Keywords
obesity, ketogenic diet, ketone bodies, protein–ligand biomarkers, in silico
This article is included in the Agriculture, Food and Nutrition gateway.
obesity, ketogenic diet, ketone bodies, protein–ligand biomarkers, in silico
Obesity has always been a pre-existing problem and has been recognized as a global pandemic in the 21st century (Ryan et al., 2021). It is described as the anomalous, irregular or disproportionate gain of weight as a result of fat buildup in the body, which adversely leads to the risk of several health-related problems such as hypertension, type 2 diabetes, cardiovascular diseases and certain cancer types, amongst others (Aronne, 2002; Natalia et al., 2021).
Obesity calculation is based on body mass index ≥30 kg/m2, which is the measurement of fat buildup calculated by dividing body mass (kg) by the square of the body height (m2). In 2016, the World Health Organization (WHO) acknowledged that nearly 2-billion adults worldwide were considered to be overweight and 650 million of that population were found to be obese. The WHO also estimated that 50% of the population found in Europe had a preeminent body weight which could lead to obesity over time (https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight).
Furthermore, in 2002, certain rural areas in Nigeria were recorded to have a high rate of obesity, at 33.7% (Ogah et al., 2013). Obesity involves a complex process, and certain physical and biochemical factors have been found to induce this condition some of which include unhealthy diets and eating habits, high calorie food consumption, and genetic, epigenetic, and ecological factors. All these factors combined together with the lack of physical activity to burn excess body weight then lead to energy disproportion and fat deposition (Williams et al., 2015; Lin et al., 2017). Due to obesity prevalence over time, several solutions and control measures have been brought up to ameliorate as well as control the condition. Some of these measures include physical activity programmes, behavioural lifestyle programmes, pharmacotherapy, diet management, and in severe obese conditions bariatric surgery is recommended (Gonzalez-Muniesa et al., 2017).
Caloric inhibition, a diet management scheme and a nutritional strategy are the commonly used weight loss mechanisms as far as diet management is concerned, and this research focuses on the use of a ketogenic diet, which has been used successfully in the therapeutic treatment of epilepsy, as a control measure for obesity (Anton et al., 2017; Ułamek-Koziol et al., 2019).
A ketogenic diet is made up of a high fat content, very minimal carbohydrate (not exceeding 4 percent) and a sufficient amount of protein. As a result of the proportionate amount of nutrients in the diet composition, there is reduced metabolism of carbohydrate and protein but increased metabolism of fat (Kayode et al., 2020a, 2020b, 2021). Consequent fat breakdown and reduced carbohydrate and protein breakdown then leads to reduced blood glucose levels and the stimulation of ketogenesis in the liver, which produces increased levels of ketone bodies and fatty acids (Kulak and Polotsky, 2013). The resulting ketone bodies are then transported to the blood–brain barrier to make available energy for the brain, and increased levels of the ketone bodies lead to increased levels of substrates such as creatine, adenine triphosphate, and phosphocreatine that are essential in the brain (Kayode et al., 2021). This study aims at exploring the efficiency of ketogenic-diet-generated ketone bodies in the prevention and amelioration of obesity using in silico simulations.
The data retrieval and computation of the entire work design utilised Discovery Studio (DS) version 21.1 (RRID:SCR_015651), Open Babel (RRID:SCR_014920), Python enhanced molecular graphics tool 1.3 (PyMOL 1.3) (RRID:SCR_000305), PyRx (RRID:SCR_018548), Chimera (RRID:SCR_002959), PubChem (RRID:SCR_004284), Protein Data Bank, SWISS-MODEL (RRID:SCR_013032), and Universal Protein resource (UniProt) (RRID:SCR_002380).
The X-ray crystallographic structures of the human target proteins were downloaded from the Research Collaboratory for Structural Bioinformatics Protein Data Bank (RCSB PDB) (www.pdb.org) (RRID:SCR_012820) and prepared for molecular docking simulation using DS v. 21.1., and Chimera software. The protein targets not readily available on RCSB PDB were developed via homology modelling using the SWISS-MODEL webserver and further prepared for docking analysis.
Docking analysis was performed according to Sharma et al.’s (2019) protocol. The active binding sites of the protein targets, listed in Table 1, were mapped out using the native ligand interaction and the models without native ligands were determined using the Computed Atlas of Surface Topography of proteins (CASTP) webserver. Molecular docking was performed by using AutoDock Vina (RRID:SCR_011958) software (Trott and Olson, 1995) in PyRx platform (GUI version 0.8).
| SN | Proteins/Ligands | Orlistat (SD) 3034010 | Cetilistat (SD) 9952916 | Beta-hydroxybutyrate complex 3541112 | Acetone 180 | Acetoacetate 6971017 | NL |
|---|---|---|---|---|---|---|---|
| 1 | Leptin (1AX8) | -4.7 | -5 | -3.7 | -2.5 | - 3.3 | * |
| 2 | Ghrelin (7W2Z) | -5.2 | -6.3 | -3.1 | -2.3 | -3 | -7.7 |
| 3 | Catalase (IDGB) | -6.8 | -6.9 | -4.7 | -3.1 | -4.6 | -8.4 |
| 4 | Superoxide dismutase (MODELLED) | -5 | -5.7 | -3.7 | -2.5 | -3.6 | * |
| 5 | FTO (3LFM) | 1 | 0.3 | -4.7 | -3.2 | -4.5 | -5.5 |
| 6 | A HMG-CoA reductase (1T02) | -4.7 | -4.7 | -3.4 | -2.5 | -3.4 | -6 |
Determination of the structural interactions of the protein–ligand complex result was performed using DS v. 21.1. software (Kayode et al., 2023).
Leptin aids hunger inhibition and energy intake-energy output balance. In the docking analysis results collated from the molecular docking of the protein, leptin was shown to exhibit a positive binding interaction with the ketogenic diet by-products (see Figures 1, 2 and 3 (β-hydroxybutyrate -3.7, acetoacetate -3.3, acetone -2.5)), as well as the standard drugs orlistat, -4.7, and cetilistat, -5 (Figures 4 and 5) used during this research study, although interactions were higher with standard drugs than with the ketone bodies. Therefore, the interaction between the standard drugs and ketone bodies with leptin indicates that greater levels of hunger inhibition, which will culminate in weight reduction, may emanate from standard drug and ketone bodies treatment since leptin’s function of inhibiting hunger and inducing starvation is highly effective in obesity treatment.
Stimulation of appetite through the hypothalamic arcuate nucleus, which controls food input and output, is achieved by the ghrelin protein (Kojima and Kangawa, 2005), as shown in the docking analysis results in Figures 6–10. The standard drugs (orlistat, -5.2, and cetilistat, -6.3) and ketone bodies (β-hydroxybutyrate -3.1, acetoacetate -3, acetone -2.3) exhibited positive binding interactions with the ghrelin protein, with the standard drugs having an increased interaction compared with the ketone bodies, which had minimal binding interaction. This connotes that the ghrelin function in the presence of these ligands (standard drugs and ketone bodies) might be inhibited and thus results in the lowering of appetite and thus food consumption, leading to weight loss in individuals.
The docking analysis of catalase interaction with standard drugs and ketone bodies (Figures 11–15) revealed the catalase exhibited a positive binding interaction with standard drugs at a higher level than ketone bodies, thereby showing that the catalase function of catalysing the disproportionate amounts of hydrogen peroxide synthesised in cells by certain enzymes and oxidases into a single oxygen (O2) molecule and water (H2O) molecule, and maintaining optimal compound levels which induce cell signalling by deactivating hydrogen peroxide are activated by the ketone bodies. This will enhance the anti-oxidative role of the diet and, since oxidative stress has been implicated in obesity progression, an enhanced cell anti-oxidative status by the ketone bodies will lead to the amelioration of obesity (Everse, 2013).
The docking analysis result of superoxide dismutase (SOD) (Figures 16–20), which is similar to that of catalase, showed positive binding interactions with both standard drugs (orlistat, -5.0, and cetilistat, -5.7) and ketone bodies (β-hydroxybutyrate -3.7, acetoacetate -3.6, and acetone -2.5), hence the SOD function of promoting the defence mechanism of cells against Reactive Oxygen Species (ROS) by catalysing the oxidative deamination of superoxide radicals (O2) into oxygen molecules (O2) and hydroxyl radicals (H2O2) are activated in vivo by the standard drugs and ketone bodies. This will also enhance the reduction of oxidative stress thus promoting amelioration of metabolic syndromes associated with obesity.
The docking analysis result collated for the FTO interaction with standard drugs (orlistat, 1, and cetilistat, 0.3) and ketone bodies (β-hydroxybutyrate -4.7, acetoacetate -4.5, acetone -3.2), shown in Figures 21–25, showed minimal binding interactions with the standard drugs but showed increased binding interactions with the ketone bodies ligands. This indicates that orlistat and cetilistat in the biological system may have minimal inhibitory action against FTO, while the ketone bodies exhibit greater inhibition of FTO. which can lead to the downregulation of fatty acid synthesis and storage, leading to significant reduction in body fat and obesity.
The docking analysis for the 3-hydroxyl-3-methylgluatarate Co-A (HMG-CoA) reductase interaction with the standard drugs (orlistat, -4.7, and cetilistat, -4.7) and ketone bodies (β-hydroxybutyrate -3.4, acetoacetate -3.4, acetone -2.5) is shown in Figures 26–30. HMG-CoA exhibited binding interactions with the standard drugs and ketone bodies. The interactions may result in inhibition of HMG-CoA reductase, hence leading to reduction in the levels of mevalonate and cholesterol synthesised in vivo This will consequently result in body fat reduction over a period of time.
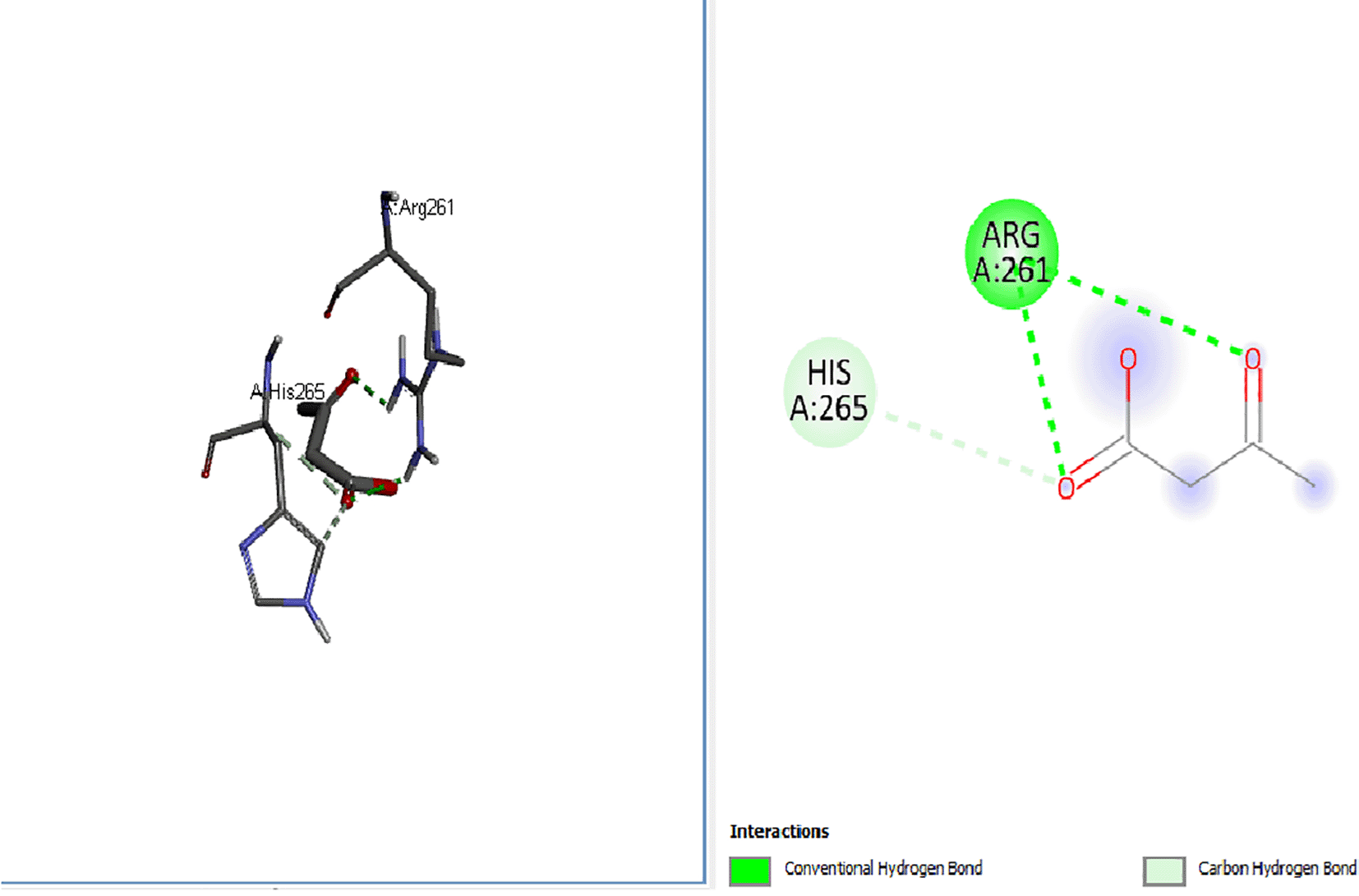
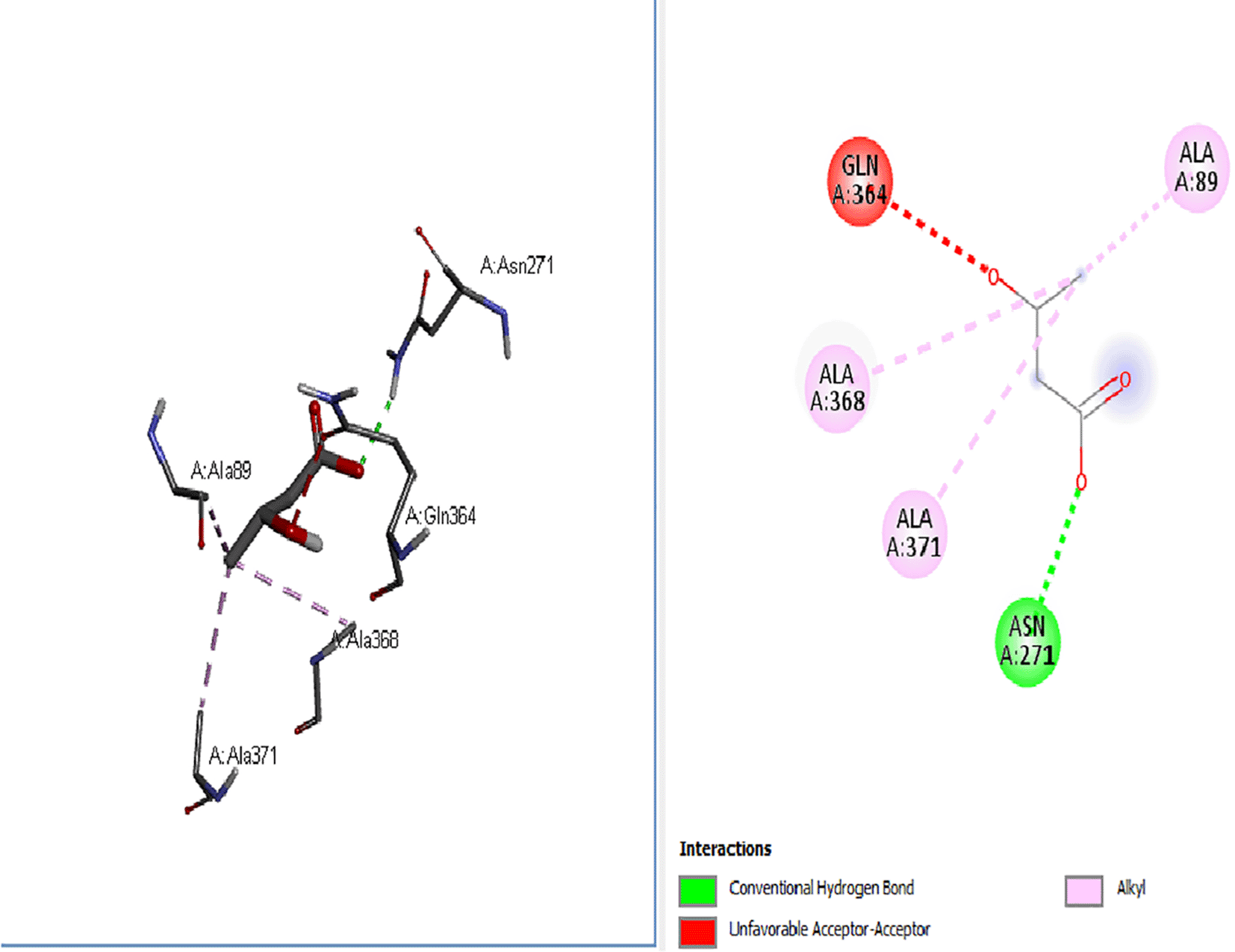
The ketone bodies exhibited varied positive interactions with the obesity-associated proteins and thus, sequel to further in vivo studies, the ketogenic diet may emerge as a therapeutic remedy for weight maintenance and obesity inhibition, which works via the ketone bodies’ interaction with obesity-associated proteins besides other mechanisms.
Figshare: Underlying data for ‘Evaluation of the interaction between ketone bodies and obesity-associated proteins: an in silico approach’. moleculardockingand2d3dinteractionsresults.zip. https://doi.org/10.6084/m9.figshare.21804411.v1. (Kayode, 2023).
This project contains the following underlying data:
- LIGAND and PROTEINS for docking 2.docx
- MOLECULAR DOCKING AND 2D-3D INTERACTIONS RESULTS.docx
- 3 HYDROXYL RESULT.csv
- AROMATES RESULT.csv
- CATALASE RESULT.csv
- FMO RESULT.csv
- FOLLICLE RESULT.csv
- GHRELIN RESULT.csv
- INHIBIN A.csv
- INHIBIN B.csv
- LEPTIN RESULT.csv
- LUTEINISING RESULT.csv
- OESTROGEN RESULT.csv
- SUPEROXIDASE DIMUTASE.csv
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors wish to thank the management of Mountain Top University for financial support for the payment of the article processing fee of this publication.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Adipose tissue biology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Bioinformatics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 30 Mar 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)