Keywords
Breast Neoplasm, Survival, Inflammatory markers, prognostic factors
This article is included in the Oncology gateway.
Breast Neoplasm, Survival, Inflammatory markers, prognostic factors
Breast cancer represents 16% of all female cancers, causing 2.3 million cases and 685,000 deaths worldwide in 2020.1,2 In Latin America, this pathology is the most important cause of death by neoplasms among women, with nearly 92,000 deaths,3 while in Peru it represents the most common primary neoplasm among women.4 Therefore, it is fundamental to determine the factors associated to overall survival (OS), such as age, comorbidities, metastasis, and laboratory analytical determinations such as neutrophil-lymphocyte ratio (NLR).5,6
While research exists on an international level that associates an elevated NLR with lower OS in patients with breast cancer, these were developed in populations with early-stage diagnosis, as opposed to countries with middle and low incomes, where a high proportion are diagnosed in advanced stages. Furthermore, differences exist in breast cancer phenotype, prevalence of oncological infections,7 and the presence of social risk factors such as marked poverty and reduced access to healthcare services between Latin-American countries8 and others.9 This highlights the need for studying the OS associated with breast cancer and its prognostic indices in this context.
Since in developing countries, the rate of clinical oncologists per million inhabitants is below the recommended value,7 simple, accessible, true, and low-cost methods, such as NLR, are required to potentially improve OS in these patients. Some research in Latin America has studied the NLR as an adverse prognostic factor in patients with different types of cancer, with few related to breast cancer.10,11 However, these studies were conducted only in patients with triple-negative breast cancer, which, although more aggressive, is not the most frequent of those presented in the region; therefore, it is necessary to perform studies including other cancer subtypes such as Luminal A, Luminal B or HER 2. In this sense, the general objetive of this study is to determine the association between NLR and OS in patients with breast cancer in the gynecology department of Hospital Nacional Hipólito Unanue (HNHU), Lima, Peru between 2012 and 2014. The specific objectives were to evaluate the association between OS and stage, molecular subtype and age. We hope to find an association between the NLR and the OS, so that the higher the NLR, the lower the OS.
An observational, longitudinal, analytical survival study was carried out in female patients diagnosed with breast cancer, seen in HNHU, between 2012 and 2014. The research protocol was carried out and uploaded to protocols.io platform, explaining step by step how the study was carried out.12 The exposed group consisted of those patients with high NLR and their OS was compared with that of the group of patients with normal NLR. Both cohorts were women who were diagnosed at HNHU between 1st January 2012 and 31st December 2014. Follow-up began at the time of breast cancer diagnosis and exposure was measured on the blood cell count that was measured at the same time as the diagnosis. The follow-up period was up to eight years. The limitations were considered to be those patients who stopped attending their follow-ups without dying or without giving notice of death in the medical record. HNHU is the only III-I establishment in East Lima, Peru, which offers specialized and comprehensive care to cancer patients, and is characterized by having highly qualified staff, innovative health technology, and high-quality standards. The oncology department has a chemotherapy room equipped with nine service areas, one procedure room, and another of oncological combinations; these services are offered to the entire population, mainly to the patients most in need from Seguro Integral de Salud (SIS).13 Data was collected from medical records during the months of January and February 2020.
We did not have a sample since we worked with the entire population, with a total of 241 female patients. We found a statistical power of 98% for this number of participants to find an expected OS difference, as reported by a similar study,14 of 86.2% for the exposed group and 97.9% for the unexposed group. This gave us a low probability of making a type 2 error when testing the hypothesis of our main objective, which was to compare the OS of those exposed and not exposed participants. To be included in the study, patients had to be over 18 years of age, with complete and follow-up clinical information from the moment of diagnosis until the last consultation or death. Patients with immune-suppressing diseases or a HIV/AIDS infection were excluded. Follow-up was performed retrospectively from medical assessments recorded in the medical history.
The outcome variable of this study was the OS of the patients diagnosed with breast cancer, time was measured in months, from the time of diagnosis until death or date of last follow-up. A participant was considered dead if it was labeled as such in the medical record and corroborated with the death certificate. Additionally, the exposure variable was NLR, which was measured from the first blood count obtained at the time of diagnosis that appears in the medical record. This rate is defined as the division of absolute neutrophil count over absolute lymphocyte count. The rate was categorized as low if NLR<3 and high if NLR≥3, according to Enríquez et al.,10 since said cut-off point predicted OS and complete pathological response in this study, in a very similar context. These measurements were made in the same way in the exposed and unexposed group.
As confounders variables, we considered age, categorized in age groups for descriptive analysis and dichotomized (>55 years), for survival analysis. Likewise, we took into account the clinical stage of breast cancer from the imaging analysis registered in the medical record at the time of diagnosis. The classifications were stage I: small and invasive tumor, with a capacity to spread to lymph nodes; stage II: cancer spread to lymph nodes without evidence of tumor in breasts; stage III: cancer spread to 4-9 axillary or internal mammary lymph nodes, a tumor greater than 50 mm may be found; stage IV: presence of metastasis.15 We also considered the molecular subtype classified as Luminal A, Luminal B, HER 2, and triple-negative.16 These variables were considered in the multivariate model only if they were associated with the bivariate analysis outcome.
To reduce the selection bias, participants were selected from the same hospital, which contributes to the groups having fairly similar clinical and social characteristics. In addition, the clinical history was reviewed exhaustively to avoid information bias. Finally, an analysis adjusted for confounders was considered to avoid erroneous conclusions.
The data collection technique and follow-up were carried out from the documentation, through a review of medical records of each patient and filled out in a data collection sheet. This research instrument, designed specifically for this study (Extended data33), was filled out with the patient’s information, such as age, date of breast cancer diagnosis, molecular subtype, clinical stage, absolute neutrophil count, absolute lymphocyte count, NLR, date of last follow-up, and, if applicable, the date of death and cause of death. Once data was collected, the clinical records were entered into a data matrix in the Microsoft Excel (RRID: SCR_016137), which was used as a database. Later, this data was used to obtain calculations and graphs through the Stata (RRID: SCR_012763) 15.0 statistical program.
First, we found the general characteristics of the entire population using frequencies and percentages for the qualitative variables, and central tendency and dispersion measures for quantitative variables, prior evaluation of its normalcy using the Shapiro-Wilk test.
Later the Kaplan–Meier method was applied to generate survival curves, which were compared using the log-rank test. Likewise, Cox regression was used to find crude and adjusted Hazard Ratios, with its respective 95% confidence intervals. The multivariate model included, as confounding variables, those variables that had statistical significance in the bivariate model, since they could influence the outcome. A p-value<0.05 was considered statistically significant.
There was only one observed missing observation in the molecular subtype variable and no extra modifications were made before the analysis. This missing number was not relevant to the conclusions of the study as this variable was not included in the multivariate model. Subjects who did not complete all follow-up were also included in the analysis. No sensitivity analyses were performed.
This study was approved by the research ethical committee of Hospital Nacional Hipólito Unanue (file N° 39993; 12th December 2019. All the patients’ data remained in absolute confidentiality by encrypting their personal identification. Patients, or their relatives, signed a written informed consent at the time of admission to the hospital, in favor of performing medical procedures and using their data for teaching and research purposes.
Of the 324 initial medical records, we excluded 35 not found and 48 incomplete medical records, leaving a total of 241 medical records for analysis (Figure 1). A follow-up of patients was performed in a maximum period of 8 years, with a mean follow-up of 3.5±1.8 years total, 2.7±1.8 years in the exposed group, and 3.9±1.7 years in the non-exposed group. Seventy-five patients (31.1%) were diagnosed in 2012, 84 (34.9%) in 2013, and 82 (34%) in 2014, with 130 patients dying during the study (Table 1). The age range was 27 to 85 years old (mean: 56.1 years±11.6), the most frequent molecular subtype of breast cancer was HER2 (39.2%), while the most frequent clinical stage was II (42.7%). Furthermore, 144 (59.8%) patients had NLR≥3. The other general population characteristics are presented in Table 2.
When evaluating OS according to the year of follow-up, we observed that patients with NLR≥3 had a lower OS (Figure 2A), compared to those who had an NLR<3. This association was statistically significant (p<0.001). When evaluating OS by molecular subtype, no statistically significant differences are observed (p=0.528) (Figure 2B). While in the clinical stage (Figure 2C), OS decreases in stage IV (p<0.001). Furthermore, we observed that the age group >55 years (Figure 2D) presented a lower OS within the follow-up years (p=0.017), compared to patients up to 55 years of age.
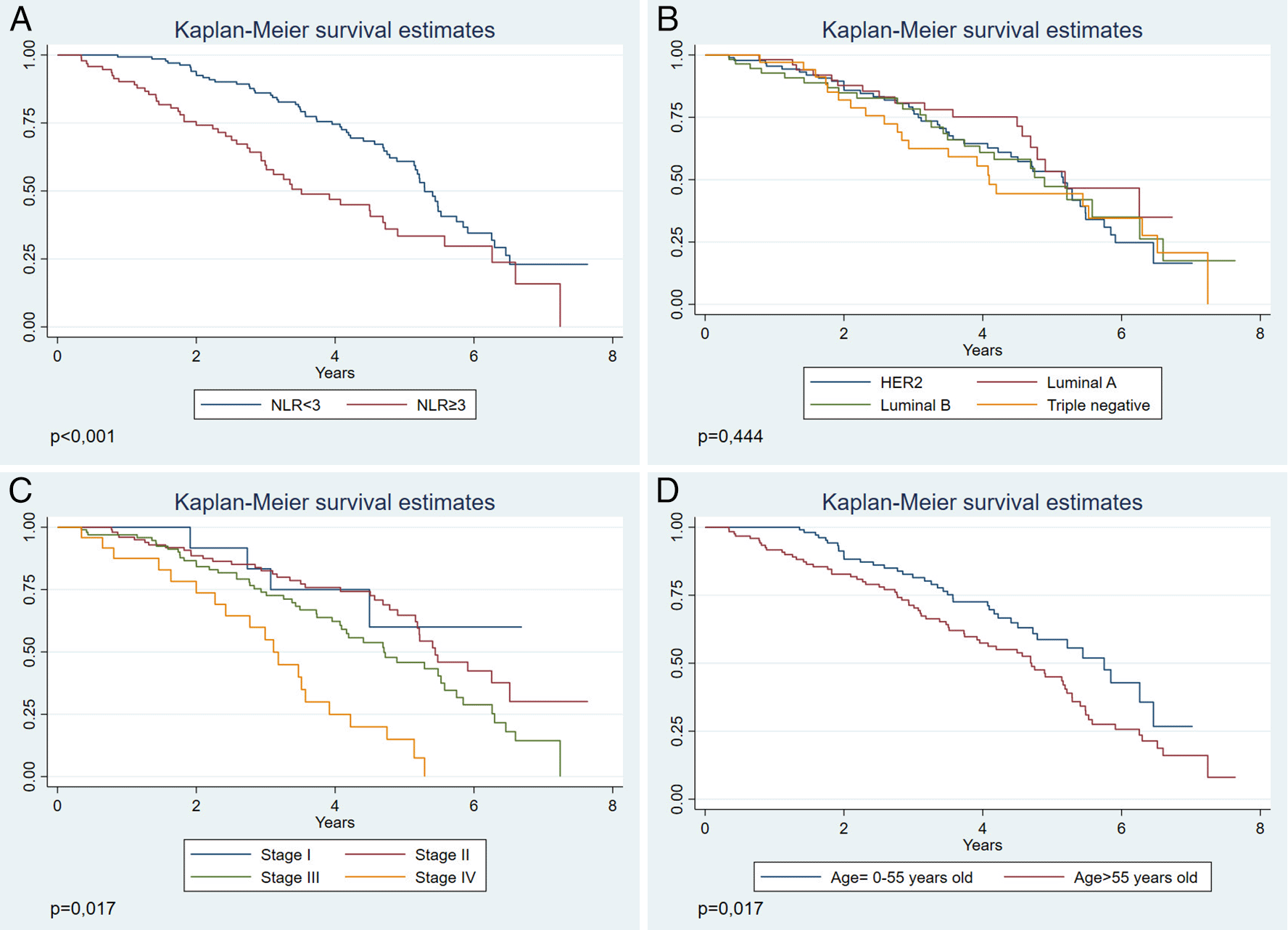
(A) According to NLR. (B) According to molecular subtype. (C) According to stage. (D) According to age.
In the multivariate analysis, the NLR≥3 (p<0.001) was a risk factor for mortality, adjusted for confounder variables of age and clinical stage. Additionally, age (p=0.016) and clinical stage IV (p=0.001) were also risk factors for mortality (Table 3).
In our study, we found that NLR≥3 (p<0.001), age>55 years (p=0.039), and clinical stage (p<0.001), were associated with a lower OS. In the multivariate analysis, the factors significantly associated to a lower OS were NLR≥3, age>55 years, and clinical stage IV. Although there have been international studies, we must consider that in all of them the population characteristics, such as risk factors, prevalence of infections, genetic susceptibility, and existing polymorphisms, are different from the Latin American context,9,17,18 which makes it difficult to generalize its results in this context. This study is especially important given that a broad follow-up of 8 years for the NLR evaluation is a prognostic factor of OS in patients in a Latin American hospital. In this regard, this type of cancer is considered a health problem of high priority,19 and the lack of resources requires cost-effective tools of easy access to orient the prognosis and need for treatment by a specialist.
Regarding OS, we found that an NLR>3 was related to a lower OS. Similar results were found by a Peruvian study, only in breast cancer with a triple-negative molecular subtype, while in our study we evidenced the use of this ratio in other molecular subtypes. Similar findings have been found in studies carried out in other regions of the world.20 This is probably because a greater NLR reflects a greater number of neutrophils, whose high infiltration has been associated with the aggressiveness of the disease and resistance to therapy, in addition to promoting pro-cancerous factors and suppressing the cytotoxic activity of lymphocytes.21–23 On the other hand, a decrease in lymphocyte count may decrease the effectiveness of the anti-tumoral immune response, which is why the combination of these conditions reflected in the NLR seems to be an excellent prognostic tool. Furthermore, it can be obtained through an accessible exam such as blood count, which is why its incorporation into the established prediction of risk justifies a larger study.24
We observed that patients with NLR≥3 had a lower OS, however, as of the fifth follow-up year the survival curves in both NLR categories tended to approach each other. This is similar to other inflammatory indices such as the combination of the NLR with the lymphocyte platelet index (NLR/LPR) studied by Hirahara et al.25 They also observed that survival was greater as this rate decreased (p<0.0001); however, from approximately the fifth follow-up year, we observed that survival functions were similar. This indicates that NLR or other hematological rates are likely not useful in predicting OS after a certain number of years. Likewise, in a Peruvian study,11 the survival functions with an NLR<2.5 and NLR≥2 were different during a 2-year follow-up in patients with triple negative breast cancer. It is probable that if it they had a longer follow-up, the behavior of the curves would have been similar to our study. Breast cancer mortality after 5 years could be high due to diverse factors foreign to what NLR might reflect, such as tumoral growth, comorbidities, and age-related functional impairment.
We found that over half of patients had an NLR≥3, similar to that found in other studies.26 On the other hand, the stages that were most frequently found during diagnosis were stages II and III, unlike the United States, where the most frequent stages were I and II.27 This could reflect a late diagnosis, typical of developing countries with deficits in their health system.7 Conversely, the average age at the time of diagnosis is comparable to that of other international reports.28 The most frequent molecular subtype was HER2 with a 39.2%, unlike the Asiatic indigenous population, where the most frequent was the luminal A type (33%). This correlates with greater risk and worse results in cancer among the indigenous communities.29
Furthermore, OS decreased noticeably in stage IV, probably since cancer diagnosis in advanced stages is more biologically aggressive and presents high recurrence rates.30 Likewise, we found that NLR was associated with the disease staging, which could imply a confusing role of that variable when evaluating the predictive capacity of the NLR. Additionally, we found that the greater the age, the lower the OS. This finding is related to the study by Tao et al.31 where older patients with breast cancer had 17% greater mortality than younger patients. This age group also had a lower OS, since breast cancer at an older age may have late diagnosis and insufficient treatments.32
By studying the entire population of a referral hospital that accepts patients from all over the country, these results could be generalized to the urban context of Peru and other cities in Latin America, since the sociodemographic characteristics and the environment where the study was made is similar to that of other Latin American cities. On the other hand, although there was a long follow-up of eight years, during this period there was no important change in the treatment that significantly affected the survival of the patients evaluated, so the results are still useful.
Among the study’s limitations, reporting bias likely exists due to the use of secondary sources such as medical records. Likewise, the patients’ treatment was not reported, a covariable that could influence OS. However, patients were treated in the same hospital, therefore it is probable that they received the same treatment regimen directed towards their type of cancer.
We conclude that NLR≥3 is a risk factor for mortality, adjusted by age and clinical stage. Additionally, age and clinical stage IV could also be risk factors for mortality. A total of 59.3% of breast cancer patients had an NLR≥3, while the most frequently diagnosed stage was stage II with 42.74%, and the most frequent molecular subtype was HER2 with 39.2%.
Figshare: Underlying data for ‘Neutrophil/lymphocyte ratio and overall survival in patients with breast cancer: a cohort study in a Latin-American hospital’, https://doi.org/10.6084/m9.figshare.20419401.v2. 33
The project contains the following underlying data:
Figshare: Underlying data for ‘Neutrophil/lymphocyte ratio and overall survival in patients with breast cancer: a cohort study in a Latin-American hospital’, https://doi.org/10.6084/m9.figshare.20419401.v2. 33
The project contains the following extended data:
Figshare: STROBE checklist for ‘Neutrophil/lymphocyte ratio and overall survival in patients with breast cancer: a cohort study in a Latin-American hospital’, https://doi.org/10.6084/m9.figshare.20419401.v2. 33
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Breast cancer, immunology, hypoxia, exercise
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 24 Mar 23 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)