Keywords
Doxorubicin, Omega-7, lactate dehydrogenase, Tumor necrosis factor-alpha, Interleukin-6.
Doxorubicin, Omega-7, lactate dehydrogenase, Tumor necrosis factor-alpha, Interleukin-6.
Doxorubicin is a highly effective chemotherapeutic agent used to treat several types of cancer, such as hematogenous and solid cancers.1 Unfortunately, this medication's clinical utility is constrained due to its adverse effects, particularly cardiotoxicity, which may include cardiac arrhythmias, congestive heart failure, left ventricular dysfunction, and cardiomyopathy.2 It has been suggested that oxidative stress, apoptosis, autophagy, and Endoplasmic reticulum (ER) stress are the mediators of Doxorubicin-induced cardiac damage.3 Doxorubicin-induced cardiac damage is also influenced by inflammation. It has been demonstrated that doxorubicin therapy significantly increases the expression of tumor necrosis factor-alpha (TNFα), interleukin (IL)-1β, and IL-6.4 In addition, phosphokinase C (PKC) has been suggested to act as an intracellular signaling mediator for TNFα activity, which causes cytotoxicity and apoptosis in various cell types.5 The membranes of cells and organelles are usually permeable to fatty acids, and the administration of unsaturated fatty acids such as omega-3, -6, and -9 fatty acids, or various combinations of these fatty acids, has been studied for its potential health benefits.6 It has also been suggested that omega-7 fatty acid supplements may affect serum inflammatory biomarkers.6 The primary components of omega-7 are palmitoleic acid (16:1, cis-9-hexadecenoic acid) and vaccenic acid [(11E)-11-octadecenoic acid] ERER, which are primarily found in cold-water fish and sea buckthorn berries.7 Omega-7 is a non-essential fatty acid in humans as it can be produced endogenously.8 The consumption of omega-7 fatty acids has been related to better health outcomes by increasing HDL cholesterol levels and decreasing LDL cholesterol levels.7 Earlier research has demonstrated that omega-7 fatty acids efficiently suppress H2O2-induced inflammatory factors such as prostaglandin E2 (PGE2), tumor necrosis factor- (TNF-α), and interleukin-1 (IL-1β).8 Nevertheless, the effects of omega-7 on the integrity of cardiac tissue are yet to be established. As a result, we want to look into whether omega-7 may prevent against doxorubicin-induced cardiotoxicity by reducing inflammation.
The investigations were conducted in accordance with the criteria set by the University of Baghdad, College of Pharmacy Ethics Committee, with permission number 490, on February 9, 2022. The animal investigation was conducted in compliance with the ARRIVE principles 2.0 and the preclinical ARRIVE Essential 10 checklist.9 In addition, every effort was made to make rats as comfortable as possible during tests and sampling.
An observational study was done employing a convenient sample of male rats from 1/4/2022 to 30/6/2022.
In this investigation, the medications doxorubicin hydrochloride and omega-7 were utilized. Doxorubicin was obtained from Pfizer Laboratories, New York, United States, with the batch number FE0746, and omega-7 was purchased from Source Naturals, United States, Batch number SN 2552.
Twenty-eight male Wister rats weighing 150 and 250 grams were kept in polypropylene cages under regulated circumstances, including a regular light/dark cycle and a temperature of 22 2 °C. Rats were given commercial pellets and water from the tap.
The rats were split into four groups using simple random selection; including seven rats in each group as the following:
1. Group 1 (negative control): healthy animals received normal saline orally as the vehicle for eight successive days and were sacrificed on day nine.
2. Group 2 (positive control): Animals that received a single dose of doxorubicin hydrochloride (IP 15 milligrams/kilogram) and were sacrificed the next day.
3. Group 3: the animals were administered omega-7 orally at a 100 milligrams/kilogram/day dose for eight days. A single injection of doxorubicin IP (15 milligrams/kilogram) was given on day nine. The animals were sacrificed on day 10.
4. Group 4: the animal was administered omega-7 orally at a 300 milligrams/kilogram/day dose for eight days. A single injection of doxorubicin IP (15 milligrams/kilogram) was given on day nine. The animals were sacrificed on day 10.
Twenty-four hours after the last administration of the drug, gel-activated tubes were used to collect blood samples after they were drawn from the carotid artery and let to stand for 30 minutes to clot. Then, the blood was spun in a centrifuge for 15 minutes at 3000 rpm to gain the serum.10 The collected serum was used to determine the cardiac biomarkers (LDH and CK-MB) and the pro-inflammatory mediators (TNFα, IL-6, and IL-1β). Bioassay Technology Laboratory, China provided all ELISA kits; details are presented in Table 1 below.
Version 27 of IBM SPSS Statistics (RRID: SCR 016479) for Windows Operating System was utilized all through the statistical analysis method. The mean and standard deviation of all study data were reported (SD). The Shapiro-Wilk test was used to determine if the findings were normal. Furthermore, an unpaired T-test was used to determine the significance of the difference in means between two independent samples. P values less than 0.05 were considered statistically significant.
Analysis of the data in Table 1 revealed a significant increase in lactate dehydrogenase (LDH) levels in group 2 (positive control) compared to group 1 (negative control) at p < 0.05. Despite the absence of a significant difference p > 0.05 in the level of LDH in group 3 (100 mg) when compared to group 2, the level of LDH decreased significantly p < 0.05 in group 4 (300 mg/kg omega-7) when compared to group 2. The level of creatinine kinase-MB (CK-MB) was significantly elevated in group 2 (positive control) compared to group 1 (p < 0.05). While there was no significant difference in the level of CK-MB between groups 3 (100 mg) and 2 (p > 0.05), the higher dose of omega-7 (300 mg/kg) in group 4 significantly decreased the level of CK-MB compared to group 2 (p < 0.05; Table 2).
| Group 1 (negative control) | Group 2 (positive control) at a dose of 15 mg/kg | Group 3 (omega-7 at dose 100 mg/kg) + doxorubicin | Group 4 (omega-7 at dose 300 mg/kg) + doxorubicin | |
|---|---|---|---|---|
| LDH U/L | 1777.71 ± 559.37 | 2527.43 ± 383.979* | 2304.57 ± 332.507 | 1876 ± 358.488# |
| CK-MB U/L | 658.714 ± 68.368 | 1021.29 ± 110.36* | 987.857 ± 145.763 | 755.714 ± 108.144# |
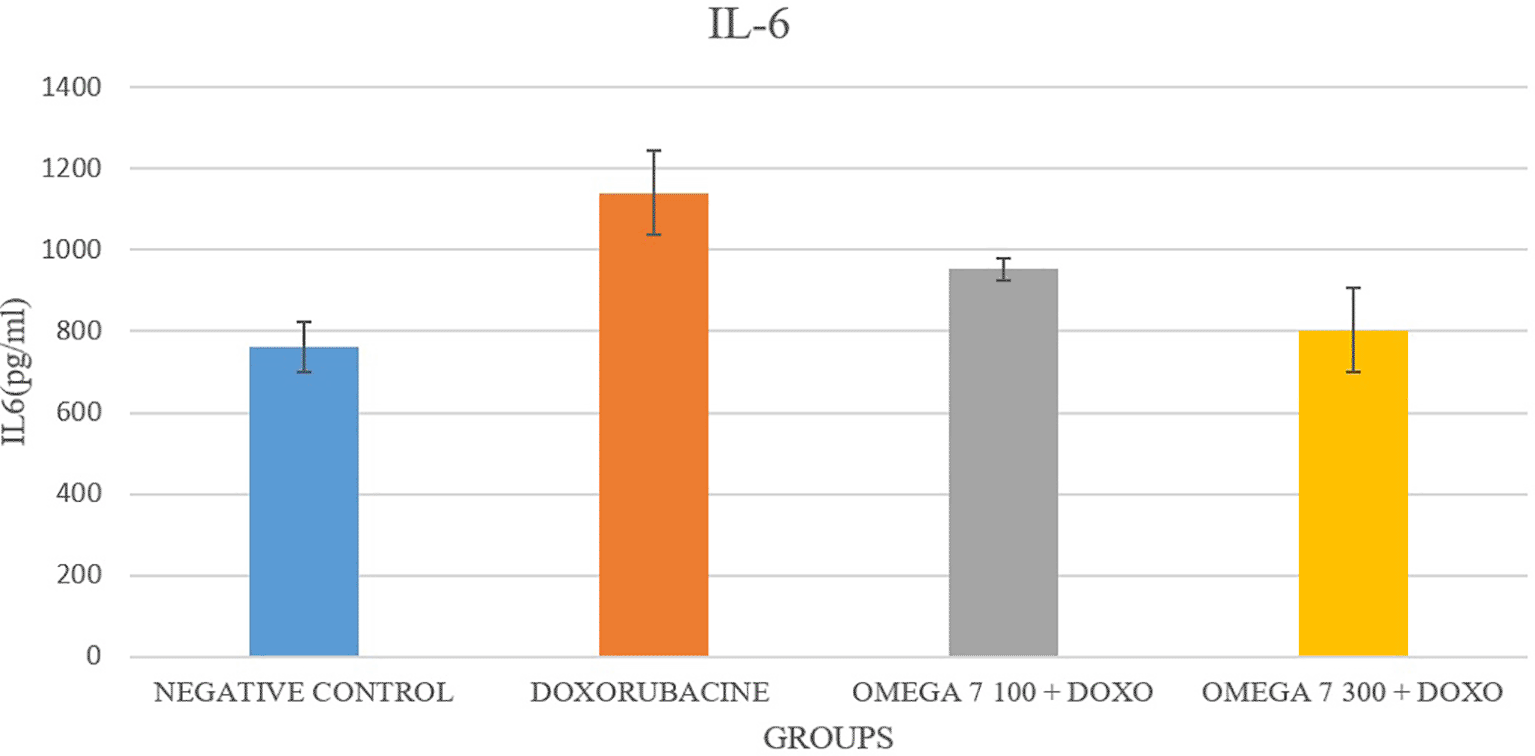
The serum concentration of TNF-α was substantially elevated in the doxorubicin-treated group compared with the control group (p < 0.05; Figure 1). In contrast, the co-administration of omega-7 in groups 3 (100 mg/kg) and 4 (300 mg/kg) decreased TNF-α levels considerably compared to group 2 (positive control) p < 0.05. IL-6 levels were considerably elevated in group 2 (positive control) compared to group 1 (negative control) in Figure 2 (p < 0.05). In contrast, the co-administration of omega-7 in groups 3 (100 mg/kg) and 4 (300 mg/kg) substantially decreased IL-6 levels compared to group 2 (positive control) p < 0.05. Significantly more IL-1 was detected in group 2 (positive control) than in group 1 (positive control), p < 0.05. As demonstrated in Figure 3, the co-administration of omega 7 (100 mg/kg) in group 3 and (300 mg/kg) in group 4 decreased IL-1 considerably p < 0.05 compared to group 2 (positive control).
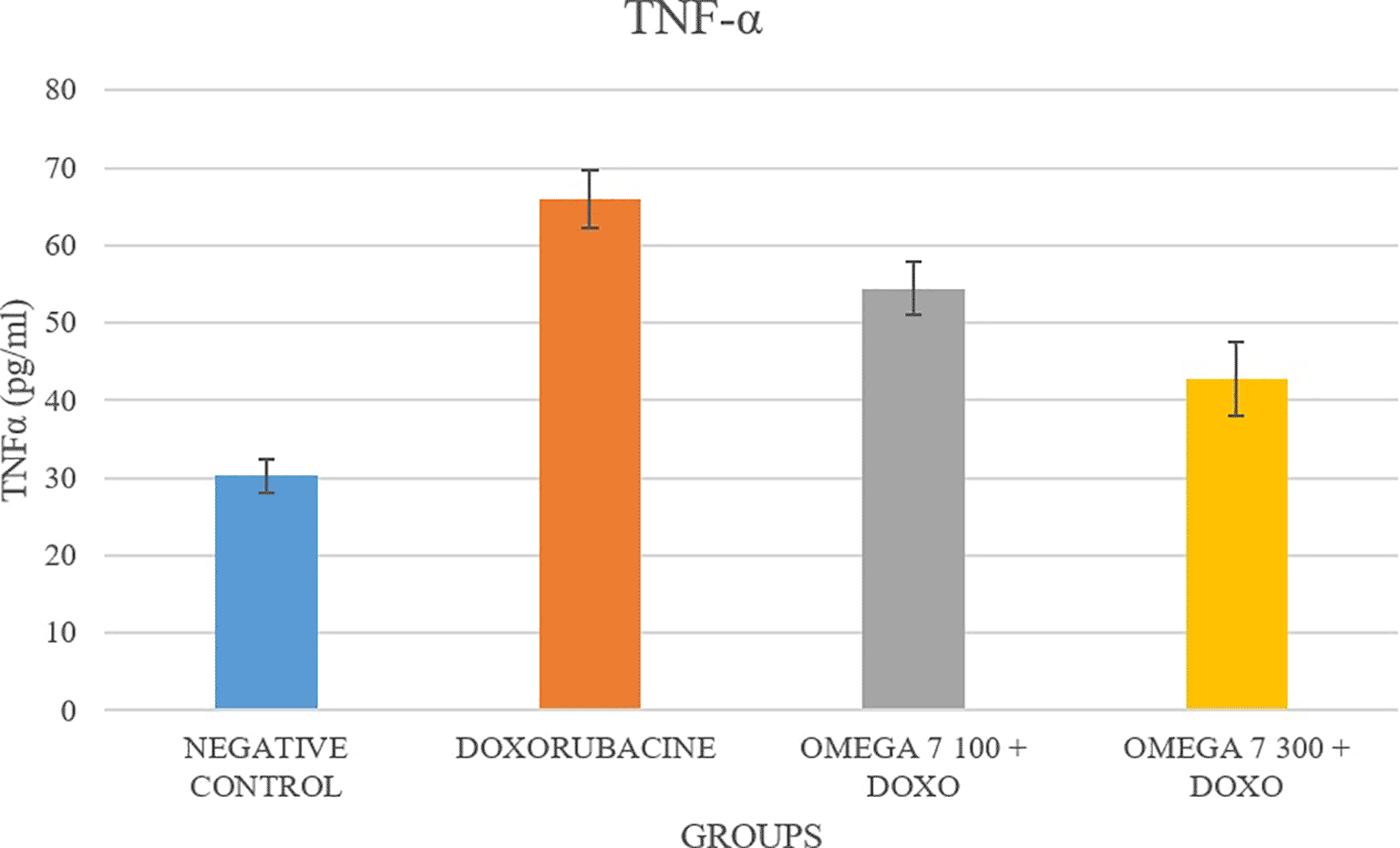
The high prevalence of cardiomyopathy and heart failure is the main side effect of doxorubicin usage.11 According to several investigations, the production of reactive oxygen species (ROS) from doxorubicin redox activation contributes significantly to the cytotoxicity of doxorubicin.11 Numerous other studies have concentrated on the signaling mechanism that causes doxorubicin-induced apoptosis.12,13 Moreover, pro-inflammatory cytokines such as IL-6, TNFα, and IL-1β are implicated in activating inflammatory reactions.14 It has previously been noted that doxorubicin causes heart tissue to release more pro-inflammatory cytokines.15 In the present study, the administration of doxorubicin to rats resulted in cardiotoxicity, as evidenced by significant increases in LDH and CK-MB serum levels (Table 1), which have been utilized in various preclinical investigations to evaluate heart injury,16,17 combined with a significant increase of serum levels of pro-inflammatory cytokines including TNFα, IL-1β, and IL-6; similar to other findings.15,18
Pre-treatment with omega-7 in group 3 (100 mg/kg) caused a non-significant difference in LDH and CK-MB levels compared to the positive control group. However, pre-treatment with a larger dose (300 mg/kg) showed a significant reduction in LDH and CK-MB levels compared to group 2. This outcome is in line with earlier investigations into the effectiveness of polyunsaturated fatty acid as a cardioprotective subsistence.19
TNFα is an inflammatory cytokine produced by macrophages and monocytes during acute inflammation and is in charge of several cell-signaling processes that result in necrosis or apoptosis.20 From the study, the level of TNFα was significantly reduced in the omega-7 pretreated group (100 mg and 300 mg/kg), which is in line with other research about the anti-inflammatory effect of omega-7 in H2O2-treated cells.8 Interleukin-6 (IL-6) is known to regulate immune responses and is considered a potentially helpful indicator of immune system activity.21 Inflammation, infection, and cardiovascular disease may cause an increase in IL-6. IL-1β is a cytokine that plays a role in pain, inflammation, and autoimmune diseases.22 From the study, the levels of both IL-6 and IL-1β were significantly reduced in omega-7 treated groups (100, 300 mg/kg) compared to the positive control group. The result is consistent with another published study on omega-3 concerning those biomarkers.23 However, the current study is the first to demonstrate the efficacy of omega-7 in lowering the elevated levels of IL -6 and IL -1β associated with doxorubicin-induced cardiotoxicity. The production of several cytokines, including TNFα, during the oxidative stress brought on by the doxorubicin therapy, served as a mediator for cytotoxicity.24 It was discovered that omega-7, which was used in this study to protect the myocardial tissue from doxorubicin toxicity, inhibited the excessive release of TNFα through a mechanism linked to the suppression of the PKC enzyme responsible for the production of NF-kβ, the crucial signaling molecule in the pathway that releases TNFα.25 Therefore, an appealing treatment strategy for treating doxorubicin-induced cardiotoxicity is to modify the expression of the cytokines.26 The presentation of TNF and other inflammatory mediators during oxidative stress situations is inhibited by substances like omega-7, which may have potential therapeutic utility in this area.
The current discovery suggests that omega-7 may shield the myocardium against doxorubicin-induced damage by reducing the inflammatory response.
Zenodo: Measurement of TNF-α, CK-MB, LDH, IL-1 and IL-6 levels for the anti-inflammatory effect of omega-7 against doxorubicin-induced cardiotoxicity in male rats: an observational study. https://doi.org/10.5281/zenodo.7353404. 27
This project contains the following underlying data:
Zenodo: The ARRIVE Essential 10: author checklist – Anti-inflammatory effect of omega-7 against doxorubicin-induced cardiotoxicity in male rats: an observational study. https://doi.org/10.5281/zenodo.7360390. 9
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Zanwar A, Hegde M, Bodhankar S: Protective role of concomitant administration of flax lignan concentrate and omega-3-fatty acid on myocardial damage in doxorubicin-induced cardiotoxicity. Food Science and Human Wellness. 2013; 2 (1): 29-38 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: clinical chemistry, clinical pharmacy
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 10 Jan 23 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)