Keywords
anti-angiogenic, Ficus deltoidea, green synthesize, nanomaterials
This article is included in the Nanoscience & Nanotechnology gateway.
anti-angiogenic, Ficus deltoidea, green synthesize, nanomaterials
Ficus deltoidea is a well-known medicinal plant that has been historically used by various ethnic groups in Indonesia. This plant, from the Moraceae family, is an epiphyte with a shrub habit that is found in secondary forests. The F. deltoidea leaf is used to treat diabetes mellitus,1 for anti-inflammation,2 as an antibacterial3 and for cancer inhibition.4 Additionally, F. deltoidea is reported to be antinociceptive, anti-inflammatory, antidiabetic, anti-obesity, anti-melanogenic, antioxidative and a free radical scavenger.5
Furthermore, F. deltoidea leaf extract may suppress cancer development through three mechanisms: apoptosis via the intrinsic route, migration and invasion inhibition, and angiogenesis inhibition.6,7 The aqueous and ethanol extracts of F. deltoidea are known to have anticancer activities on human ovarian carcinoma cells. The F. deltoidea aqueous extract has an IC50 of 224.39±6.24 g/mL, while the F. deltoidea ethanolic extract has an IC50 of 143.03±20.21 g/mL. However, cell growth is affected differently by exposure to the different extracts. Cell release is triggered with the aqueous extract, while cell growth is reduced with the ethanol extract.8
Recently, technology surrounding nanoparticles, which can be biologically synthesized, has grown, since the nano-size items exhibit different characteristics of its bulk components due to variations in their surface–volume ratio.9 Most nanomaterials have demonstrated outstanding quantum confinement with unique catalytic features in many biological reactions and a variety of applications in various sectors that enhance the approach for electrical, environmental and medicinal objectives.10 Nanomaterials are employed in biological imaging, diagnostics, biosensing, gene therapy, and antibacterial and anticancer drugs.11
“Greener” nanoparticle synthesis is advantageous over previous approaches as it is simple, inexpensive, and relatively repeatable, typically resulting in more stable compounds. Nanoparticles can also be created using microorganisms. However, the rate of synthesis is slower, and the process is limited regarding accessible sizes and forms when compared with approaches employing plant-based components. The green synthesis process does not require high pressure, energy, temperature, or harmful ingredients; as a result, many scientists have abandoned synthetic approaches. Moreover, plants generate more stable nanoparticles than other technologies and are easily scaled up.11
Previous studies revealed that silver nanoparticles synthesized using Ficus deltoidea extract (AgNPs) have been biosynthesized using various plant extracts, including Salvia spinosa,12 Origanum vulgare L.,13 Araucaria angustifolia,14 Myrmecodia sp bulb,15 Eleutherine americana,16 and Citrus medica.17 The biosynthesis of AgNPs using various plant extracts is a simple and rapid method, with its success easily determined by color changes and UV-Vis spectrophotometry. Furthermore, to characterize the resulting AgNPs, X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and transmission electron microscopy (TEM) are employed.18
Several studies using plant derived AgNPs involving cancer and angiogenesis have been performed. Previous research reported that the green synthesis of AgNPs using rapeseed flower pollen potentially reduced angiogenesis.19 Similar findings were also described by Vimalraj and Ashokkumar,20 who stated that green synthesis of gold nanoparticles mediated by Mangifera indica seed water extracts demonstrated anti-angiogenic properties. Baharara and Namvar21 revealed that AgNPs generated from Achillea biebersteinii flower extracts were well-dispersed and stable using green techniques and exhibited potential therapeutic advantages against angiogenesis. In addition, the biosynthesized-AgNPs successfully reduced angiogenesis in an embryonated chicken model, and this anti-angiogenic property of AgNPs can be investigated as a potential therapeutic against pathological angiogenesis and solid tumors by targeting the vasculature.22
Various in vivo angiogenesis assays have been conducted to study the angiogenic processes and to discover new therapeutic agents that inhibit or trigger angiogenesis. One of the most common laboratory methods used to study angiogenesis and anti-angiogenesis is the chorioallantoic membrane (CAM) assay of chicken embryos. The CAM methodology has several advantages, such as a high embryo survival rate, simple procedure, not requiring high sterility, low cost and a high level of reproducibility and reliability. The CAM assay was first performed by Judah Folkman and colleagues for testing angiogenic activity in tumor tissue.23–25
Though several studies of AgNPs and anti-angiogenic properties of plant extracts have been conducted, the anti-angiogenic properties of AgNPs biosynthesized using F. deltoidea (AgNPs-Fd) has yet to be defined. Thus, the present research aimed to evaluate the anti-angiogenic properties of AgNPs-Fd using the CAM method. The biosynthesis of AgNPs-Fd was characterized by observing the color change, Tyndall effect, UV-Vis spectrophotometry, TEM, XRD and FTIR. Phytochemical analysis of AgNPs-Fd was also conducted using gas chromatography-mass spectrometry (GC-MS). For quantitative analysis of the vascular network in the CAM assay, AngioTool open-source software was used.26
Present methods has been deposited step-by-step on protocols.io with DOI: dx.doi.org/10.17504/protocols.io.14egn2nj6g5d/v1.
The Ethics Research and Community Service Mulawarman Universitas approved the use of chicken embryos to be used in the chorioallantoic membrane assay (Contract Number: 464/UN17.L1/HK/2022; 10 May 2022). This study is reported in line with ARRIVE guidelines.53
Leaves of the F. deltoidea were collected from a local farmer at East Kalimantan, Indonesia. The collected leaves were identified using a floras identification manual. The dry leaves were cut into small pieces and ground using a mechanical blender. The leaf powder was extracted using ethanol, concentrated using a rotary evaporator and stored at 4°C until further use.
Aqueous silver nitrate (AgNO3) (100 mL, 1 mM) was combined with 100 mL 0.1% F. deltoidea leaf extract. The pH of the solution was adjusted to 7.0, and it was subsequently incubated on a rotary shaker at 150 rpm for 48 h at 28°C. The fabrication of AgNPs-Fd was observed macroscopically by color change and the Tyndall effect.
The resulting AgNPs-Fd were confirmed using UV-Vis spectrophotometry (Shimadzu, UV-1280, Japan) in the range of 350–750 nm. The TEM (MIRA3 model, Czech Republic) was operated to display the surface morphology of the AgNPs-Fd. XRD was applied to evaluate the chemical characterization of AgNPs-Fd. To determine phytochemicals surrounding the AgNPs-Fd, FTIR spectroscopy (Agilent, Cary 630 model, US) was performed.27
Phytochemical analysis of the AgNPs-Fd was performed using GC-MS (HP-5MS UI, Agilent, USA) to evaluate the chemical compounds that potentially serve as anti-angiogenic. For this, samples of AgNPs-Fd were dissolved in ethanol in a microtube, vortexed and centrifuged for three minutes at 9,500 rpm. The resulting supernatant was used for identification and injected into the GC-MS apparatus. The condition of the GC-MS is as follows: column: HP-5MS UI, gas carrier: helium UHP (He), injector temperature: 290°C, split flow: 10 ml/min, split ratio: 10; front inlet flow: 1.00 ml/min, MS transfer line temp: 230°C, ion source temp: 200°C, mass list range (amu): 40–500, purge flow: 3 ml/min, gas saver flow: 5 ml/min, and gas saver time: five minutes.
To analyze AgNPs-Fd for anti-angiogenic properties, a CAM assay was performed following previous methods by Ribatti,24 Camposano and Torre,28 and Gamallo and Espere.29 In total, 24 chicken eggs were collected in preparation for the CAM analysis and were dosed with F. deltoidea extract (Fd) or AgNPs-Fd. The doses used in the paper disk for the CAM assay were as follows: negative control (30 ng basic fibroblast growth factor (bFGF); positive control (30 μg cortisone acetate with 30 ng bFGF); treatment groups (30 ng bFGF with AgNPs-Fd at 45, 60, 75 and 90 μg, respectively). A basic fibroblast growth factor (bFGF-Thermo Fisher Scientific, USA) is a group of proteins secreted by tissues to regulate cell metabolism, proliferation, differentiation, and survival. The bFGF was used to induce neovascularization.30
Before incubation, eggshells were cleansed with 70% alcohol. The eggs were incubated in for six days at 37−39°C with 50−60% humidity. The boundaries of the air space, which indicated the location of the embryo, were marked on all eggshells with a pencil (1 × 1 cm). Candling, with egg binoculars, was used to determine the location of the embryo. By using a povidone-iodine solution, the eggshell was cleaned at the pole containing the air space and the part above the embryo. Using a needle, a small hole was made in the air chamber and vacuumed until the air moved from the pole to the top of the egg.
Next, a window measuring 1×1 cm was made on the marked area using a mini drill. Paper discs for each treatment were then embedded onto the CAM through this window. The holes in the polar regions and 1×1 cm windows were sealed with paraffin film after they had been planted according to the treatment. The eggs were then incubated for 48 h at 37−39°C with 50−60% humidity. After, the eggs were removed and subsequently killed by freezing for 24 h. The eggs were then opened by cutting the eggshell into two parts, beginning with the part closest to the air cavity. The egg contents were slowly and carefully removed, keeping the CAM attached to the shell. Each CAM was photographed and analyzed using AngioTool 0.6 software (RRID:SCR_016393).
AngioTool is a small, easy-to-use application that allows for the quick, hands-free and reproducible measurement of vascular networks in micrographs. AngioTool calculates a variety of morphological and geographical data, such as the area covered by a vascular network, the number of vessels, vessel length, vascular density and lacunarity. AngioTool also computes the “branching index” (branch points/unit area), which quantifies specimen sprouting activity.26
Data from the AgNPs-Fd characterization were evaluated as graphs and images. Notations and arrow marks were added to the images, and they were cropped and the resolution was increased to 300 dpi, all of which were performed using Adobe photoshop (RRID:SCR_014199) (Adobe Photoshop, adobe, Inc., USA). Meanwhile, data from the CAM assays, such as vessel area, total number of junctions, junction density, total vessel length, average vessel length and total number of end points, were presented as the mean±standard errors. Significant differences among the treatment groups were assessed using analysis of variance (ANOVA) with IBM SPSS Statistics (RRID:SCR_016479) ver. 22 (IBM Corp., USA). A Duncan’s multiple range test (DMRT) was performed if significant differences were detected from ANOVA analysis. Significance was defined as p<0.05.
The solution began to change color once the plant extract was added to the AgNO3 solution. When the reaction began, the solution was light brown. After 30 minutes of constant stirring, the solution gradually darkened (Figure 1).52 Further, Tyndall scattering was performed to confirm the biosynthesis of AgNPs-Fd. As depicted in Figure 2, the solution of colloidal AgNPs-Fd (Figure 2b) demonstrated the Tyndall effect (beam of light visible from the side) due to the presence of lyophobic sol particles large enough for light dispersion, while genuine solutions (Figure 2a and c) did not exhibit light dispersion. Thus, the presence of AgNPs-Fd in aqueous solution as colloids was deduced.

(A) Plant extract; (B) colloidal AgNPs-Fd; (C) AgNO3 solution. AgNPs-Fd, silver nanoparticles synthesized using Ficus deltoidea extract; AgNO3, aqueous silver nitrate.
A peak in the UV-Vis absorption spectra of AgNPs-Fd at 430 nm with respect to 2.25 absorbance was observed, whereas the ethanol extract of the F. deltoidea leaf absorbed at 490 nm (Figure 3). This finding is similar to previous studies reporting that the UV-Vis maximum absorbance of AgNPs was 430 nm when mediated by the Pedalium murex leaf extract,31 Alternanthera dentata leaf extract32 and Tecomella undulata leaf extract.33
The morphology and size of the AgNPs-Fd were analyzed using TEM images. Figure 4 shows a TEM image of AgNPs biosynthesized from ethanolic extract of F. deltoidea leaves. The AgNPs-Fd shape was spherical in nature. The AgNPs-Fd were surrounded by a faint, thin layer of other substances, which were assumed to be organic material from the F. deltoidea extract. Few particles were agglomerated among the 20 nm nanoparticles that were produced. A recent study performed by Ref. 34 mentioned that the TEM results of the AgNPs mediated using Terminalia arjuna leaf extract have spherically-shaped nanoparticles approximately 10–50 nm in size.
The crystalline nature of the biosynthesized AgNPs-Fd was evaluated using XRD. The XRD pattern obtained revealed that AgNPs-Fd had a face-centered spherical structure in nature (Figure 5), and there were several characteristic diffraction peaks indexed from 21.386 to 76.655.
Biomolecules for the capping and effective stability of the metal nanoparticles produced using the ethanolic extract of F. deltoidea leaves were identified through FTIR analysis. Figure 6 presents the FTIR spectrum of AgNPs-Fd. The bands at 3,356, 3,200, and 3,243 cm-1 correspond to H-bonded alcohols and phenols with O-H stretching. The signals at 2,917 and 2,849 cm-1 correspond to carboxylic acids with an O-H stretch. The assignments in 1,711, 1,610, 1,516, and 1,449 cm-1 correspond to primary amines with a bent N-H. The peak at 1,376 cm-1 relates to C-N stretching of an aromatic amine group, and the band at 1,242 cm-1 pertains to a C-N bond, which is either an amine or an amide molecule.
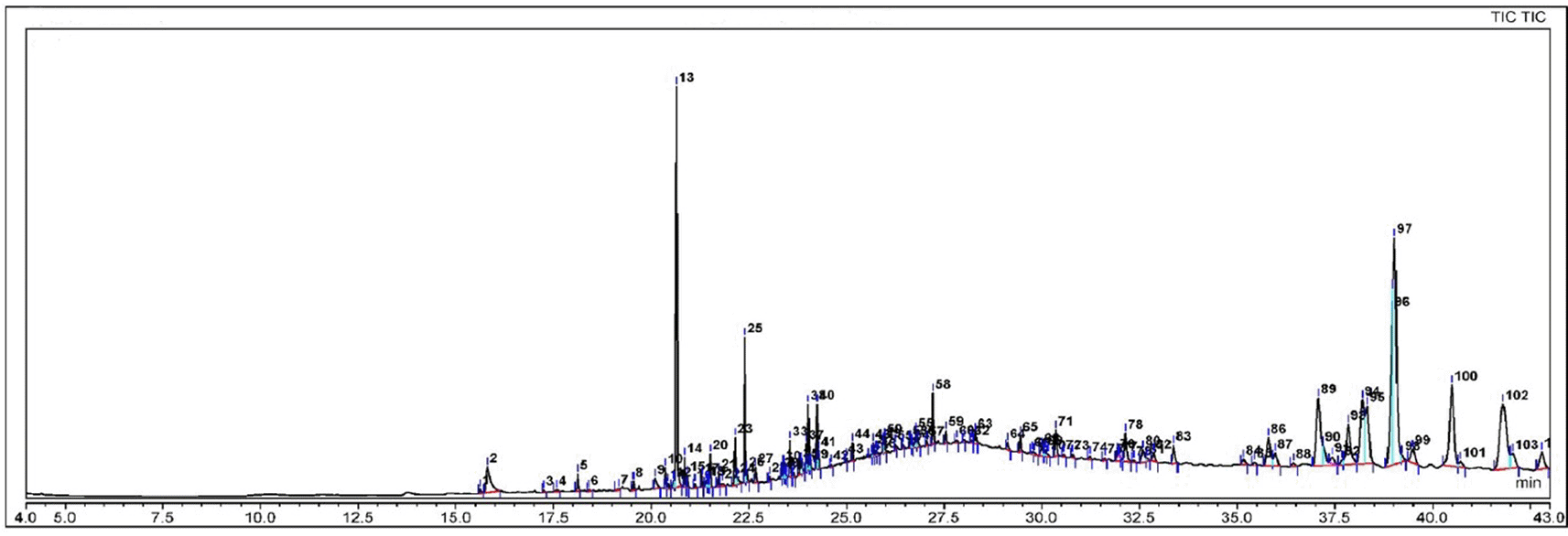
Phytosterols appear to suppress carcinogen generation, cancer cell development, angiogenesis, invasion, and metastasis, as well as promote apoptosis in malignant cells through a variety of methods. Phytosterol consumption potentially increases the activity of antioxidant enzymes, lowering oxidative stress.35 The present study found several phytosterols in the AgNPs-Fd biosynthesized using ethanolic extract of Ficus deltoidea leaves (Figure 7 and Table 1).
AgNPs-Fd, silver nanoparticles synthesized using Ficus deltoidea extract.
AgNPs-Fd, silver nanoparticles synthesized using Ficus deltoidea extract.
The CAM assay is commonly used as an in vivo testing system for anti-angiogenesis research. It has the advantage of being cost-efficient and lends itself to large-scale screening by testing the efficacy of an inhibitor using various stimulators alone or in combination with an anti-angiogenic medication. The present study used AgNPs-Fd as an anti-angiogenic compound and found that AgNPs-Fd at 90 μg in the CAM analysis resulted in higher anti-angiogenic activities than other treatment groups. The anti-angiogenic activities were demonstrated through the reduction of vessel area, total number of junctions, junction density, total vessel length, average vessel length and the total number of endpoints (Table 2).
The green synthesis of nanoparticles using biological systems, particularly plant extracts, is an emerging subject in nanotechnology. Various plant extracts and several methodologies have been investigated to explore the benefits of these nanomaterials. In the field of medicine, specifically angiogenesis, which is an essential physiological process and important in several pathological diseases, including tumor development and metastasis, the utilization of plant extracts is a low-cost and environmentally friendly method for producing nanoparticles. The present work aimed to investigate the anti-angiogenic properties of AgNPs biosynthesized using the ethanolic extract of F. deltoidea leaves on chick CAMs.
In the biosynthesis of the nanoparticles, the color change in is an indicator of AgNPs-Fd formation. Similar findings were revealed by Anju and Parvathy,36 who mentioned that the visual confirmation of AgNPs generation via green synthesis is possible by evaluating the color shift. The color shift is caused by the reduction of silver ions (Ag+) to AgNPs37 and is facilitated by bioactive phytochemicals in the F. deltoidea leaf extract, such as flavonoids.38 Flavonoids may be responsible for the rapid reduction and capping of Ag+ into AgNPs-Fd observed in this research. The flavonoids in F. deltoidea leaf extracts are potent reducing agents, suggesting that AgNPs-Fd may be synthesized by AgNO3 reduction. The flavonoid components of F. deltoidea ethanolic extract may actively be involved in and liable for Ag+ to silver (II) oxide (Ag0) reduction.39 Another investigation also found that water-soluble flavonoids were involved in the reduction of metal ions utilizing plant extracts.40
Meanwhile, the Tyndall effect was especially relevant to colloidal suspensions at room temperature, with the production of AgNPs-Fd exhibiting an immediate pale brown color. It also demonstrated that the AgNPs-Fd were strongly formed and stable in the aqueous phase, with no precipitation.41 Furthermore, previous reports on AgNPs revealed that absorbance at approximately 430 nm UV-Vis spectrophotometer is a hallmark of the noble metal particles.42 It was noticed that the surface plasmon resonance at 430 nm indicated the retention of AgNPs-Fd in the colloidal solutions due to the emergence of the pale brown color for particle stabilization.
Characterization of resulting AgNPs-Fd using TEM found few particles had a size of about 20 nm. A past study performed previously34 mentioned that the TEM results of the AgNPs mediated using Terminalia arjuna leaf extract have spherically shaped nanoparticles approximately 10–50 nm in size. In addition, the crystalline nature of the biosynthesized AgNPs-Fd detected using XRD revealed that AgNPs-Fd have a face-centered spherical structure in nature, and there were several characteristic diffraction peaks indexed from 21.386 to 76.655, which contained some functional groups.
Amines/proteins and metabolites comprising functional groups of alcohols, ketones, aldehydes and carboxylic acids surrounded the generated AgNPs-Fd. The carbonyl group of amino acid residues and proteins has the highest capacity to bind metal, suggesting that proteins may be able to encapsulate metal nanoparticles, including AgNPs, to avoid agglomeration and stabilize the medium.43 The present study identified an assortment of chemicals compounds of AgNPs-Fd that were detected through GC-MS analysis. From Figure 7 and Table 1, the GC-MS of AgNPs-Fd contains several bioactive compounds that have anti-angiogenic and anticancer activities, such as phytol (PHY), stigmasterol, lupeol and sitosterol.
Previous studies revealed that PHY, or 3,7,11,15-tetramethylhexadec-2-en-1-ol, has anticancer properties and a variety of pharmacological features, including toxicity and cytotoxicity. Furthermore, PHY modulates pro-carcinogens as well as produced genotoxicity and death in breast cancer cells. It has also demonstrated DNA damage repair capabilities in mouse lymphocytes.44 Additionally, PHY increases the activity of natural killer cells, which identify and eliminate cancer cells, and stimulates macrophage roles in immunity.45 Phytol exhibits anti-angiogenic properties by inducing apoptosis in cancer cells, such as lung adenocarcinoma cells.46
Meanwhile, stigmasterol and lupeol are potent compounds that exhibit anti-angiogenic properties. Stigmasterol and lupeol are two major phytosterols found in many herbal plants, with anti-inflammatory properties and have been proposed as anticancer agents. Though their mechanisms in inhibiting cancer are unclear, past studies report that cell survival, migration, and morphogenesis of human umbilical vein endothelial cells, but not cholangiocarcinoma cells, were inhibited by stigmasterol and lupeol. Both compounds lowered the transcript level of tumor necrosis factor-α considerably in expression studies.47
Furthermore, a growing body of research suggests that β-sitosterol (BSS) may have anti-angiogenic properties. BSS impedes synovial angiogenesis by inhibiting the proliferation and migration of endothelial cells.48 Current findings align with previous research performed by Nurhidayati et al.49 who used the ethanol extract of Ficus septica Burm. f. leaves, revealing that the number of new blood vessels had been decreased as the dose of the extract increased. The anti-angiogenic effects of AgNPs-Fd also involved the phytochemical contents in the nanomaterial, which was produced during the biosynthesis of the AgNPs.
Further, the application of 90 μg AgNPs-Fd in the CAM assay had similar effects of anti-angiogenic activities to the positive control (cortisone acetate 30 μg and 30 ng bFGF), which confirms its anti-angiogenic properties. Cortisone acetate acts as an inhibitor of angiogenesis in CAM chicken embryos. Cortisone acetate is a corticosteroid compound in the glucocorticoid class, which is typically used in the treatment of angiogenesis-related diseases such as diabetic retinopathy and solid tumors. Meanwhile, glucocorticoids also regulate angiogenesis through several mechanisms, namely suppressing the proliferation, migration and growth of endothelial cells and reducing the secretion or expression of cytokines.50,51
F. deltoidea leaves can be used as a reductor agent for the biosynthesis of AgNPs. Color change and Tyndall effects were an indicator of green synthesis of AgNPs-Fd. The surface plasmon resonance, which was identified at 430 nm, is the optimum peak of AgNPs-Fd. Several phytochemically active groups were identified in AgNPs-Fd using FTIR analysis, and it was observed that the morphology of the AgNPs-Fd had particle sizes of 20 nm, based on TEM characterization. The GC-MS analysis of the Fd leaf extract and AgNPs-Fd highlighted potential anti-angiogenic compounds, such as phytol, stigmasterol, lupeol and sitosterol. Fd or AgNPs-Fd treatments at 90 μg doses demonstrated significant anti-angiogenesis in CAM chicken embryo analysis, indicating greater effectiveness in controlling vessel formation. The AgNPs-Fd displayed anti-angiogenesis properties as indicated by the reduction of vessels in the CAM model. The anti-angiogenic properties of AgNPs-Fd in the CAM model were exhibited through the parameters of vessel area, total number of junctions, junction density, average vessel length and total number of end points. The 90 μg of AgNPs-Fd provided optimal anti-angiogenic effects. From a technical standpoint, the resulting AgNPs-Fd offer a simple technique to synthesize AgNPs. This method contains various benefits, such as cost-effectiveness, suitability for medical and pharmaceutical applications, and large-scale commercial manufacturing potential. The present research also suggested that AgNPs mediated by F. deltoidea leaf ethanolic extract have potential as anti-angiogenic agents.
Figshare: Raw data antiangiogenic 2022, https://doi.org/10.6084/m9.figshare.22256071. 52
This project contains the following underlying data:
Figshare: ARRIVE checklist for ‘Anti-angiogenic activity of Ficus deltoidea L. Jack silver nanoparticles using chorioallantoic membrane assay’, https://doi.org/10.6084/m9.figshare.22090673. 53
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
All authors thank Ministry of Education, Culture, Research and Technology, Indonesia for grant funding financial year: 2022. Our gratitude expressed to Department of Biology Faculty of Mathematics and Natural Sciences, Mulawarman University, Samarinda, East Kalimantan, Indonesia for all support.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Phytochemistry, natural products isolation
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 24 May 23 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)