Keywords
REMINERALISATION, NANO SILVER FLUORIDE, SODIUM FLUORIDE, INCIPIENT CARIES
This article is included in the Nanoscience & Nanotechnology gateway.
REMINERALISATION, NANO SILVER FLUORIDE, SODIUM FLUORIDE, INCIPIENT CARIES
The professional application of topical fluoride varnishes remains the most preferred method for treatment of demineralisation. Regularly available topical fluoride varnishes are sodium fluoride, stannous fluoride, acidulated phosphate fluoride.1 However, these remineralising agents have their shortcomings. Sodium fluoride varnish has to be applied four times at weekly intervals. Stannous fluoride varnish may cause brownish discoloration of the teeth and adjacent tissues and reversible tissue irritation. It has a sour taste and needs to be prepared freshly before use.2
Silver diamine fluoride (SDF) is an effective agent in remineralising initial carious lesions and arresting advanced caries. Annual application of SDF has shown successful results in caries prevention. However, staining caused by SDF on the tooth and adjacent structures is a significant drawback.3 A substituted silver-based preparation, nano silver fluoride (NSF), was developed by Targino AG et al. to overcome SDF’s limitations.4 The potency of NSF in arresting carious lesions is owed to the synergistic action of silver nanoparticles and fluoride. It is currently mulled over silver diamine fluoride to arrest caries without discoloring the permeable dental tissues dark.5
In recent times, the use of low pH fluoride delivery systems is on an upheaval. In the year 1988, Saxegaard et al. asserted that acidic pH improves the deposition of calcium fluoride.6 Cruz et al. in 1992 further studied this fact and emphasized on the crystalline change that occurs to enhance ion incorporation.7 Various studies comparing the remineralising potential of acidulated phosphate fluoride gel (APF) and sodium fluoride varnish suggested better results with APF gel.8,9 Olympio et al. suggested that low pH dentifrices increase fluoride concentration in saliva and delay its salivary clearance.10
Thus, this research was conducted to assess the remineralising potential of 5% Sodium fluoride varnish, neutral nano silver fluoride and acidulated nano silver fluoride in artificially induced enamel caries of primary teeth.
The in vitro study was carried out in the Department of Pediatric and Preventive Dentistry in the year 2020 after obtaining approval from the Institutional Ethical Committee (DMIMS/DU/IEC/2018-19/4799) on September 30th 2018. The sample size was calculated using 95% probability, showing a statistically significant difference using the alpha level and power 80% by using N master software. According to this software, the minimum sample size calculated was 12 for each group and total 36 samples were taken. The teeth included in the study were caries free primary anterior teeth without any cracks or defects near to exfoliation obtained from healthy children between 5 to 7 years of age. The teeth of children with any systemic condition and teeth with cracks, caries or any defect were excluded from the study. The parents were informed about the procedure and a written consent was obtained. The parents and patients were explained how the teeth will be extracted after application of topical anaesthetic agent and injecting local anaesthetic agent in the required region. The tooth was then extracted and bleeding was stopped by applying finger pressure to the socket using gauze piece. After giving post-operative instructions the patients were sent home. The extracted samples were stored in 0.1% thymol solution at 4°C and were utilised for the study within three months.
The primary anterior teeth were placed in 5.25% sodium hypochlorite for 1 minute for cleaning, and scaling using Woodpecker ultrasound scaler (UDS-J) on the surface for 2 minutes. The samples were stored in 0.1% thymol solution at 4°C for not more than three months. The teeth samples were sliced 2 mm below the cemento-enamel junction (CEJ) and mounted in self-cure acrylic resin, and polished using silicon carbide paper sequentially. The samples were then cleaned using distilled water for 20 seconds. An area of 5 × 5 mm was exposed on the buccal surface of all the samples and two coats of nail varnish were applied on the remaining portion of the teeth.
Five grams of nano silver fluoride powder was manufactured by wet chemical route synthesis at Nano Research Elements, Haryana, India. Nano silver fluoride varnish was prepared by dispersing 4.16% NSF in a colophony base. The pH was adjusted to 7 for the neutral varnish and 4 for the acidulated varnish using ascorbic acid. Further, both the varnish were separately stored in amber colored glass bottles at room temperature.
Demineralisation solution was prepared using 2.2 mM calcium chloride (CaCl2), 2.2 mM potassium dihydrogen phosphate (KH2PO4), 0.05 M acetic acid and 0.25 ppmF, and stirred on a magnetic stirrer. After which, 1M KOH was added to the solution for adjusting the pH at 4.5. The remineralising solution consisted of 20 mMol l−1 HEPES(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 130 mM potassium chloride (KCl),1.5 mM calcium chloride (CaCl2), 0.9 mM potassium dihydrogen phosphate (KH2PO4) and 1 mM sodium azide (NaN3). Potassium hydroxide (KOH) was used to adjust the pH at 7. Each sample was immersed in 30 ml of demineralisation solution for three days to induce incipient caries. Next, the samples were washed with distilled water for 20 seconds.
Primary surface microhardness (SMH) of the samples was tested by Vickers micro hardness testing machine (MITUTOYA, 810-401 D) under a load of 50 grams for 10 seconds, at 3 different sites. The mean surface microhardness value was calculated for each sample. The samples were washed with distilled water, dried and remineralising agents were applied on them as follows:
Group 1: Sodium fluoride varnish was dispensed on the paper pad and applied on the exposed enamel surface unidirectionally using an applicator brush.
Group 2: Two drops of neutral nano silver fluoride varnish applied on the exposed enamel surface unidirectionally using an applicator brush.
Group 3: Two drops of acidulated nano silver fluoride varnish applied on the exposed enamel surface unidirectionally using an applicator brush.
Simulation of the oral condition was accomplished by following a 7 day pH cycling protocol.11 Samples were immersed in remineralising solution for 21 hours and then in the demineralising solution for 3 hours. This cycle was repeated for 7 days. The solutions were changed on every third day. The samples were washed with distilled water and subjected to post treatment SMH testing, same as before.
One sample representative of each group was prepared to carry out Scanning electron microscopy and Energy Dispersive Spectroscopy (SEM EDS).
Scanning electron microscope (JEOL JSM-6380A) was used to examine the surface changes after 7 days of remineralising agent application. The samples were coated with platinum sputter in the Auto fine coater (JEOL JFC-1600) for better visualization. The samples were scanned at 50×, 500× and 1000× magnification and photomicrographs were taken.
Energy-dispersive X-ray spectroscopy (JEOL JSM-6380A) was used to analyse the elemental concentration in the teeth samples. The concentrations of silver, fluoride, calcium and phosphorus ions were assessed in the sample after application of the remineralising agents. The proportion of the individual concentration of elements present on the enamel surface were measured in weight percentage.
Descriptive and inferential statistics were to be obtained from the data. Student’s paired t-test was used to compare the mean surface microhardness values before and after application of each test materials. Comparison of difference in mean surface microhardness values after application of 5% Sodium fluoride varnish, neutral nano silver fluoride and acidulated nano silver fluoride using one way ANOVA. Whereas, comparison of difference in the p values of mean surface microhardness values after application using 5% Sodium fluoride varnish, neutral nano silver fluoride and acidulated nano silver fluoride using HSD Tukey test. Software used for the analysis were SPSS 24.0 and GraphPad Prism 7.0 version, and P<0.05 is considered as level of significance.
There was a significant difference (HSD Tukey) in all the groups after demineralisation and after remineralisation, showing that all the agents increased the surface microhardness values (Table 1).35–37
The effectiveness of all the remineralisaing agents differed (One Way ANOVA). The highest increase in the surface microhardness was observed in group 3 followed by group 2 and group 1. The mean difference between group 1 and group 2 was statistically insignificant. Whereas, the mean difference between group 1 and group 3 and also, group 2 and group 3 was statistically significant (P<0.01) (Table 2).
The elemental composition of enamel surface after application of 5% sodium fluoride varnish, neutral nano silver fluoride and acidulated nano silver fluoride in Wt% is suggestive of increase in the concentration of fluoride in all the three samples and presence of silver in group 2 and 3 (Table 3).
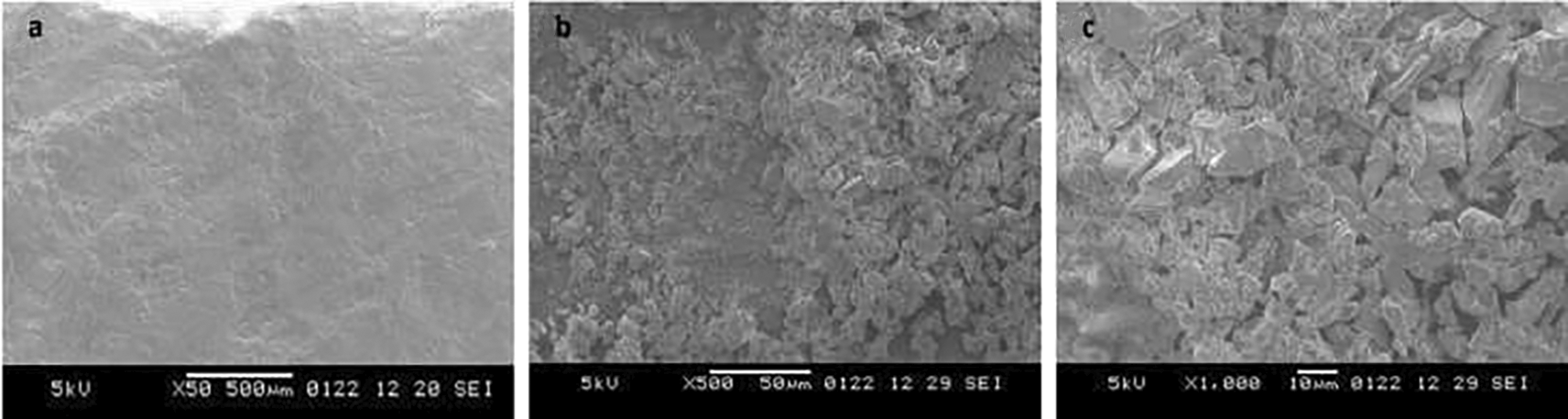
Sodium fluoride (NaF) varnish is the most widely used topical fluoride agent containing 22,600ppm fluoride.12 On application, NaF varnish reacts with hydroxyapatite crystals and deposits a layer of calcium fluoride over the HAP lattice and its choking off phenomenon provides a sustained fluoride release.13,14 Sodium fluoride varnishes are easy to apply and have moisture tolerance.15 According to a systematic review, 5% sodium fluoride varnish demonstrated acceptable remineralising ability and is considered as the gold standard.16
Under the broad umbrella of nanotechnology, silver nanoparticles have emerged as a promising tool and this is attributed to two major properties; quantum size effect which is elicited because of the small size, providing a larger surface area for interactions and quantum tunnelling effect which depicts the ability of the particle to cross any barrier to cause membrane disintegration and show antibacterial property.17 Augmentation of broad range of interaction and molecular reactions, modify the physical properties of materials by re-organization of the particles.18,19 The positive results of AgNPs encouraged its incorporation in various dental materials for diagnostic and therapeutic purposes.20
Recent studies have emphasized the synergistic action of silver nanoparticles with high fluoride concentration in prevention of spread of dental caries.21,22 A new compound, nano silver fluoride was introduced by Targino AG. et al. for anticariogenic action.4 This new agent has a high safety index and amalgamates the antibacterial properties of silver and remineralising efficacy of fluoride.
In the present study, the remineralising efficacy of 5% sodium fluoride varnish was evaluated before and after varnish application. Baseline value was not assessed to avoid damage to the samples and mean ranging from 320 to 350 VHN mentioned in literature was considered.23 The results depicted a significant increase in the surface microhardness of carious lesion (Table 1) The remineralisation of samples can be attributed to the formation of fluorapatite which has a low solubility and increases the precipitation of calcium and phosphate ions on the teeth surface as mentioned by Shen C. et al.24 and Vicente A. et al.25
Two indigenously prepared varnishes of nano silver fluoride in neutral and acidulated forms were used in the current study at a concentration of 4.16% conforming its minimum inhibition concentration and minimum bactericidal concentration.26,27 Neutral nano silver fluoride group showed a statistically significant difference in the remineralising efficacy before and after its application (Table 1). The increase in surface microhardness of the carious lesion can be attributed to the deposition of fluoride and silver nano particles, which precipitated into the demineralised lesion, and is in congruence with the study conducted by Nozari A et al.23 According to Zhi QH et al., silver fluoride promotes remineralisation by getting incorporated into the crystalline lattice. It also suggests increased calcium fluoride formation when more than 100 ppm of fluoride is present in the delivery system, which tilts the demineralising curve towards remineralisation. This favours the results of the present study.
On comparing the remineralising efficacy of neutral NSF and 5% sodium fluoride, it was observed that the mean microhardness of samples treated with neutral NSF was more but the difference was statistically insignificant (P=0.356674). These results are in equivalence with the studies carried out by Silva A. et al.26 and Nozari A. et al.23 These studies stated that as silver nanoparticles are inherently stable, they penetrate deep into the demineralized structures without interfering with the action of fluoride. In a study conducted Burns J. et al., NSF was applied showed improved efficacy than water.6 The results of a trial conducted by Tirupathi S. et al. concluded that 5% NSF showed better efficacy in caries reduction as compared to 38% SDF when applied annually due to the higher concentration of fluoride used in NSF group.5
In our study, statistically significant increase was obtained in the mean value of the surface microhardness of enamel caries after applying acidulated NSF. Under the influence of acid, hydroxyapatite released calcium, aiding the formation of calcium fluoride. Alongside this, the nano size of the particles improved the penetration of NSF into the enamel as suggested by Alves K. et al.27
A comparison between 5% Sodium fluoride and acidulated NSF was revealed a statistically significant difference (P=0.001005) with 188.46 VHN in 5% Sodium fluoride group and 202.66 VHN in acidulated nano silver fluoride group, in our study. However, a study comparing the remineralising potential of acidic NSF and NaF claimed better results after application of NaF varnish. The difference in the results can be attributed to chitosan coating over the NSF particles, which precluded the ionic bonding between NSF and hydroxyapatite.28 In the present study, NSF was produced by wet chemical route synthesis without chitosan. Another reason making a difference in the results, can be the amount of fluoride used in the formulation. Akylidiz M. et al. used 10,147 ppm of fluoride28 whereas, in our study 1,45,000 ppm of fluoride is NSF was used, which improved the efficacy of the experimental formulations.
A comparison between neutral NSF and acidulated NSF was also carried out in the present study. The mean surface microhardness of acidulated NSF statistically more (P=0.002056) than neutral NSF with 194.26 VHN. Similarly, in a study conducted Lee Y. et al. neutral sodium fluoride and acidulated phosphate fluoride was evaluated and acidulated phosphate fluoride showed better efficacy. This study’s results are owed to the fact that acidic pH etched the surface of the teeth and assisted deeper incorporation of fluoride ions. Although the fluoride concentration was less in APF gel than neutral sodium fluoride, it showed better results.29 Thus, the acidulated formulations of topical fluorides augment the fluoridation of teeth.6
Researchers have attempted to decode the mechanism by which nano silver fluoride acts on the dental tissues and have suggested that caries prevention occurs by the virtue compound supplementary action of silver and fluoride.30 Literature suggests that there is precipitation of silver ions on the tooth surface, increasing the microhardness.31 Noronha et al. proposed a hypothesis that silver nanoparticles in NSF have inherent ionic stability, which prevents it from interfering with the action of fluoride.32 A striking revelation has been made by Zhi QH et al. regarding the mechanism of NSF in remineralisation, which suggests the formation of silver apatite. On application of silver fluoride, there may be precipitation of silver salts that decrease the permeability and aids in calcification of the teeth.21
A study conducted by Zhao et al. mentions that the interaction between the hydroxyapatite lattice and the fluoride compounds used depends on fluoride concentration. A high concentration of fluoride leads to the formation of calcium fluoride, whereas at lesser concentration, fluorapatite is formed.33 According to this, presence of calcium fluoride can be anticipated after NSF application.
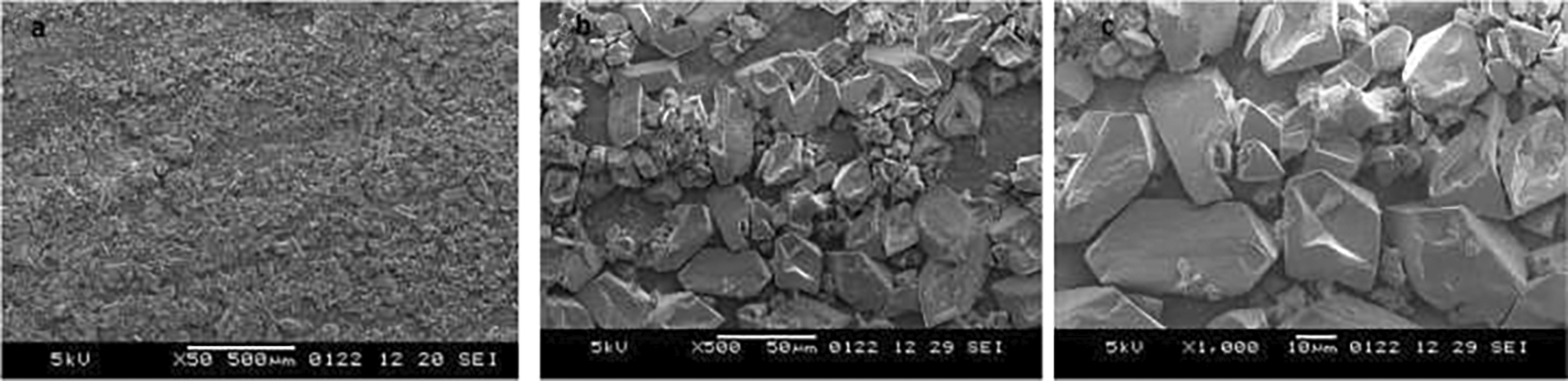
The positive results of the surface microhardness assessment encouraged us to investigate their clinical applicability further. A qualitative correlation of the results was carried out by analysing the samples under scanning electron microscope (SEM). The 5% sodium fluoride sample depicts a fine granular layer of precipitated minerals. Some amount of the deposits is retained, but cracks are still visible, representing a partially mineralized structure (Figure 1). The sample treated with neutral nano silver fluoride illustrates irregular polyhedral deposits with a fine layer of precipitated minerals (Figure 2). The application of acidulated nano silver fluoride shows presence of polyhedral deposits in abundance, depicting a mineral phase precipitation without any debris, owed to the acidic pH (Figure 3). An extra sample was prepared to obtain SEM image on the 3rd day after application of acidulated nano silver fluoride for evaluating initial remineralisation (Figure 4). The image depicts polyhedral deposits of mineral phase with a flower-field like appearance depicting initial remineralisation phase, as suggested by Gjorgievska et al.34

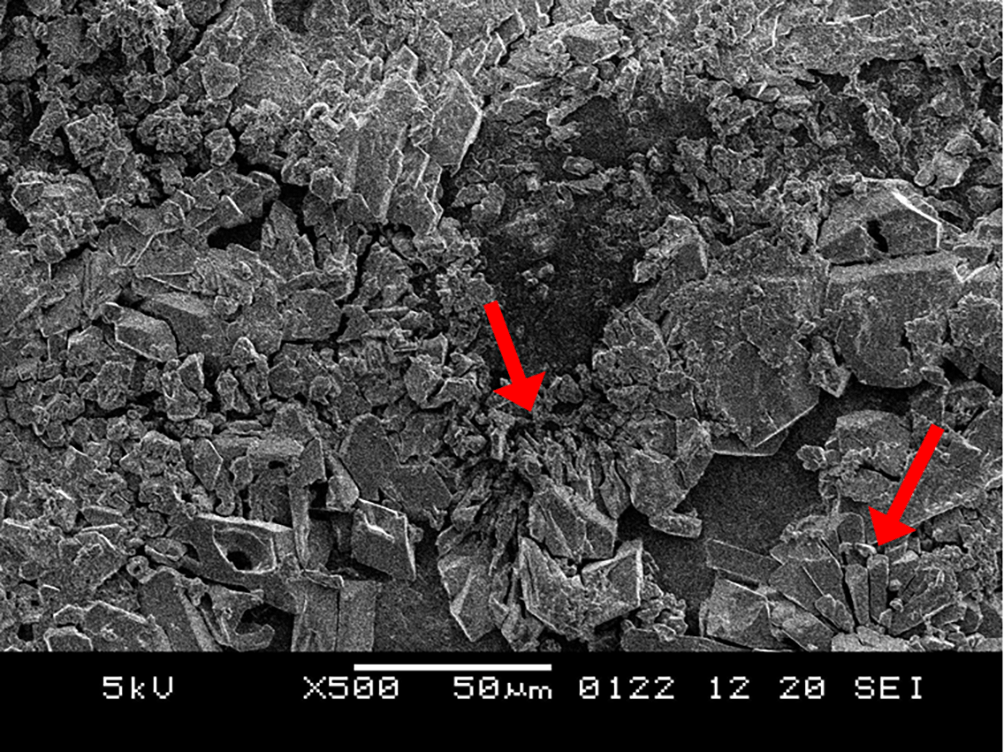
The sample displays flower-field appearance in few regions. This represents an initial phase of remineralisation.
The present study’s Energy dispersive X-ray spectrophotometry (EDS) analysis detected fluoride concentration of 5.45 wt% in the 5% Sodium fluoride group without any silver content. The neutral nano silver fluoride group showed 14.64% of fluoride and 13.57% of silver. The acidulated nano silver fluoride group values were approximately in the same range as neutral nano silver fluoride with 14.94% of fluoride and 16% of silver. These fluoride and silver values on the teeth surface confirm the deposition of minerals on the tooth surface, initiating the process of remineralisation.
The results of the study prove the superiority of acidulated nano silver fluoride followed by neutral nano silver fluoride and 5% sodium fluoride in remineralisation of enamel caries in primary teeth.
All the remineralising agents were capable to remineralise the enamel of primary teeth. However, acidulated nano silver fluoride varnish depicted more efficacy than others; whereas, 5% sodium fluoride and neutral nano silver fluoride varnish had close results.
Figshare: IMPACT OF SODIUM FLUORIDE AND NANO SILVER FLUORIDE-BASED VARNISHES ON REMINERALISATION OF ENAMEL CARIES: AN IN-VITRO STUDY. https://doi.org/10.6084/m9.figshare.22800479.v1. 35
This project contains the following underlying data:
Figshare: IMPACT OF SODIUM FLUORIDE AND NANO SILVER FLUORIDE-BASED VARNISHES ON REMINERALISATION OF ENAMEL CARIES: AN IN-VITRO STUDY. https://doi.org/10.6084/m9.figshare.22592200.v1. 36
This project contains:
Figshare: IMPACT OF SODIUM FLUORIDE AND NANO SILVER FLUORIDE-BASED VARNISHES ON REMINERALISATION OF ENAMEL CARIES: AN IN-VITRO STUDY. https://doi.org/10.6084/m9.figshare.22827947.v1. 37
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 2 (revision) 27 Jul 23 |
read |
|
Version 1 12 Jun 23 |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)