Keywords
EMG detectors, Low SNR EMG, Movement intent detection, EMG-BCI, Robot-assisted therapy, Neurorehabilitation
Active participation of stroke survivors during robot-assisted movement therapy is essential for sensorimotor recovery. Robot-assisted therapy contingent on movement intention is an effective way to encourage patients’ active engagement. For severely impaired stroke patients with no residual movements, a surface electromyogram (EMG) has been shown to be a viable option for detecting movement intention. Although numerous algorithms for EMG detection exist, the detector with the highest accuracy and lowest latency for low signal-to-noise ratio (SNR) remains unknown.
This study, therefore, investigates the performance of 13 existing EMG detection algorithms on simulated low SNR (0dB and -3dB) EMG signals generated using three different EMG signal models: Gaussian, Laplacian, and biophysical model. The detector performance was quantified using the false positive rate (FPR), false negative rate (FNR), and detection latency. Any detector that consistently showed FPR and FNR of no more than 20%, and latency of no more than 50ms, was considered an appropriate detector for use in robot-assisted therapy.
The results indicate that the Modified Hodges detector – a simplified version of the threshold-based Hodges detector introduced in the current study – was the most consistent detector across the different signal models and SNRs. It consistently performed for ~90% and ~40% of the tested trials for 0dB and -3dB SNR, respectively. The two statistical detectors (Gaussian and Laplacian Approximate Generalized Likelihood Ratio) and the Fuzzy Entropy detectors have a slightly lower performance than Modified Hodges.
Overall, the Modified Hodges, Gaussian and Laplacian Approximate Generalized Likelihood Ratio, and the Fuzzy Entropy detectors were identified as the potential candidates that warrant further investigation with real surface EMG data since they had consistent detection performance on low SNR EMG data.
EMG detectors, Low SNR EMG, Movement intent detection, EMG-BCI, Robot-assisted therapy, Neurorehabilitation
We have modified the manuscript based on the suggestions from the reviewer. We have: (a) used the surface electromyogram acronym throughout the paper, (b) made the introduction concise by shortening the text on BCI drawbacks.
The oversight in Table 1 on the signal conditioning methods for the Lidierth detector has been rectified. In Figure 1, the amplitude of the biophysical model signal has been scaled to have similar amplitudes to that of the phenomenological model to avoid any confusion.
We have modified the term EMG-triggered robot-assisted therapy to EMG-driven robot-assisted therapy to since we want robot-assisted movements to be contingent on the presence or absence of sEMG; we have also changed “sEMG-triggered” to “sEMG-driven” in the manuscript.
We have also included a paragraph in the discussion section to explain the choice of the 20% cut-off for defining a good detector. This cost will permit up to 20% FPR resulting in a sensitive detector. This is not a major concern, because some form of time-based filtering will be applied on the binary output of the EMG detector before using it to drive robotic assistance. The choice of amount of time-based filtering can be determined through patient feedback from patients about their sense of agency when interacting with the robot device.
See the authors' detailed response to the review by Werner Wolf
See the authors' detailed response to the review by Vincent Crocher
Substantial recovery of sensory-motor function after a stroke is possible with high-intensity and high-dosage movement training.1 Rehabilitation robots can facilitate such high-intensity movement training while providing physical assistance to the user for completing movements consistently and precisely. About 30% of stroke survivors are severely impaired2,3 and require physical assistance to actively engage in movement training. While physical assistance from a robot can motivate such subjects to attempt and train movements, they can also provide inappropriately timed or too much assistance4,5 leading to slacking, where patients reduce their voluntary effort and exploit robotic assistance to perform the movements. Inappropriately timed robotic assistance also alters the patient’s sense of agency or subjective awareness of control. This could lead to a lack of intrinsic motivation and attention, affecting motor learning and performance.6 Positive therapeutic effects have been observed only when patients actively engage in therapy.7–10 Jo et al.11 reported no improvement in clinical scales with passive range of motion therapy. Thus, active patient participation during movement therapy is an essential ingredient for sensorimotor recovery.
Robotic assistance contingent on a subject’s intention to move is an effective way to guide neuroplasticity.12 However, it can be difficult to detect the intention to move in stroke patients with no residual movement. Electroencephalogram (EEG) based brain-computer interface (BCI) has been used to detect movement intention in order to trigger robotic movement assistance and the effectiveness of EEG-BCI in robot-assisted rehabilitation has been investigated in several studies.13–19 Ramos et al. reported a 3 point improvement in the Fugl-Meyer assessment (FMA) score of the experimental group who were given robot-assisted therapy contingent on movement intention.17 There are, however, several drawbacks to EEG-BCI systems. They often exhibit a poor signal-to-noise ratio, with significant trial-to-trial intra-subject variability.20 Unlike electromyography systems, EEG-BCI modalities lack task specificity.21 Moreover, their complexity and time-consuming nature make them less suitable for routine clinical use.22
Surface Electromyogram (sEMG) could be a viable alternative to address these drawbacks of the EEG-BCI modality for robot-assisted therapy. sEMG is a simple, robust, compact modality suitable for routine clinical use. In a recent study, we identified sEMG as a potential alternative to EEG-BCI to detect movement intention from severely affected stroke patients without residual movement.22 About 70% (22 out of the 30) of the study participants had residual sEMG in the forearm muscles that showed a consistent increase in amplitude with wrist/finger movement attempts.
However, our study reported poor agreement between the EEG and sEMG modalities for detecting movement intention. The authors suggested that this discrepancy could be because of the simple root mean square detector with temporal thresholding that was used. This detector may not optimally pick up low SNR surface sEMG signals22 expected from severe patients with no residual movements.
Numerous sEMG detection algorithms have been proposed for the automatic detection of sEMG onset.23–30 The review article by Staude et al.23 compared different sEMG detector types to identify the best detectors for detecting sEMG onset.23 However, this work was done on high SNR (3dB and 6dB) simulated sEMG signals generated by bandpass-filtering white Gaussian noise, and it only investigated detectors reported till 2001. Other detectors have been reported in the last 20 years, and a systematic characterization of existing sEMG detector types on low SNR sEMG signals (generated using different signal models) is still lacking. Identifying an optimal detector is essential for further exploring the use of sEMG-based movement intent detection for robot-assisted therapy in severely affected stroke subjects with no residual movements.
Our goal in this study is to systematically compare the detection accuracy and latency of existing sEMG detectors on low SNR sEMG signals to identify the most promising detectors, while eliminating the ones with poor performance, for further investigation. To this end, we will here:
• Generate simulated low SNR (0dB and -3dB) sEMG data using two phenomenological (Gaussian and Laplacian) models and a biophysical model to evaluate the performance of the different detector types.
• Define an appropriate cost function considering the detection accuracy and latency to evaluate the performance of the different detector types.
• Compare the performance of the different detector types on simulated surface sEMG signals from the three signal models for two different SNRs (0dB and -3dB) and identify the most appropriate detector type(s).
We note that the detectors studied in this work produce a binary output suitable for on-off control of robotic assistance during therapy. This is in contrast to previous studies exploring continuous control paradigms using sEMG amplitude estimates on less severely affected stroke patients with some residual limb movements31,32 However, for severely affected patients with no residual movements, who may or may not have detectable sEMG, an on-off control scheme might be the easiest modality for human-robot interaction. Continuous control schemes might be appropriate as patients gain better control of their muscles that are still too weak to produce residual movements.
Neurorehabilitation training consists of repeating specific movements of interest punctuated by periods of rest.17,33 A typical training session will involve several “trials” of a particular movement, each with a period of rest (rest-phase) followed by a period of movement/movement attempt (move-phase). In sEMG-driven robot-assisted therapy, the robot remains inactive during the rest phase, while in the move phase robot-assisted movement of the subject’s limb is contingent upon the presence or absence of sEMG at any given time instant; continued sEMG is required to continuously receive robotic assistance. The rest of this section starts with the formal definition of the signal processing problem solved by an sEMG detector, followed by the details of the simulated sEMG signals, a description of the general structure of sEMG detectors, and the approach used to compare the performance of the different detectors.
Let be the recorded signal from a target muscle during the trial; is the sampling instant, where is the start of a trial, is the number of data points from each trial. Let and be the number of samples in the rest- and move-phases of a trial, respectively, then . The time segments and correspond to the rest-phase and move-phase of the trial, respectively.
Problem definition: To detect the presence of EMG in real-time in the move-phase of a trial using only the current and past EMG data from the start of the trial. Let represent the binary output of the sEMG detector at the current sampling instant ,
where, is the detector function that maps the sEMG signal , from the start of a trial to a binary output corresponding to the presence or absence of sEMG at the current time instant ; is the set of detector parameters that control the detector’s performance. The function is often a complex mathematical operation consisting of a series of simpler operations performed on the sEMG data to produce the binary output. This binary output can be used as a simple on/off control of robotic assistance by severely affected patients to relearn movement initiation.34,35
The analysis of the different sEMG detectors was performed using simulated sEMG data. To generate this simulated sEMG, we assume that:
• the measurement noise has a fixed variance throughout the experiment,
• the muscle is fully relaxed in the rest-phase of any trial, i.e., there is no sEMG activity from the target muscle during the rest-phase, and
• the muscle is activated at a constant level for the entire duration of the move-phase.
These assumptions were made to evaluate the sEMG detectors under the conditions that: (a) the sEMG signal has a fixed signal-to-noise ratio (SNR) in the move-phase, and (b) all other intra- and inter-trial variabilities in the sEMG signal characteristics are minimized. The detectors that perform poorly under these ideal conditions will likely perform worse with real sEMG data from patients, since real sEMG data from severely affected stroke patients might occur in random bursts and is likely to have time-varying amplitude.
We simulated 100 trials of sEMG data with an individual trial duration of 13 seconds (8 s and 5 s for rest- and move-phases, respectively). The sampling frequency of the simulated signal was set to 1000 Hz. Three different sEMG signal generation models were employed in the current analysis – two phenomenological (Gaussian and Laplacian) models and one biophysical model.
The phenomenological models were based on the work of De Luca36 where a surface sEMG signal from a muscle activated at a fixed level can be treated as zero-mean white noise followed by a shaping filter (electrode properties); this model is widely accepted in the literature.23,37–40 The exact probability density of the sEMG signal depends on the muscle activation level, with high levels of activation following a Gaussian distribution.37,38 However, at low levels of muscle activation, sEMG signals have been reported to follow a distribution that lies between a Gaussian and a Laplacian distribution.41 Therefore, to ensure that the detectors are tested with the appropriate signals, we generated data using both white Gaussian and white Laplacian signals, resulting in two phenomenological models.
The first step in this model is the generation of the zero-mean unit-variance white Gaussian and Laplacian noise . A step change in the signal variance, within a trial, at the transition between the rest- and move-phases of a trial was obtained by multiplying by :
In addition to the phenomenological models, we also wanted to test the detectors on more realistic data based on the biophysics of the sEMG signal, accounting for the physiological origin of the electrical muscle activity and the recording electrode geometry. In this paper, the biophysical model proposed in Ref. 42 was employed to generate the simulated sEMG data. Assuming a linear, isotropic volume conduction model, a simple muscle geometry with parallel muscles fibres ignoring the effects due to the finite muscle fibre length, the sEMG recorded by a bipolar electrode configuration can be approximated using the following expression:
The sEMG simulator developed by Devasahayam40 was employed in the current work to generate the simulated sEMG signals.42 A bipolar surface electrode configuration with a 10 mm interelectrode distance was considered. The simulator takes in the muscle force level as its input and computes the corresponding firing pattern for the motor units. In the current study, the force levels from the muscle were set to 0N in the rest-phase (no muscle activation) and 10N in the move-phase (average firing rate of 16.4 Hz for the muscle). The simulator generated pure muscle activity recorded by the chosen electrode configuration. The force level for the muscle in the move-phase was chosen empirically to ensure that the temporal profile of the simulated sEMG signal visually resembled that of real surface sEMG signals. A zero-mean white Gaussian noise of fixed noise variance was added to to introduce measurement noise. The noise variance was chosen based on the signal power () of in the move-phase to obtain a signal with the desired SNR:
Following this, the noisy signal is bandpass filtered (8th order non-causal FIR filter) between 10 Hz and 450 Hz cut-off frequencies:
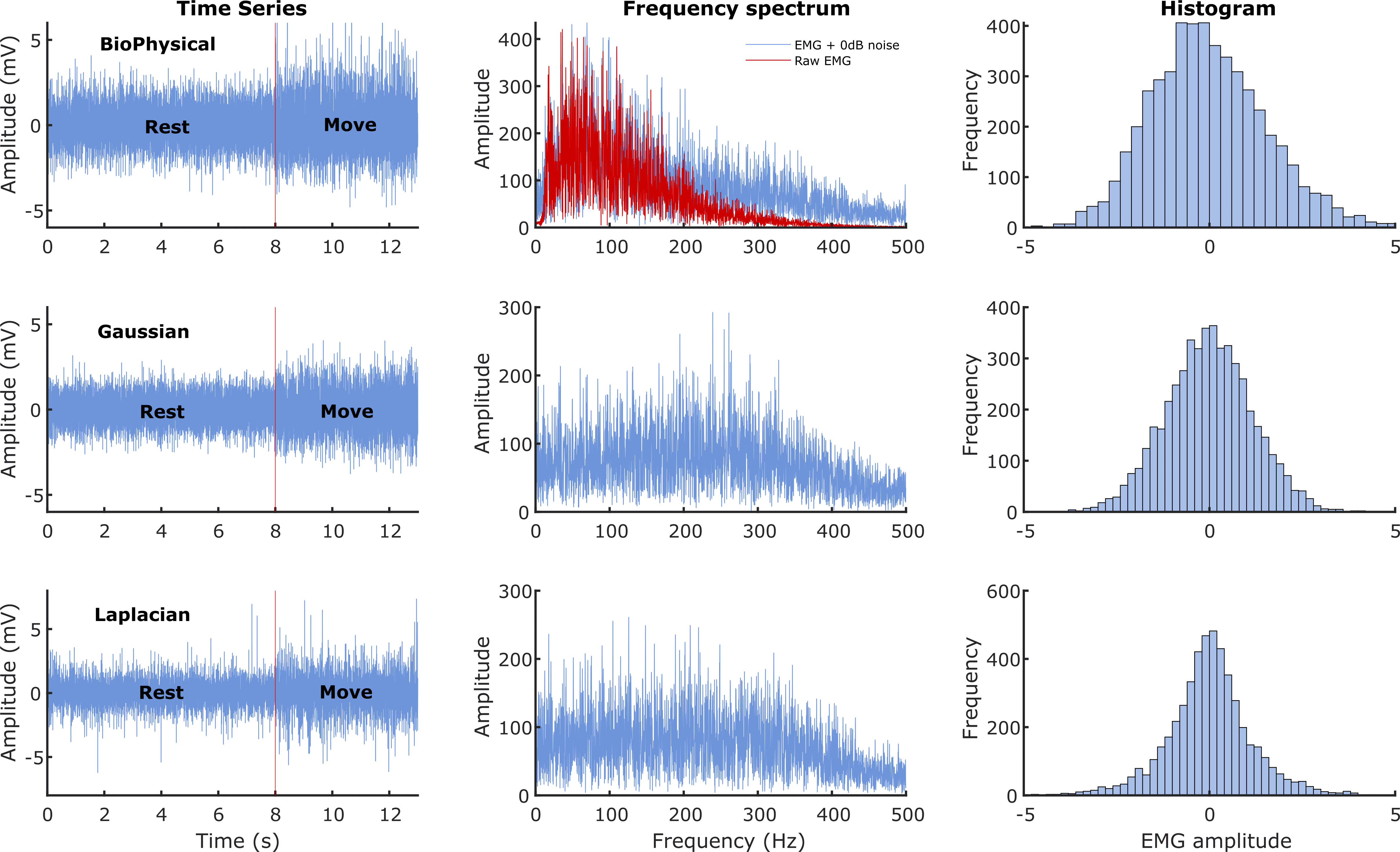
The three rows correspond to the three different signal models: Biophysical in the top row, Gaussian in the middle, and Laplacian in the bottom row. The left most column shows the time series of the simulated 13 seconds of data with the first 8 seconds corresponding to the rest phase and the next 5 seconds to the move phase. The middle column shows the corresponding Fourier magnitude spectrum of the 5 seconds of move phase data. The right column displays the estimate of the probability density functions of the 5 seconds of move phase data from the three models.
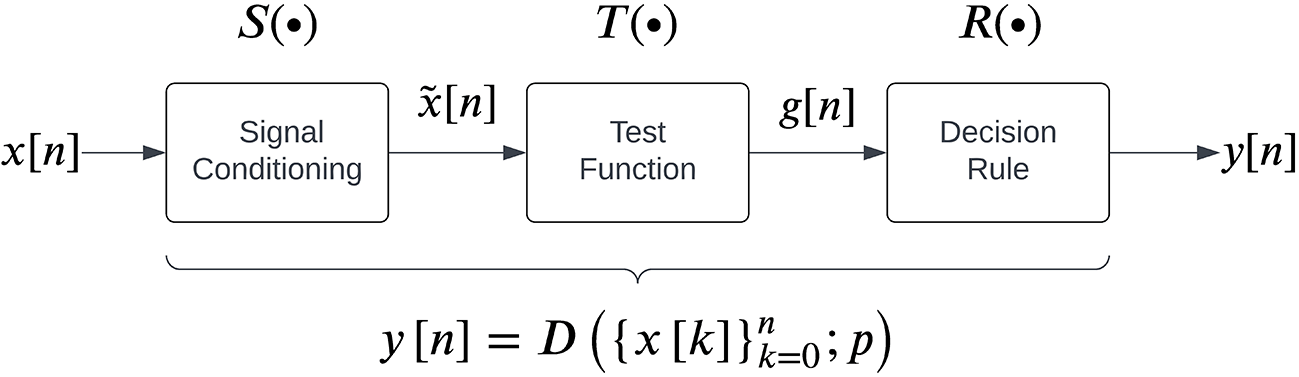
The general structure for sEMG detectors proposed by Staude et al.23 is shown in Figure 2, which consists of three steps carried out sequentially to map the given real-time sEMG data into a binary output:
1. Signal conditioning is the first step to improve sEMG signal quality for better detection, often involving high-pass filtering for movement artefact removal. Some detectors might employ additional filtering operations, such as adaptive whitening for stable sEMG amplitude estimation.43,44 The conditioned signal is represented by
2. Test function computation transforms into a scalar variable or feature that can distinguish the presence or absence of sEMG. The test function is computed at the current time instant over a causal window of size :
Some examples of test functions in the literature include the moving average of , test variable,45 likelihood ratio46 etc.
3. A decision rule is applied on the test function by comparing it to a threshold to identify the presence/absence of an sEMG signal:
The threshold h is adaptive and is calculated for each trial by adding α times the standard deviation of first 3 seconds data (Figure 3) to the mean. α is termed as the weight for threshold in this paper. Some detectors employ a more sophisticated decision rule, such as double thresholding, to control the false positive rates of detection.45,47
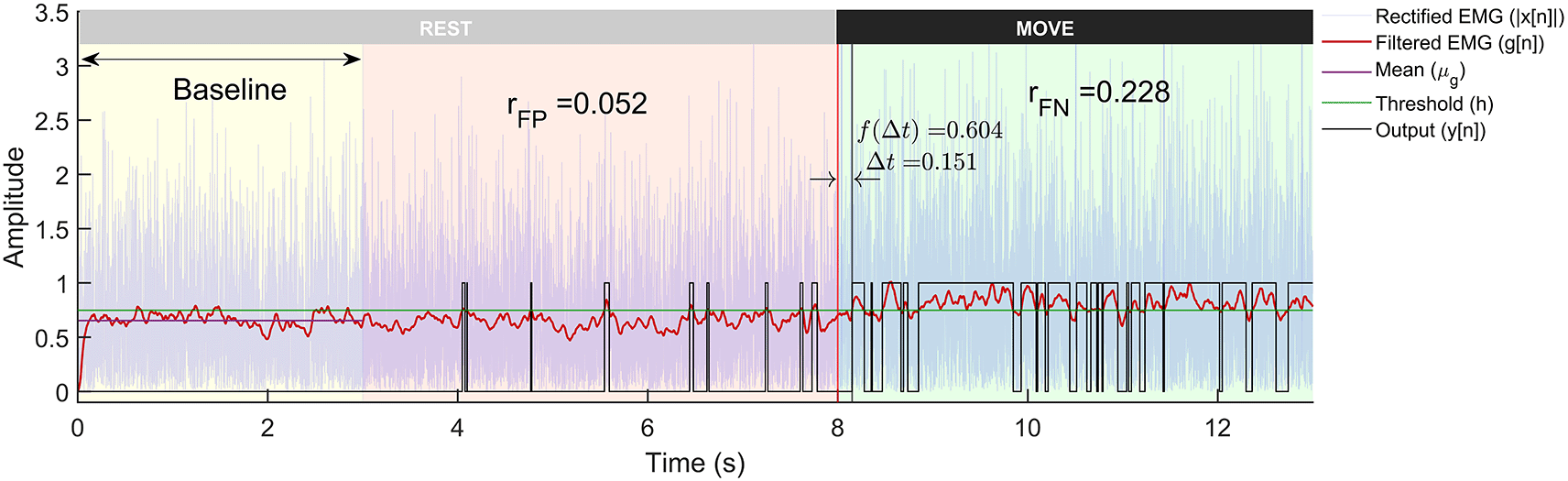
The plot shows the rectified sEMG signals, its lowpass filtered output, and the binary output from the detector. The trial is 13 seconds long with first 8 seconds corresponding to the rest-phase and the next 5 seconds to the move-phase. The rest-phase is further divided into the baseline phase (yellow background) that is used for computing the threshold , and the remaining rest-phase (red background) is used for computing . The move-phase (green background) is used to compute and .
We note that each detector has a set of parameters associated with it. The current study compares the performance of 13 detector types reported in the literature which can be implemented in real-time, listed in Table 1. Each detector type fits into the general structure shown in Figure 2. The different parameters associated with these detector types are also provided in Table 2. A detailed description of the individual detector types and the algorithms for their implementation are provided in the extended data (Table S1). All detector algorithms were obtained from the literature and implemented in MATLAB50 with appropriate modifications required for real-time detection.
| Detectors | Signal Conditioning | Test function | Parameters |
|---|---|---|---|
| Modified Hodges | Rectification Low pass filter | Cut-off frequency of LPF ( Weight (α) | |
| AGLR - G23,48 | Adaptive whitening | Likelihood Ratio test | Window size (W) Weight (α) |
| AGLR - L | Adaptive whitening | Likelihood Ratio test | Window size (W) Weight (α) |
| Fuzzy27 Entropy | - | Chebyshev distance Fuzzy function | Window size (W) Weight (α) |
| Modified Lidierth | Rectification Low pass filter | Cut-off frequency of LPF ( Weight (α) Double threshold params: m, T1 | |
| Hodges23 | Rectification Low pass filter | Cut-off frequency of LPF ( Window size (W) Weight (α) | |
| RMS22 | Band pass filter | Root mean square () | Window size (W) Weights (α) Window shift (p) Temporal threshold (m) |
| Lidierth23 | Rectification | ) | Window size (W) Weight (α) Double threshold params: m, T1 |
| TKEO28 | High pass filter | Cut-off frequency of HPF ( Window size (W) Weight (α) Double threshold params: m = 1, T1 | |
| Bonato45 | Adaptive whitening | Weight (α) Double threshold params: m, T1 | |
| Sample30 Entropy | - | Chebyshev distance Local tolerance for the distance | Window size (W) Weight (α) Tolerance |
| CWT29 | - | Correlation of signal with Wavelet representing shape of MUAP | Scaling parameter of the mother wavelet (a) Weight (α) |
| SSA49 | - | Computation of detection statistics using Euclidean distance. | Window size (W) |
The simulated sEMG data from the three different signal models and the two different SNRs were used to evaluate the performance of the different detector types. Each trial of sEMG signal (13 seconds long) was input to the different detectors to compute binary output indicating the presence or absence of sEMG signal. An optimal EMG detector designed for use in EMG-driven robot-assisted therapy should possess the capability to quickly identify the onset of sEMG, efficiently eliminate false positives, and consistently detect sEMG when it is present (lower false negative). Such a detector might be essential for maintaining the user’s motivation and sense of agency. These are computed from the output of each trial (Figure 3), where the sEMG signal from each trial was analysed in the following three steps:
1. the first three seconds (0–3s) of the rest-phase data is used for estimating the threshold for detection:
2. the remaining 5 seconds of the rest-phase are used to estimate the false positive rate .
3. the 5 seconds of the move-phase are used to estimate the false negative rate and the detection latency .
We defined a performance measure to compute a single number referred to as the cost of detection that considers the false positive rate , the false negative rate , and the detection latency . Let be the cost vector associated with the output of the detector for a particular trial. We define the cost of detection as the infinity norm of the cost vector .
The false positive rate is defined as the ratio of the number of 1s in the detector output in the rest-phase of a trial, and the false negative rate is defined as the ratio of the number of 0s in in the move-phase.
From Figure 3, the detection latency is defined as the time delay from the start of the move-phase to when the detector output goes to 1:
Latencies between 0 to 250 ms have linearly increasing costs while the ones above 250 ms are considered as bad as 250 ms. Based on the definitions of , , and , . A detector with a consistently lower cost of detection would be considered a better detector.
A detector’s performance or cost is determined by the SNR of the input signal, the detector type, and its associated parameters. Thus, for a fixed SNR input signal, comparing two detector types must be done only after controlling for the influence of their corresponding detector parameters. In the current work, this was done by first choosing the optimal parameters for each detector type, before comparing different detector types. The optimal parameters for a detector type were selected by first splitting the 100 movement trials of simulated sEMG data of the three models (which was generated as explained above) into training and validation datasets with 50 trials each. This was done for both SNRs (0dB and -3dB) and for all three signal models (Gaussian, Laplacian, and biophysical). The training dataset was used to identify the optimal parameter values for the different detector types, i.e. the values of the parameter combination that consistently resulted in the least cost for the detector on the training dataset. The exact procedure is given in Algorithm 1 (end of the document), while details are provided in the extended data.
After identifying the optimal parameter combination for each detector type, the optimal parameter values were used to run the detector on the 50 trials of the validation dataset, which resulted in the validation cost set for the detector type . The cost set from the different detector types were compared using two-way ANOVA with the detector type and signal SNR as the two factors for each of the three-signal model. The complete code for the analysis can be found here.
The entire analysis – generation of the simulated data, the various detection algorithms, optimization51 of the detector parameters, and analysis of the results – reported in this paper were implemented in MATLAB R2020 (RRID:SCR_001622) (alternative languages could also recreate this study i.e. Python [RRID:SCR_008394] or GNU Octave [RRID:SCR_014398]). A sample of the individual trials from the three sEMG signal models is depicted in Figure 1. A sample output of the different processing stages of the Modified Hodges detector from a Gaussian sEMG signal trial is shown in Figure 3. The Modified Hodges detector, filters (2nd order Butterworth low pass filter) the rectified raw sEMG signal (blue coloured trace in Figure 3). This lowpass filtered signal is the test function of the detector (red-coloured trace in Figure 3). The threshold for this trial is shown by the green-coloured horizontal line in Figure 3. The output of the detector (black coloured trace in Figure 3) is 1 whenever the test function crosses the threshold, it is 0 otherwise. The figure also shows the values of , , , and for the trial.
The 13 detector types were compared after choosing the optimal parameter set for each detector type using the training dataset of 50 trials for each of the six combinations of the three signal models and two SNRs.52 This procedure is depicted in Figure 4 for the Modified Hodges detector for the 0 dB SNR Gaussian signal model, which shows the outcomes from the different steps in the optimization process described in Algorithm 1. Figure 4(a) shows the histograms of the cost for the different parameter combinations, in light blue traces. These histograms are estimated from the cost values obtained from the 50 trials in the training dataset for the different combinations of the detector parameters. The scatter plot of the median and inter-quartile range of these histograms are shown in Figure 4(b). The choice of the best parameter for the detector was determined to be the one with the least Euclidean norm which is shown as the red circle in Figure 4(b); its corresponding histogram is shown in the thick red trace in Figure 4(a). Figure 4(c) shows the marginal histograms of the individual contributors to the cost for the optimal parameter combination for the Modified Hodges detector. The values of the optimal parameters for the different detector types are listed in Table 2.
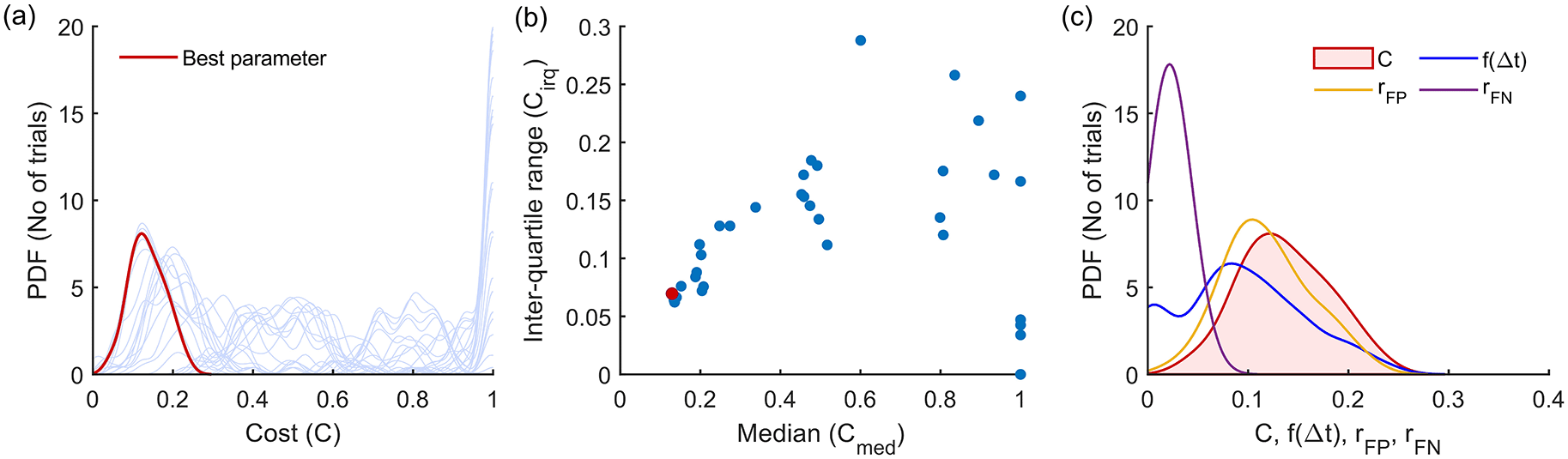
(a) Estimated probability density function of cost for the different combinations of parameters (in light blue traces). The red trace corresponds to the cost of the optimum parameter combination. (b) Scatter plot of median vs IQR for the cost of different parameter combinations. (c) Estimated histograms of latency, false positive rate, false negative rate and cost of the optimum parameter.
The performance of the different “optimal” detector types, i.e., detectors using the optimal parameter values, were compared using the 50 trials from the validation datasets. The boxplot of the performance of these different detector types for the three different signal models – Gaussian, Laplacian, and biophysical – are shown in Figure 5(a), (b), (c), respectively; each of these subplots displays the performance for the 0dB and -3dB SNRs in red and blue boxplots, respectively. Note that the order of the depiction of the different detectors is in terms of the increasing average cost across the three signal models and two SNRs; the detectors on the left are better than the ones on the right in an average sense. A two-way ANOVA on the effect of the detector type and SNR on performance revealed a significant difference between the detector types (p < 0.001) and SNRs (p < 0.001) for all three signal models. The test revealed a significant interaction between the factors for all three models (biophysical: p < 0.0001; Gaussian: p < 0.0001; Laplacian: p < 0.0001). These statistical results confirm the results shown in the boxplots in Figure 5, where the performance is different among the detector types, with consistently poorer performance for -3dB compared to 0dB. The costs for both 0dB and -3dB appear to be lower for the biophysical model compared to the Gaussian and Laplacian models.
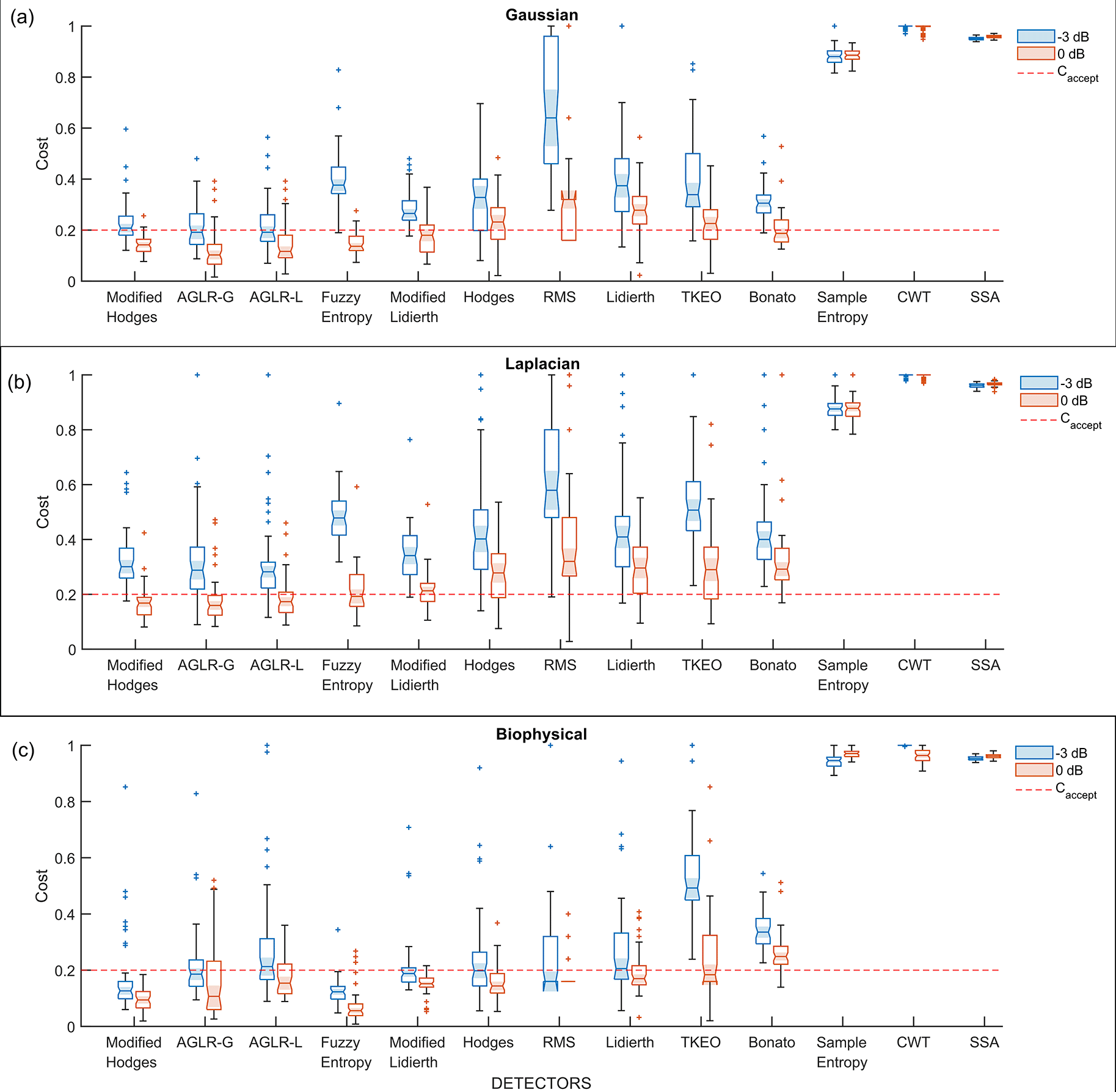
All detectors shown in this plot use the optimal detector parameter sets optimized on the training dataset. (a) Cost of detection for the Gaussian signal model, (b) Laplacian signal model and (c) Biophysical signal model. The red and blue colored boxplots are of 0dB and -3 dB SNR, respectively. The red dashed line is the acceptable cost .
Most detector types perform similarly except for the Sample entropy, Continuous wavelet transform (CWT), and Singular spectrum analysis (SSA) detector types which perform worse across the different signal models and SNRs. Among the other detector types – the Modified Hodges, the approximate generalise likelihood ratio test- Gaussian (AGLR-G), and the approximate generalise likelihood ratio test- Laplacian (AGLR-L) detectors – have almost similar costs for the different signal models and SNRs. The other detector types – Root mean square (RMS), Hodges, Bonato, Lidierth, Modified Lidierth, Teager Kaiser Energy Operator (TKEO), and Fuzzy entropy – have slightly higher costs for one or more specific signal models and SNRs. We note that the Fuzzy entropy detector performs very well on the biophysical signal model for both SNRs with an acceptable cost for more than 95% of the validation trials.
The choice of appropriate detector type(s) for use in robot-assisted therapy requires the specification of an acceptable cost of detection . To this end, we specify the upper limits for the false positive rate, false negative rate, and the latency of detection as , which corresponds to a detector with the following cost components:
We believe that these upper limits are a reasonable compromise among the three competing factors determining the cost. Any detector type with costs consistently lower than would be deemed an appropriate detector for use in robot-assisted therapy. To determine the detector types with consistently lower costs than , we computed the proportion of the 50 validation trials with acceptable for the detector for the three signal models and two SNRs using
1. All detectors perform poorly for the -3dB Laplacian signal model. The highest value of is 0.22 for this signal model, which interestingly is from the AGLR-L detector designed for the Laplacian signal. Many detectors perform a little better with higher values for the -3dB Gaussian and biophysical signal models.
2. The Modified Hodges detector is the most consistent detector across the different signal models and SNRs. It has an for the three signal models at 0dB and the biophysical model at -3dB SNR.
3. The Fuzzy Entropy detector performs as well as the Modified Hodges detector for the Gaussian and biophysical signal models, but not on the Laplacian model.
4. In terms of the average value of , across the three signal models (last two columns of Table 3), the Modified Hodges detector performs the best for the 0dB signals, followed by the AGLR-G, AGLR-L, and Fuzzy Entropy detectors which have slightly lower but similar performance. For -3dB signals, the Modified Hodges, AGLR-G, and AGLR-L detectors result in similar performances.
The cells with proportions greater or equal to 0.8 are highlighted in gray.
Based on these observations, the Modified Hodges appears to be the most consistent detector for low SNR signal models, irrespective of the sEMG signal model. The two statistical detectors (AGLR-G, and AGLR-L) and the fuzzy entropy detectors provide similar but slightly lower performance than the Modified Hodges detector.
Movement intention-triggered robot-assisted therapy is one of the options available for severely impaired patients without residual movement. sEMG for movement intent detection is a simpler, more direct and task-specific alternative to EEG-BCI.22 The investigation of sEMG-driven robot-assisted therapy requires a sensitive and robust method for the accurate and fast detection of movement intention from residual low SNR sEMG signals. This study systematically investigated existing sEMG detection algorithms in the literature until 2018. The investigation was carried out on simulated sEMG signals using three different signal models with low SNR of 0dB and -3dB. These SNRs correspond to feeble sEMG signals compared to regular surface sEMG recordings from healthy individuals. Using three different signal models – two phenomenological and one biophysical – makes the study results robust to assumptions about the simulated sEMG data.
The study by Staude et al. published in 200123 compared different sEMG detectors for accurate sEMG onset-time detection. They employed a Gaussian signal model with ramp variance profiles (with varying slopes) at SNR of 3dB to 12dB in their analysis23 and found the AGLR statistical detector to be the best in terms of onset detection, while the Hodges detector performed poorly.23 Although there are some similarities between the current study and those of Staude et al., the two differ in several ways: (a) the current study is focused on real-time detection, while Staude et al.’s primary goal was offline analysis; (b) the current study employed lower SNR signals, which is important considering its application to detect motion intention in severally affected stroke patients; (c) the current study tested three different signal models, while Staude et al. used only the Gaussian signal model; (d) the primary performance measure in Staude et al. was onset detection latency, while the current study used a composite performance measure (or cost) consisting of the false positive rate, false negative rate, and detection latency; (e) the rationale for the choice of the specific detector parameters was not explicitly mentioned in Staude et al. In the current study, the detector parameters were optimized through a brute force search to ensure the best detectors from each detector type were compared; and (f) the current study investigates a wider class of detector types than Staude et al., including the detectors published after 2001.
In this analysis, an acceptable cost 0.2 was chosen for application in sEMG-driven robot-assisted therapy; this corresponds to a latency of 50 ms, 20% FPR or 20%FNR; the low latency, and relatively high FPR and FNR can result in a more sensitive detector being chosen as the optimal detector. We do not believe this is a problem, because the raw output of this detector is unlikely to be used directly to drive the robot-assistance. Some form of low-pass or time-based filtering (like the one employed by Ramos-Murguialday et al.17) will be employed to filter out show false positives/negative pulses before using it to drive robotic assistance. This filtering operation reduce the FPR and FNR at the expense of introducing an additional latency; a delay of 200-300ms are well tolerated when reporting for sense of agency.53 The choice of amount of filtering of the chosen detector’s output will need to be done through feedback from patients/users of the system.
The current study identified that the Modified Hodges detector performed consistently well with cost for at least 80% of the validation trials, across the different signal models and SNRs, except for the -3dB Laplacian signal model, where all detectors fail. The modified Hodges detector – a simplified version of the Hodges detector – performs better than Hodges because it does not involve the additional averaging step in computing its test function. This reduces the detection latency for the modified Hodges detector without an appreciable increase in the false positive and false negative rates (Table S4 in the extended data in figshare (RRID:SCR_004328)). The AGLR-G, AGLR-L, and fuzzy entropy detectors perform slightly lower than modified Hodges but better than the rest of the detectors. The good performance of the statistical detectors agrees with that of Staude et al. even with the lower SNRs investigated in this study. The fuzzy entropy detector also performs well, unlike its counterpart – sample entropy. The sample entropy algorithm in this study used the local estimate of the signal’s standard deviation for normalizing the data. Sample entropy’s poor performance with the local estimate of the standard deviation was previously reported by Zhang et al. Sample entropy performs well only with the global estimate of the signal’s standard deviation.30 This is not suitable for real-time implementation as estimating the global standard deviation is a non-causal operation requiring the entire signal record. The use of the fuzzy similarity measure addresses this problem with sample entropy, allowing the fuzzy entropy detector to track changes in the overall signal amplitude. Interestingly, fuzzy entropy has a low cost of detection for both 0dB and -3dB biophysical signal models, which could be due to the additional structure of the motor unit action potentials (MUAPs) in the move-phase of the biophysical signal.
Interestingly, the RMS detector we used previously to demonstrate the viability of sEMG as an alternative to detect movement intention in severely impaired chronic stroke subjects22 was not one of the best performers, as seen in Figure 5 and Table 3. We note that the observed performance was for the RMS detector with optimized parameters (Table 2) using the training dataset. This optimized RMS detector had a relatively high false negative rate and higher detection latency which resulted in its poor performance. This could possibly explain the lack of agreement between the sEMG and EEG detectors we had observed in our previous study, and a more sensitive detector might have identified sEMG activity in a larger proportion of subjects. The current study results warrant further investigation with real sEMG data from severely impaired patients using other detectors, such as the modified Hodges, AGLR-G/L, and fuzzy entropy.
In general, most detectors have a relatively lower cost of detection for the biophysical signal model, compared to the Gaussian and the Laplacian signal models. The reasons for the better performance on the biophysical model are not entirely clear, except for the fuzzy entropy detector, which might be sensitive to the temporal structure of the simulated data (MUAP) from the biophysical model. One possibility is the difference in the spectra of the signals from the biophysical model compared to the Gaussian or Laplacian modes (Figure 1); more signal energy is concentrated in the lower frequencies for the biophysical model than in the Gaussian or Laplacian models. Most detectors compute their test functions through a lowpass filtering or averaging operation, which could retain a relatively larger portion of the signal in the biophysical model compared to the Gaussian and Laplacian ones, thus resulting in improved performance with the biophysical model. If this is correct, then the difference in performances between the biophysical and the Gaussian/Laplacian models should disappear when an appropriate spectral shaping filter is used in the Gaussian and Laplacian models, yielding a spectrum like the biophysical model. Finally, among the Gaussian and Laplacian models, the relatively poorer performance with the Laplacian signal model could be due to the long tails of the Laplacian distribution.
The simulated data used in the current study relies on a step-change in the signal properties between the rest- and move-phase, and an sEMG signal of fixed amplitude during the move phase. These assumptions will be violated when dealing with feeble surface sEMG signals recorded from impaired participants with no residual movements. In such participants, movement attempts are likely to produce intermittent bursts of sEMG activity with smooth transitions between the on and off states in the target muscles. The sEMG signal might have time-varying amplitude even when the participant can continuously activate the muscle for sufficient duration. Although based on idealized simulated sEMG data, the current results do provide some idea about the detector types that can potentially work on real low SNR sEMG signals; a detector performing poorly on ideal data is likely to perform worse with real data. Furthermore, the results from the current analysis also indicate that modified Hodges, AGLR-G, AGLR-L, and fuzzy entropy detectors are likely to pick up even bursts of sEMG signals since they have small detection latency (∆t ≤ 50 ms).
The detectors studied in this paper can only be used for on-off control of robotic assistance,54 where once sEMG activity is detected, robotic assistance drives the limb towards the target in a preprogramed fashion. This is different from continuous control of the motion where robotic assistance is proportional to the level of sEMG measured from the target muscles.35 Continuous control of robotic assistance is likely to be a more natural and engaging interaction for participants than simple on-off control. The most common sEMG feature for continuous control has been the sEMG amplitude estimate,35 however, the test functions employed by the different detector types in the current study could be potential alternatives. The choice of the best control variable depends on which one of these is sensitive, robust, and provides a natural human-robot interaction with minimal lag. However, it should be noted that it is unclear how well severely impaired participants, with no residual movements, can finely modulate their sEMG activity. The choice of on-off versus continuous control of robotic assistance for a participant will require a screening procedure to evaluate the ability of the participant to modulate sEMG activity in the target muscle.
The study has limitations that are worth noting to ensure that the results are interpreted appropriately. The study entirely relies on simulated data to investigate the different detectors. The conclusions are thus only as good as the assumed signal models and how well they represent the residual surface sEMG signals of patients with no visible movements. This is the first study investigating detectors for low SNR sEMG, and thus the use of simulated data was essential to gain some understanding of the performance of the different detectors. Simulated data also allows complete control of the ground truth, which provides a more truthful characterization of different detectors’ detection accuracy and latency. The use of three different signal models to investigate the different detectors also adds some robustness to the study’s findings. Additionally, this analysis allows us to exclude the poorly performing detectors and identify the ones that warrant further investigation with real data. Another potential limitation of the use of simulated data is the availability of complete information about the ground truth against which the different detectors are compared. However, the results of the current study can’t be verified with real data because we will never know the ground truth in the surface sEMG from patients with no residual movements. This is a valid concern. Nevertheless, some form of an unsupervised approach will be required for verifying the results of the current study with real data. With real data, the best detector would be the one that consistently provides the maximum separation for the probability density function of the test function from the different detectors under the rest-phase and move-phase.
This paper systematically investigated existing sEMG detection algorithms on low SNR sEMG signals simulated using three different signal models (two phenomenological – Gaussian, Laplacian models and a biophysical model) at two different SNRs (0dB and -3dB). The Modified Hodges detector – a simplified version of the threshold-based Hodges detector, introduced in the current study – was found to be the most consistent detector across the different signal models and SNRs. This detector had false positive and false negative rates of lower than 20% and a detection latency of lower than 50 ms for almost 90% of the trials on which it was tested for 0 dB SNR and more than 40% of the trials for -3 dB SNR. The two statistical detectors (Gaussian and Laplacian Approximate Generalized Likelihood Ratio) and the Fuzzy Entropy detectors have a slightly lower performance than Modified Hodges. Overall, the modified Hodges, Gaussian and Laplacian approximate generalized likelihood ratio, and fuzzy entropy detectors were identified as potential candidates for further validation with real surface sEMG data on a population of severely impaired patients. The current study forms the first step towards developing a simpler, practical, and robust sEMG-based human-machine interface for triggered robot-assisted therapy in severely impaired patients.
Let the parameter set for the given detector type be .
Set the parameter ranges for the individual parameters in the parameter set for the detector type, which results in different parameters combinations.
For to parameter combinations:
- Compute the output of the detector for the chosen combination of parameter values for each of the 50 trials in the training dataset.
- Compute the cost for each of the 50 trials.
- Compute the median and inter-quartile range of the cost values from the 50 trials.
- Compute the overall detector performance for the trial as .
Get the best parameter combination for the detector type as the following,
SB conceived and brainstormed the idea with EB and VSKM. PR worked on the initial literature search and the implementation of some of the detectors. MY implemented the different EMG signal models, all the detectors, optimization of detector parameters, and the analysis of the results. AD and SB provided scientific inputs for the implementation and analysis done by MY. EB and VSKM provided critical feedback for the methodology and results. MY, AD, and SB wrote the first draft of the manuscript. All authors reviewed and approved the final submitted manuscript.
Figshare - Dataset: A systematic investigation of detectors for low signal-to-noise ratio EMG signal, https://doi.org/10.6084/m9.figshare.22317658.v2. 52
The data used in the analysis is saved as csv file which is named based on the SNR and signal generating model as follows:
Figshare: Supplementary material: A Systematic Investigation of Detectors for Low Signal-to-Noise Ratio EMG Signals.docx, https://doi.org/10.6084/m9.figshare.22232527.v3. 51
This project contains the following extended data:
- Supplementary material.pdf (parameters for optimizing the detector)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Source code: https://github.com/1608Moni/EMG_detectors
Archived source code: https://doi.org/10.5281/zenodo.7750951
License: MIT
The authors thank Prof. Suresh Devasahayam for providing us with the software he’d developed for simulating the EMG signals. This software was used for generating data for the biophysical EMG model.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Rehabilitation robotics
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biosignal Processing - human motor control
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Rehabilitation robotics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 4 (revision) 18 Apr 24 |
||
|
Version 3 (revision) 25 Mar 24 |
read | |
|
Version 2 (revision) 30 Jan 24 |
read | |
|
Version 1 21 Apr 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)