remained undisturbed by the energy applied. The image in Figure 2c (black-and-white) was generated using the color tool from Power Point® (Microsoft™, USA) to facilitate the estimation of percent exposed areas. Regarding such estimations, the online tool ‘Coolphptools’ was employed,11 to calculate the percentage area per color. The black-color areas, which corresponded to wax cuticle exfoliated regions, was estimated to cover as 0.61, indicating a 61% success rate in cuticle exfoliation of the total laser-irradiated area. Table 1 shows the average percentage of exfoliated area for each energy level.
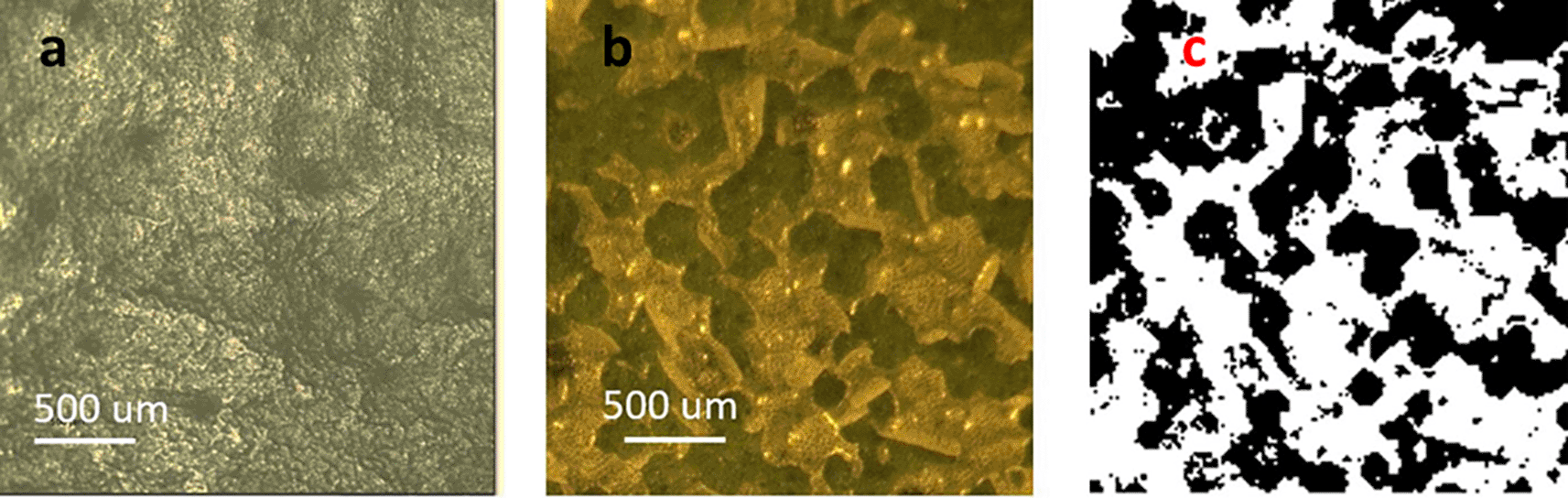
It can be appreciated that the exfoliated are is correlated to the energy density of the pulse.
| Energy density (J/cm2) | % Exfoliated area | |
|---|---|---|
| Mean | Std Dev | |
| 0.3 | 18.3 | 7 |
| 0.5 | 37.3 | 6.1 |
| 0.76 | 61 | 4.5 |
The infrared wavelength inherent to the erbium laser, which is identical to the absorption peak of water, allows for considerable laser-energy absorption by such water content. This phenomenon provokes the rapid heating and exerting of pressure that ‘pushes’ the wax cuticle outwards. Since such a laser-energy/wavelength is not absorbed within the epidermal tissue or any other plant constituent, consequently, there is no irreversible physical damage imposed upon the leaf structural integrity, apart from cuticle exfoliation (temporary separation). The mechanism of selective ablation of plant parts/elements was previously utilized for the removal of cactus spines, where it is possible to pulverize and extract such spines through rapid heating of water content present within glochids.12
Figure 3 presents a fluorescent view of a laser treated leaf using a green filter. In Figure 3a, the areas where the cuticular wax was “lifted” or exfoliated appear green in color. The fluorescent green color represents autofluorescence of undamaged epidermal cell walls. These open irregular-shaped areas range from several tens to hundreds of micrometers. The beige areas correspond to the exfoliated wax clumps. A cross section of a treated area in presented in Figure 3b. The figure distinctly shows the “lifting” of the epidermal wax on the upper side of the leaf. The bright spots represent autofluorescence of the vascular tissue.
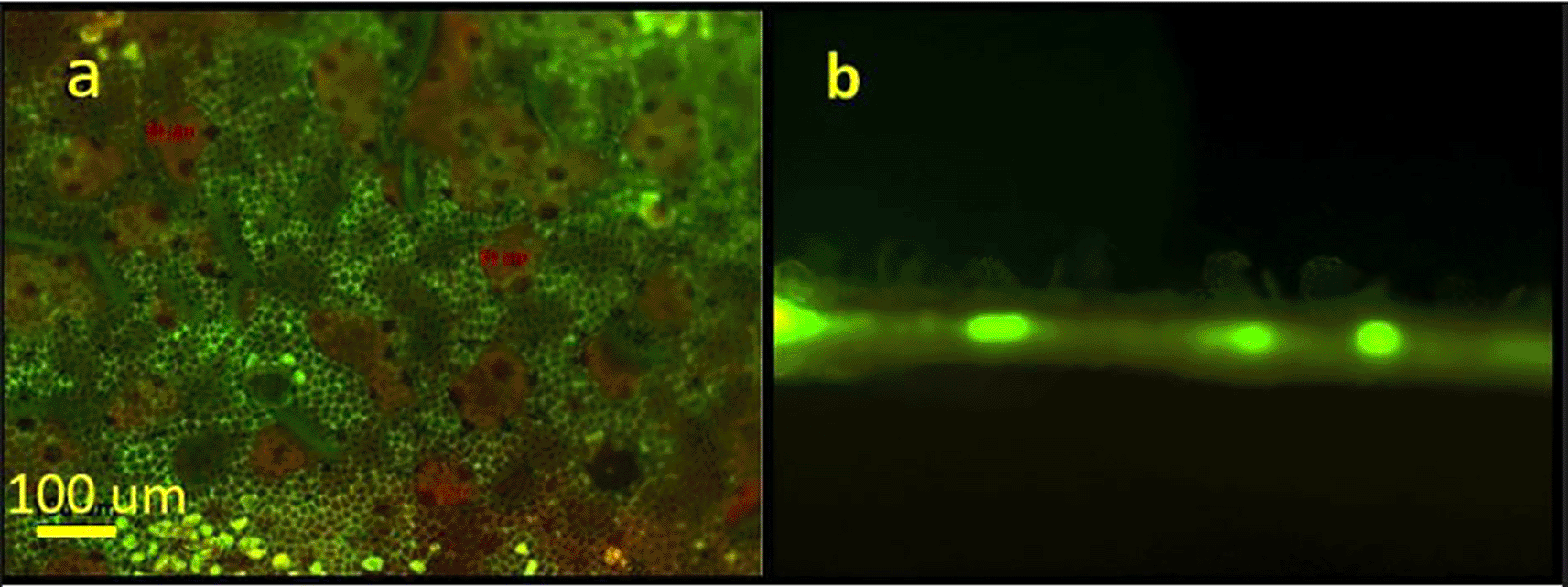
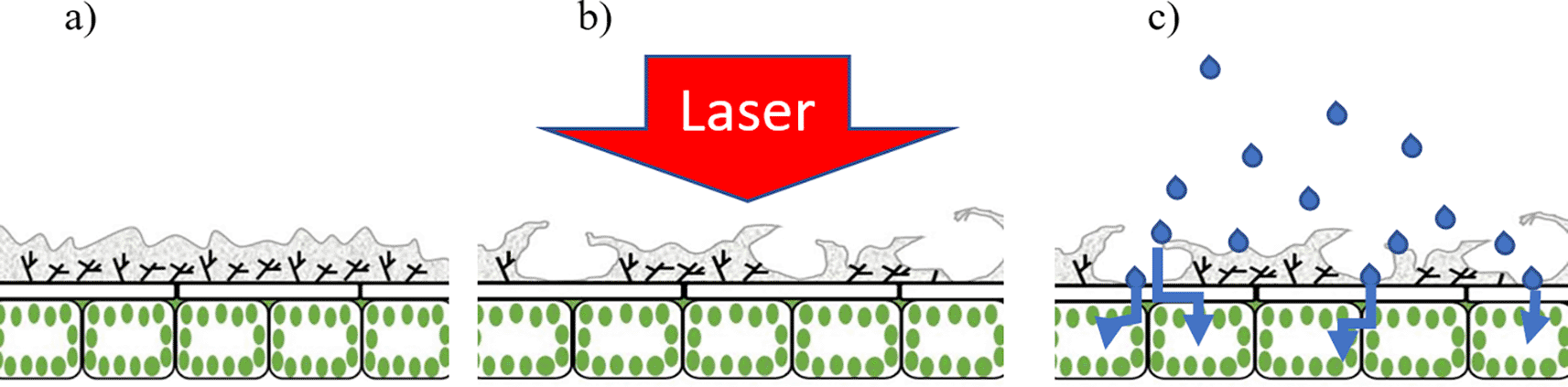
Figure 4 is a diagrammatic representation of a laser treated leaf highlighting the leaf epidermis prior to (Figure 4a) and following (Figure 4b) application of a laser pulse. In Figure 4, the grey areas represent the cuticle whereas the underlying cells include the epidermal cells and the palisade cells. Post-laser treatment, the cuticle appears ‘lifted’ (or partially detached) as a result of the pressure exerted by the laser-excited water (Figure 4b). Through such areas having detached and raised cuticles, therapeutic compounds can easily penetrate into the leaf epidermal layer and then follow its pathway to the plant transport system.
To demonstrate the effectiveness of the laser treatments in allowing externally applied hydrophobic substances to penetrate the leaf and travel throughout the plant vasculature, we applied a fluorescent NBDG to laser treated areas (Figure 5). The images are viewed under green/red control untreated leaf is presented in Figure 5a. Conversely, Figure 5b depicts a treated leaf two hours after laser treatment and NBDG application. The externally applied fluorescent NBDG is visible throughout the leaf, especially in the veins containing the plant vascular tissue, clearly indicating successful solution penetration and distribution across the majority of leaf surface area.
The application of agrochemicals through the foliar route remains a “gold-standard” therapeutic administration route for enhancing crop productivity, treatment of diseases, and pathogen/parasite circumvention and prophylaxis. However, despite its wide application, penetration through leaves remains quite inefficient, causing dramatical environmental impact as > 90% of applied agrochemical doses by the foliar route are not absorbed by the plant and eventually lead to a detrimental impact on the immediate plant environment.1 The use of laser technology for foliar application of agrochemicals contributes with a plethora of advantages. Aside from the improving foliage uptake of most therapeutic compounds/agrochemicals, it results in the reduction in agrochemical losses/wastage and eventual detrimental impact on the treated plant’s immediate environment. The high efficacy provided by the cuticle exfoliation method presented in this communication eliminates perforation of live tissue by limiting its effect to the cuticle only, therefore, providing a less intrusive method for substance penetration. Future work requires the development of prototypes for in-field application conditions. This will include testing at longer distances based on the fact that the Er:YAG laser does not require a lens for this application.
Open Science Framework: Use of non-intrusive laser exfoliation to improve substance uptake into citrus leaves. DOI: 10.17605/OSF.IO/6M43P. 13
This project contains the following underlying data:
• Statistics exfoliated area (Percentage exfoliated area and pulse energy density for all samples in this study.)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Laser, optics, photothermal spectroscopy, digital holography microscopy
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 10 May 23 |
read | read |
|
Version 2 (revision) 14 Apr 23 |
read | read |
|
Version 1 20 Mar 23 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)