Keywords
catalase, extinction risk, radical scavenging, smallholder and farm, sperm motility
This article is included in the Nanoscience & Nanotechnology gateway.
This article is included in the Agriculture, Food and Nutrition gateway.
catalase, extinction risk, radical scavenging, smallholder and farm, sperm motility
Some others study were added about additions substances in extender to increase post-thawed goats semen quality in the Introduction and Discussion section. The relevant references were added. The narration in the Abstract, Introduction, Results, Discussion, and Conclusion has been revised according to the data and suggestion of reviewers.
See the authors' detailed response to the review by Maria Madalena Pessoa Guerra
See the authors' detailed response to the review by Muhammed Duman
Smallholder farmers raise Kacang goats (Capra hircus) to increase their financial income, reduce poverty, and prevent malnutrition. However, the pure breed of this indigenous livestock species in Indonesia is at risk of extinction due to cross-breeding and must be protected.1 Artificial insemination (AI) techniques involving freeze–thawed semen are expected to increase the population of these goats. However, goat sperm is sensitive to cold shock.2 Indeed, >60% sperm death was detected in post-thawed goat semen previously frozen in skim milk–egg yolk (SM–EY) extender without antioxidants,3 which does not meet the minimum requirements for AI, i.e., motility must be >40%.4 High sperm death and low motility occurs because the freezing and thawing process leads to an excess of reactive oxygen species (ROS) production, which damages the polyunsaturated fatty acids (PUFAs) in the plasma membrane of spermatozoa, increasing malondialdehyde (MDA) levels and ultimately reducing sperm living cells and motility.5 Therefore, antioxidants are needed to counteract the effects of ROS and thereby increase post-thawed semen quality.6 In our previous studies, green tea extract was used to improve semen quality and decrease nucleotide mutations in mtDNA3 and protein-encoded mtDNA,7 presumably due to an increase in the antioxidant capacity.
The freeze–thawing process in goat semen causes lipid peroxidation in the spermatozoa membrane, which reduces semen quality.8 Semen contains endogenous antioxidants that help maintain the oxidant–antioxidant balance.9 However, the excessive production of ROS due to the freeze–thawing process cannot be overcome by endogenous antioxidants owing to the limitations of the spermatozoa cytoplasm and decreased antioxidant levels due to the addition of extenders.10 Several studies to improve the quality of post-thawing goats semen have been carried out, including adding extenders with egg yolk omega-3,11 fish semen plasma,12,13 curcumin,14 butylated hydroxytoluene,15 combination of myo-inositol and melatonin,16 and L-carnitine.17 Epigallocatechin-3-gallate (EGCG) is a powerful antioxidant extracted from green tea.18 The nanoparticle extract has a large surface area to volume ratio, so it is expected to exhibit increased penetration into cells,19 including sperm cells, and improve the quality of post-thawed semen. The addition of EGCG chitosan nanoparticles (CNPs) to the extender when freezing Kacang goat semen has not yet been studied. Thus, in the present study, the addition of EGCG CNPs to SM–EY extender and their ability to increase antioxidant capacity was investigated by assessing catalase and 2,2-diphenyl-1-picrylhydrazyl (DPPH) levels. The aim of this study was to obtain the best possible quality of post-thawed Kacang buck semen for AI according to MDA levels, sperm membrane plasma integrity (MPI), living cells, and motility.
The study was conducted in February to August 2022, at The Artificial Insemination Center of Airlangga University, Tanjung village, Kedamean, Gresik District, East Java, Indonesia, at coordinates 7° 19′ 25′′ S and 112° 32′ 54′′ E.
This study is part of a multiyear research project. The protocol was approved by the Animal Care and Use Committee of Airlangga University (number 520/HRECC.FODM/VII/2019).
Dried green tea (Camellia sinensis. Kuntze) leaves was obtained from Perkebunan Nusantara XII Malang, East Jawa Indonesia. Briefly, EGCG was isolated from C. sinensis using a thin-layer chromatography method and verified by a comparison with epigallocatechin gallate hydrate (Tokyo Chemical Co., Ltd., Japan).20 Subsequently, 5 mL of 0.1% chitosan solution [containing low molecular weight chitosan (Sigma-Aldrich) in 1% acetic acid] was added to 50 mL of EGCG solution [0.05% EGCG (Sigma-Aldrich) in distilled water], and the mixture was stirred at room temperature. Next, 0.5 mL of triphosphate (TPP) solution [0.025% TPP (Merck) in distilled water] was added drop-wise with stirring for 3 h at 112 × g. This solution was centrifuged at 21,952 × g for 10 min using a MC-10K centrifuge (Bio-Gener, Hangzhou, China) and washed three times with deionized water to obtain the EGCG CNPs, which were freeze dried for 48 h and stored at 4°C.21 The particle size of EGCG CNPs was measured using a Zetasizer Nano ZS (ZEN 3600, Malvern Instruments Ltd., Worcestershire, UK). A helium–neon ion laser at a wavelength of 633 nm was used as the incident beam at 25°C with a 90° angle.22
Samples were collected from three of Kacang bucks aged two–three years and weighing 35–40 kg. These bucks were (owned by The Artificial Insemination Center, Airlangga University) fed approximately four kg of forage and 3.5 kg of concentrate (16%–18% crude protein) daily and provided with drinking water ad libitum. Semen was collected from the bucks using an artificial vagina twice per week to obtain six ejaculate samples to process as frozen semen.
Skim milk powder (15 g; 115338; Merck) was dissolved in distilled water to a volume of 150 mL, heated for 10 min to 92°C–95°C, and then cooled to room temperature (25°C). Egg yolk (5 mL; derived from laboratory chicken eggs) was added to 95 mL of skim milk solution, then added with 1 IU/mL of penicillin (Meiji Seika Pharma, Tokyo, Japan) and 1 μg/mL of streptomycin (Thermo Fisher Scientific, Singapore).7 The solution was divided into five equal volumes without addition of EGCG CNPs for the control group (T0) and with addition of 0.5, 1.0, 1.5, and 2.0 μg of EGCG CNPs/mL extender for T1, T2, T3 and T4 groups, respectively.
Each SM–EY extender group was divided into two equal volumes. The first volume was added to fresh semen to obtain 480 million spermatozoa/mL. The second volume was added with glycerol up to 16% concentration, which was in turn added to the first mixture to obtain 240 million spermatozoa/mL. The extended semen was cooled from room temperature (25°C) to 5°C for 1 hour, then filled in 0.25 ml French straws (I.M.V., France) and sealed. The filled and sealed straws were chilled in liquid nitrogen vapor from 5°C to − 140°C for 10 min, and immediately stored in liquid nitrogen (−196°C) for 24 hours before evaluation were conducted.7
The straws allocated to each group were thawed in sterile water for 30 s at 37°C. Six replicates randomly were used to assess sperm MPI, living cells, progressive motility, MDA levels, catalase levels, and DPPH scavenging, respectively, according to methods reported in a previous study.7
MPI
A semen sample (0.1 mL) was added to 1 mL of a hypoosmotic solution [containing 7.35 g of sodium citrate (Sigma-Aldrich) and 13.52 g of fructose (Sigma-Aldrich) dissolved in distilled water to a volume of 1 L)] and incubated at 37°C for 30 min. The sperm MPI was assessed for 100 sperm under a light microscope (Olympus BX-53, Tokyo, Japan) at 400× magnification. Sperm with an MPI showed a curved tail, whereas those with a damaged plasma membrane showed a straight tail.7
Living cells
A drop of semen sample and a drop of nigrosine (Sigma-Aldrich) were mixed and smeared on a glass slide, after which the slide was dried over a flame. The slide was then examined under a light microscope (Olympus BX-53, Tokyo, Japan) at 400× magnification to evaluate the percentage of live sperm in 100 spermatozoa. Live sperm were identified by their brightly transparent heads, whereas dead sperm were colored red.7
Motility
An homogenate of a semen sample (10 μL) and a 0.9% (w/v) NaCl solution (1 mL) was dropped onto a glass slide and covered. The number of progressively motile sperm was counted for 100 sperm at 400× magnification under a light microscope (Olympus BX-53, Tokyo, Japan) equipped with Linkam Warming Stages set at 37°C–38°C (Meyer Instruments, Texas, USA).7
MDA levels
MDA levels in semen samples were determined using the thiobarbituric acid (Sigma-Aldrich) method. A semen sample (100 μL) and MDA kits (0, 1, 2, 3, 4, 5, 6, 7, and 8 μg/mL of malondialdehyde) were added to 550 μL of distilled water and 100 μL of 20% trichloroacetic acid. These mixtures were then homogenized for 30 s, and 250 μL of HCl (1 N) was then added and homogenized. Subsequently, 100 μL of 1% sodium thiobarbiturate was added and homogenized. This mixture was centrifuged at 28 × g for 10 min, and the supernatant was incubated in a 100°C water bath for 30 min before being left to room temperature (25°C). The color absorption was determined at a wavelength of 533 nm using a spectrophotometer (Thermo Fisher Scientific). MDA levels (ng/mL) were determined by extrapolating the sample absorbance values using a standard MDA curve.7
Catalase levels
A 30 mM H2O2 phosphate-buffered solution [1 mL; comprising 0.34 mL of 30% H2O2 diluted in fresh phosphate buffer (50 mM and pH 7)] was added to a semen sample (2 mL) at room temperature and assessed against a blank (not containing an enzyme) solution. The UV spectrophotometric absorbance method was used to measure catalase activity at a wavelength of 240 nm.23
DPPH radical scavenging
A 5 mL of DPPH radicals (10 mM) in methanol were added to a cuvette containing 970 mL of mixed methanol. This mixture was incubated at 20°C for 3 min, and the absorbance was measured at 517 nm (A517) using a UV-Vis Spectrophotometer (Thermo Fisher Scientific). Next, 25 mL of each sample and 25 mL of an acetonitrile solution (9.5 M; used as negative control) were added and mixed, and the mixture was incubated at 20°C for 3 min. Subsequently, the A517 decrease related to DPPH radical decomposition was measured. All experiments were performed in duplicate, and the mean DPPH scavenging effect was calculated according to the following formula: DPPH scavenging effect (%) = (1 − A517 sample/A517 negative control) × 100.24 IC50 values were calculated using a relationship curve of RSA versus concentrations of the respective sample curve.25
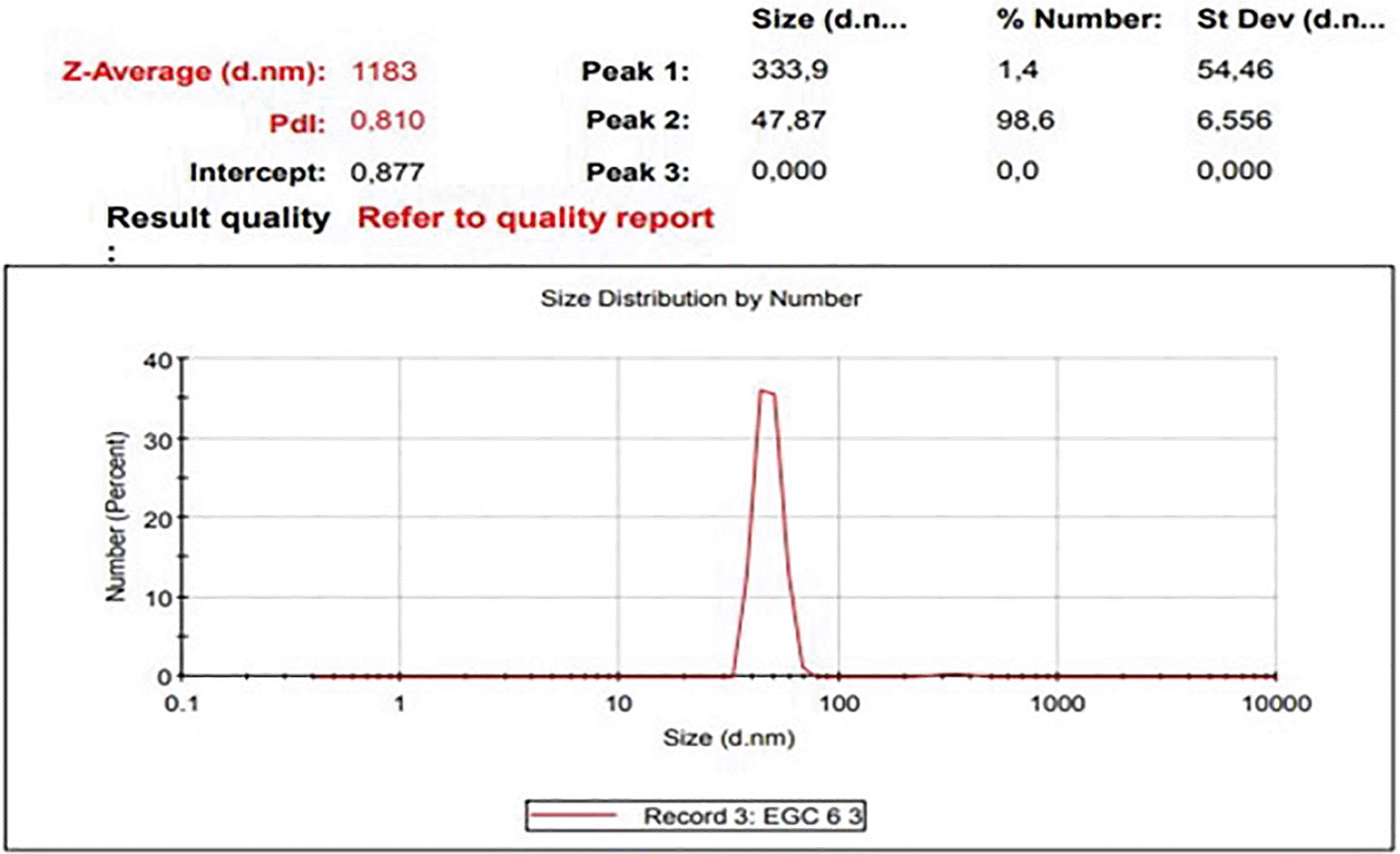
The diameters particles of EGCG CNPs was in range 41.31 – 388.36 nm with the averages as presented in Table 1 and size distribution curves of EGCG CNPs as seen in Figure 1. The progressive motility of sperm indicates the quality of fresh semen. Based on the criteria for the motility of individual spermatozoa of >70%, the obtained semen ejaculate of the Kacang goats met the requirements for freezing (Table 2).
| Range | Averages | Percentage |
|---|---|---|
| 10–100 | 47.87 ± 6.56 | 98.6% |
| 100–1000 | 333.9 ± 54.46 | 1.4% |
Post-thawed semen that was previously frozen without antioxidant EGCG CNPs in the extender (T0 group) exhibited the lowest levels of catalase and DPPH and the highest levels of MDA (p < 0.05). The addition of SM-EY extender with EGCG CNPs at doses of 1.5 or 2.0 μg/mL (the T3 or T4 group) resulted in higher catalase and DPPH, and doses of 1.0 or 1.5 or 2.0 μg/mL EGCG CNPs (the T1 or T3 or T4 group) resulted in lower of MDA (p < 0.05) than those of control group (T0) (Table 3).
Data were analyzed using ANOVA followed by the Tukey Honestly Significant Difference test at a significance level of p ≤ 0.05.
Semen that was frozen without antioxidant EGCG CNPs in the extender (T0 group) exhibited lowest sperm MPI, living cells, and motility both in the pre-freezing and post-thawed conditions (p < 0.05). The addition of SM-EY extender with EGCG CNPs 2.0 μg/mL (T4) increased the MPI, and doses of 1.5 and 2.0 μg/mL (T3 and T4) increased the living cell sperm and sperm motility (p < 0.05) than those of the control group (T0) (Table 4).
Data were analyzed using ANOVA followed by the Tukey Honestly Significant Difference test at a significance level of p ≤ 0.05.
Of the EGCG CNPs added to the SM-EY extender in the study, 98.6% were 10-100 nm in diameter, and the remaining percentage was 100-1000 nm. Nanotechnology techniques have been used to produce particles with a size scale of 0.1–1000 nm.26 The smaller particle size of nanoparticles than the microparticles, causes the NPs have larger surface areas to volume ratio and the opportunities for chemical reactions and biological activities also increase. Bioavailability is the ability of NPs to penetrate cells. The effect of NPs on the target site depends on their chemical composition, shape, surface structure, surface charge, catalytic properties, and aggregation ability with other materials.27 The NPs size causes the active compound to spread in the medium and reach the target with increased accuracy.28 One of the safest materials used in NP encapsulation technology is chitosan.29
EGCG possesses metal-chelating properties that provide antioxidant functions. The two structures that give EGCGs metal chelation properties are the ortho-3′,4′-dihydroxy moiety and the 4-keto, 3-hydroxyl, or 4-keto and 5-hydroxyl moiety. Catechins prevent the generation of potentially damaging free radicals via the chelation of metal ions. Through their ability to chelate transition metal ions, flavonoids can complex and inactivate iron ions, thereby suppressing the superoxide-driven Fenton reactions that are thought to be a crucial route to forming ROS. Electron transfer from catechins to ROS-induced radical sites on DNA and the formation of stable semiquinone free radicals are other mechanisms by which catechins exert their antioxidant effects,30 which are more pronounced than those of vitamins C and E.31
In general of this study, adding EGCG CNPs in SM-EY extenders resulted in higher catalase and DPPH and lower MDA than those of SM-EY extenders without EGCG CNPs. Previous study reported that adding 2.5 mM curcumin in a Tris-based extender did not decrease lipid peroxidation, and malondialdehyde formation on Angora buck semen compared to inositol and carnitine supplementation.14 Another study reported that lipid peroxidation can be lowered with supplementation of combined myo-inositol and melatonin,16 or 5 mM L-carnitine in the plant-based extenders.17 Semen has antioxidant enzymes, namely catalase, superoxide dismutase (SOD), and glutathione peroxidase (GPX), which maintain the oxidant–antioxidant balance32 and play fundamental roles in protecting biological systems against free radical attacks. The scavenging activity of SOD is accomplished via catalase, which reduces hydrogen peroxide to water and molecular oxygen.33 Indeed, catalase activates the decomposition of hydrogen peroxide into water and oxygen, thereby blocking the ROS-generating pathway and reducing oxidative stress.34 The addition of catalase to a commercially medium increased the total motility, membrane integrity, and living cells of postliquid goat semen35 and ram semen.36 Optimal catalase levels in the extender also reduce detrimental effects on post-thawed motility, living cells, plasma membrane, and acrosome integrity.37 In humans, catalase is used as a molecular target for diagnosing and monitoring male infertility38 and in strategies for optimizing sperm parameters.39 This study’s result is consistent with that of Papas et al.,40 who demonstrated that the specific activities of catalase, GPX, and glutathione reductase during stallion semen cryopreservation were similar between effective and ineffective freezing of ejaculates. However, SOD activity was found to be higher in ejaculate following effective freezing than in ejaculate subjected to poor freezing power.41 In stallions, the total and specific activity of catalase in seminal plasma is high; however, no correlation was observed between total catalase activity in stallion seminal plasma and sperm kinematic parameters.40
A higher value of DPPH in the T3 and T4 groups than those of the T0 group represents more effective free radical scavenging.42 A DPPH measurement has an acceptable reproducibility for determination of radical scavenging activity in several samples.43 The DPPH assay, a popular method for evaluating the kinetics and stoichiometry of antioxidative reactions, is used widely because it is easy to use, rapid, and sensitive. The assay is based on the reduction of the purple chromogen DPPH· via a hydrogen atom or electron transfer from the scavenging molecule, i.e., an antioxidant, which causes the formation of pale yellow hydrazine (i.e., DPPH).44
The highest levels of MDA in the control group indicate highest lipid peroxidation. Oxidative stress may result in an imbalance between ROS generation and endogenous antioxidant activities. Higher ROS levels cause cell damage through the peroxidation of PUFAs in the sperm plasma membrane. Lipid peroxidation generates toxic lipid aldehyde species, including MDA,45 and higher MDA levels indicate free radical attacks46 and plasma membrane damage.47 The lower MDA levels in post-thawed semen of the T2, T3, and T4 groups than those of the T0 group indicated a decrease in oxidative stress. The lower oxidative stress is followed by increased membrane fluidity and a lower percentage of acrosomal damage due to membrane lipids and protein rearrangement.34
Post-thawed semen quality in goats is a very critical subject. Several previous studies have reported the following results. Post-thawed of goat semen frozen in a citrate extender with 10% egg yolk omega-3 showed higher live sperm and sperm motility.11 Supplementation of an egg yolk extender with 1% rainbow trout plasma semen resulted in a higher of post-thawed sperm living cells of ram semen.12 Post-thawed Saanen goats frozen semen in a soy lecithin-based extender with 8% rainbow trout seminal plasma showed higher sperm motility, acrosome integrity, plasma membrane integrity, and mitochondrial function.13 Adding 2.5 mM curcumin in a Tris-based extender resulted in higher post-thawed sperm motility of Angora buck semen compared to inositol and carnitine supplementation.14 Beetal buck semen cryopreserved in tris egg yolk with butylated hydroxytoluene showed increased acrosome integrity but was not significantly different on sperm living cells, even decreased sperm motility.15 The combination of myo-inositol and melatonin improved post-thawed sperm living cells, sperm motility, and plasma membrane integrity.16 Supplementation of 5 mM L-carnitine in the plant-based extenders improves sperm living cells, sperm motility, and membrane integrity.17
In the recent study, adding of EGCG CNPs in the SM-EY extender increased the MPI, the living cell sperm, and sperm motility compared to those of the SM-EY extender without EGCG CNPs. Goat semen is sensitive to cryopreservation. Freezing semen leads to excessive ROS production48 followed by lipid peroxidation of the membrane, resulting in MDA production,49 which in turn markedly reduces sperm MPI, living cells, motility, and DNA integrity.50
Intactness of plasma membrane is essential for protecting the organelles of sperm and molecular transportation; thus, it is crucial for sperm living cells and sperm motility.51 Post-thawed semen previously frozen without antioxidant EGCG CNPs in the extender showed the lowest MPI, sperm living cells, and sperm motility levels, whereas adding EGCG CNPs to the extender increased each of these levels. Damage to the plasma membrane of the spermatozoa reduces the quality of post-thawed semen because the integrity of this membrane is essential for the survival and motility of sperm.52 Excessive ROS production in semen due to the freezing–thawing process causes lipid peroxidation in the sperm membrane,8 disrupting the structure and function of this membrane and leading to the death of the spermatozoa.50 Lipid peroxidation also damages axonemal and mitochondrial proteins, resulting in the loss of sperm motility, even though the spermatozoa remain alive.53 Membrane damage due to lipid peroxidation also results in higher MDA levels.54 Furthermore, ROS cause the opening of bonds between disulfide chains in proteins, thereby destabilizing the DNA structure and leading to DNA fragmentation.55 The ejaculate defense system, including antioxidant enzymes such as GPX, catalase, and SOD, deals with ROS.56 However, the smaller volume of the sperm cytoplasm than those of commonly cells is the challenging for the antioxidant.57 The addition of semen extender might also lead to decreases of endogenous antioxidants. Insufficient antioxidant levels to combat oxidative stress during cryopreservation can have multiple negative effects, including decreased sperm living cells, sperm motility, and plasma sperm integrity. Thus, the addition of antioxidants to the extender prior to semen freezing is necessary.
The EGCG is an antioxidant that can reduce lipid peroxidation, protein carbonylation, and sperm DNA damage.58 The NPs form of EGCG increases the ratio of surface area of membrane to volume of particles, which allows EGCG to penetrate sperm cells efficiently.59 The presence of antioxidants reduces lipid peroxidation in the plasma membrane of spermatozoa, which in turn increases sperm living cells, motility, and acrosome integrity.60 Adding EGCG CNPs at 1.5 or 2.0 μg/mL of SM–EY extender improved semen quality compared to the control group. These results are consistent with those of previous studies in which adding ethanol green tea extract in extender maintained the motility, living cells, MPI, and DNA integrity of Simmental bull sperm.61
In this study, EGCG CNPs were used for the first time in SM–EY extender to improve the antioxidant capacity and quality of post-thawed semen. Indeed, adding 1.5 μg/mL or 2.0 μg/mL EGCG CNPs to the SM–EY extender increased the antioxidant capacity and quality of Kacang buck post-thawed semen. Future studies are required to validate this finding for AI under farm conditions.
Imam Mustofa (IM), Suherni Susilowat I (SS), Tri Wahyu Suparyogi (TWS), Adeyinka Oye Akintunde (AOA), Yudit Oktanella (YO), Djoko Agus Purwanto (DAP)
IM, SS and TWS conceived the idea, designed the mainframe of this study, and conceived in detail the manuscript. IM, TWS collected, prepared extender and freezing semen process. DAP: extracted EGCG from green tea leaves. YO: processing EGCG nanoparticles and measuring the particle size of extenders. SS, YO: evaluated semen quality. IM: interpreted the data and statistical analysi. AOA, YO, DAP: read critically and revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Biostudies. Epigallocatechin-3-gallate chitosan nanoparticles in an extender improve the antioxidant capacity and post-thawed quality of Kacang Goat semen. DOI: https://www.ebi.ac.uk/biostudies/studies/S-BSST924 62
This project contains the following data:
- This study aimed to determine the addition of epigallocatechin-3-gallate chitosan nanoparticles (EGCG CNPs) to skim milk–egg yolk (SM–EY) extender to obtain the best possible quality of post-thawed Kacang buck semen for AI
ARRIVE checklist. Figshare. DOI: https://doi.org/10.6084/m9.figshare.21531213.v1 63
The authors thank Dikky Eka Mandala Putranto, DMV, M.Sc., the Chairman of the Insemination Center, Airlangga University, Subchan Aziz, and Agus Purwanto for technical support.
Approval of support_EGCG CNPs, DOI: 10.6084/m9.figshare.21532326. https://figshare.com/articles/poster/Approval_of_support_EGCG_CNPs/21532326.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Andrology, Buck semen cryopreservation
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Semen quality and additives
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: -
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Ustuner B, Alcay S, Toker MB, Nur Z, et al.: Effect of rainbow trout (Oncorhynchus mykiss) seminal plasma on the post-thaw quality of ram semen cryopreserved in a soybean lecithin-based or egg yolk-based extender.Anim Reprod Sci. 2016; 164: 97-104 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Semen quality and additives
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
|
Version 3 (revision) 05 Sep 25 |
read | read | |||
|
Version 2 (revision) 04 Apr 23 |
read | read | |||
|
Version 1 09 Jan 23 |
read | read | |||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)