Keywords
Cannabis, CBD, Cannabidiol, Anti-inflammatory, Immunosuppressant
This article is included in the Cell & Molecular Biology gateway.
Cannabis, CBD, Cannabidiol, Anti-inflammatory, Immunosuppressant
Cannabis sativa (Cannabaceae) has been one of the most used herbaceous plants in traditional medicine for decades. There are many bioactive compounds in this plant: around 750 compounds have been identified, such as cannabinoids, flavonoids, terpenoids, alkaloids, among others.1 Among its bioactive compounds, cannabinoids are the main pharmacological phytocannabinoid compounds and include cannabidiol (CBD), cannabinol (CBN), Δ9-tetrahydrocannabinol (Δ9-THC), and Δ8-tetrahydrocannabinol (Δ8-THC). These compounds have been studied in cannabinoid-based medicine. CBD and THC interact in the endocannabinoid system (ECS), but they have different effects. THC compound has psychoactive properties, mediated by activating cannabinoid receptor type 1 (CB1).2 Unlike THC, CBD, a non-intoxicating cannabinoid, has pharmacological anti-inflammatory properties, and lacks psychoactive characteristics because of the low binding affinity of cannabinoid receptors.3 Therefore, CBD exhibits more beneficial properties than THC. Due to the complex mechanism action of CBD, pharmacological studies are required for use in clinical practice. CBD has been studied in animal models for anti-inflammatory effects such as reduced inflammation in arthritis and paw edema.4 Anti-inflammatory properties can be found in CBD, as well as antioxidant activity.5 The pharmacodynamics of CBD probably involve the interaction with host molecular molecules, including PPARγ, GPR3/6/12/18/55, TRPV1/2, TRPA1, 5-hydroxytryptamine receptor, and mitochondrial proteins.6–9 A report has confirmed that CBD is able to reduce arthritis by raising intracellular calcium levels, reducing cell viability, and producing IL-6/IL-8/MMP-3 rheumatoid arthritis synovial fibroblasts.10 In addition, CBD can also minimize inflammation in carrageenan-induced paw inflammation in rat models. Costa et al. demonstrated that CBD could be used for the reduction of paw edema and inflammatory biomarkers levels. However, the study still lacks information on pro-inflammatory cytokine levels.11 Regarding pharmacology dosing titration, CBD is well tolerated even when given in high doses-concentration in the long-term, according to clinical studies.12,13 Recently, a research group also provided evidence that CBD allowed improvements in pain physical function and sleep quality.14 However, no one reported a study of systemic immune changes in an acute inflammatory response during short-term oral CBD administration in an animal model. Conversely, CBD use in animal species is an area of growing interest, especially for its anti-inflammatory and immune modulation effects, even though all its biological effects are still not fully understood.15
In the present study, we therefore, studied an acute inflammation response by monitoring information regarding the degree of edema, pathophysiology, prostaglandin E2 (PGE2), serotonin, cyclooxygenase (COX) 1 and 2 activities, chemokines, and cytokines at baseline and during 6 hours of various oral CBD administrating doses (5, 10, 20, and 40 mg/kg) in a carrageenan-induced edema rat model.
A total of 48 male Sprague Dawley rats (300-320 g, Nomura Siam International, Thailand) were used. The animals were housed in the Laboratory Animal Canter Thammasat University under standard conditions of strict hygienic conventional temperature (22±1°C), relative humidity (30-70%), light (130-325 Lux), and a light/dark cycle (12 h/12 h). Rats were kept under laboratory conditions for one week prior to the experiments. The protocols and methods used in this study were approved by the Institutional Animal Care and Use Committee of Thammasat University, Thailand (Protocol Approval No. 010/2021).
We complied with the standard practices and guidelines to treat rats respectfully and compassionately. After six hours post-carrageenan injection, rats were sacrificed with isoflurane and confirmed with cardiac puncture. All tissues and blood samples were collected after being sacrificed with isoflurane by veterinarian staff at Animal Laboratory Animal Center, Thammasat University, Thailand. The center employs trained veterinarians knowledgeable about animal behavior and welfare to ensure the sacrifice is conducted efficiently and with minimal stress to the rat.
Cannabidiol powder was purchased from Suranaree University of Technology, Thailand, and was dissolved in sunflower oil. Lambda-carrageenan, hematoxylin and eosin (H&E) staining solution, absolute ethanol, acetic acid, and ethylenediaminetetraacetic acid were purchased from Sigma-Aldrich, Milano, Italy. Diclofenac and formaldehyde were purchased from Tokyo Chemical Industry, Tokyo, Japan.
Carrageenan-induced edema was conducted using the procedure by Morris (2003).16 The original protocol of Morris (2003) used a mouse animal model, and indomethacin (Non-steroidal anti-inflammatory drugs; NSAIDs) was used as a positive control in the experiment. However, we modified the Morris (2003) protocol to use a rat animal model and diclofenac (Non-steroidal anti-inflammatory drugs; NSAIDs) as a positive control. Rats were orally administered with either olive oil placebo (olive oil) (10 mg/kg), diclofenac (10 mg/kg), or CBD (5, 10, 20, and 40 mg/kg). Paw edema was induced by injection of 0.1 mL carrageenan (1% w/v in saline) into the plantar of the right hind paw. Paw edema was measured using a plethysmometer (Ugo Basile, Varese, Italy). After carrageenan injection, the volume of paws was recorded at 0, 1, 2, 3, 4, 5, and 6 h. Paw volume was expressed as the difference in volume between before and after carrageenan induces inflammation.
After six hours post-carrageenan injection, rats were sacrificed with isoflurane and confirmed with cardiac puncture. The right hind paw of each rat was cut and fixed in a 10% neutral buffered formalin solution for 12 h. Each sample was decalcified with 10% EDTA solution, dehydrated with ethanol, and embedded in paraffin. Each sample was cut into 2 μm thick sections and stained with H&E. A senior pathologist performed the histologic analysis with a light microscope.
The degree of inflammation was assessed by choosing the area of maximal infiltration with inflammatory cells in each case and grading the density of inflammatory cells into three tier grades 1, 2, and 3. The criteria for grading inflammation started from grade 1, showing sparse inflammatory cells. In contrast, grade 3 shows distinct high cellularity of inflammatory cells, and in grade 2, the number of inflammatory cells lies between these two grades. The assessment was performed blindly without knowing the information regarding each specimen.
Plasma samples were used for serotonin, prostaglandin E2, chemokine, and cytokine assays, whereas tissue samples were used for cyclooxygenase activity assay. Samples were collected after rats were euthanized using isoflurane. Blood samples (6 mL) were collected in EDTA K2/gel tubes (BD Vacutainer BD Diagnostic, Milan, Italy) via cardiac puncture. Plasma samples were collected by centrifugation for 5 min at 10,000 revolutions per minute. The rats’ plasma samples were stored at -20°C until used. For tissue samples preparation, samples were prepared according to the COX activity assay kit instructions. Briefly, the claws of rat paws were cut and then washed three times with phosphate-buffered saline buffer (PBS). Tissue samples were ground in liquid nitrogen and then homogenized in a lysis buffer with protease inhibitors using a pestle. The supernatant was collected after centrifugation at 12,000×g for 3 min at 4°C.
Serotonin levels in serum samples were measured by a competitive enzyme-linked immunosorbent assay (ELISA) using a commercial test kit (Abcam, UK #ab133053) following the manufacturer’s instruction. Briefly, serum samples or serotonin standards were prepared and mixed into an alkaline phosphatase (AP)-conjugated serotonin solution. A specific antibody to serotonin was then added to each well of the 96-well plate, which was pre-coated with goat anti-rabbit antibody. A mixture solution was incubated at room temperature (RT) for two hours with plate shaking before washing three times with washing buffer. A substrate solution for AP (n p-nitrophenyl phosphate disodium salt; pNpp) was added and incubated for one hour. Followed by a stop reaction step by adding a stop solution before measuring absorbance at 405 nm using a microplate reader (SpectraMax M5 microplate reader, Molecular device). The concentrations of serotonin in samples were calculated from the standard curve.
Prostaglandin E2 in serum samples was tested using a competitive ELISA kit (Abcam, UK, #ab133021). Briefly, samples or standards were mixed with alkaline phosphatase-conjugated PGE2 in each well of the 96-well plate. A specific antibody to PGE2 was added and incubated at RT for two hours with shaking. Then, the plate was washed with washing solution three times. An enzyme (pNpp substrate) was added and incubated for 45 min before stopping the reaction with the kit’s stop solution reagent. Absorbance at 405 nm was measured using a microplate reader. The concentrations of PGE2 in samples were calculated from the standard curve.
To understand an immune response after carrageenan-induced edema, serum 14 cytokines (namely G-CDF/CSF-3, GM-CSF, IFN-gamma, IL-10, IL-12p70, IL-13, IL-17A, IL-1alpha, IL-1beta, IL-2, IL-4, IL-5, IL-6, and TNF-alpha) and eight chemokines (namely Eotaxin, Gro-alpha/KC, IP-10, MCP-1, MCP-3, MIP-1alpha, MIP-2, and RANTES) were measured using the commercial bead array assay (ThermoFisher Scientific, #EPX220-30122-901). Samples were prepared and tested according to manual instructions. Briefly, samples or standards were added to each well of the 96-well plate. Then, a mixture of antibodies-coated beads was added and incubated at RT for two hours. After incubation, the unbound beads were removed, and beads were washed twice with a washing buffer. Next, a rat anti-cytokine/chemokine detecting antibodies cocktail was added. The reaction was incubated at RT for one hour. The washing was repeated before phycoerythrin-conjugated streptavidin was added and incubated for 30 min. Then, beads were washed twice before readout the signal using a MAGPIX detector (Luminex, Austin, TX).
The cyclooxygenase (COX) activity in tissue serum samples was quantitated using a commercial test kit (Abcam, UK, #ab204699). Samples were tested following the manufacturer’s instructions. Briefly, samples were mixed with a COX probe and COX factor. Then, the mixture solution was added to celecoxib or SC560 solution to measure COX-1 or COX-2 activity, respectively. After that, the arachidonic acid solution was added to each mixture solution and immediately measured fluorescence (Excitation at 535 nm and Emission at 587 nm) in a kinetic mode (15-s interval, 30 min). The protein concentration of each sample was measured by a Bradford protein assay method. The amount of resorufin in samples was calculated from the standard curve. The COX reactivities were calculated from the amount of resorufin per reaction time and the amount of protein in the sample. The COX reactivity was reported in pmol/min/mg or μU/mg.
Each group was constituted of at least eight rats. All results were expressed as mean ± standard deviation (SD) or standard error of the mean (SEM) using Graphpad Prism version 9 (Graphpad Software, San Diego, CA). The experiments were compared using analysis of variance (ANOVA) and Tukey’s post hoc test for multiple comparisons. The correlation between data sets was analyzed using the Spearman r-test and plotted by simple linear regression. The Mann-Whitney U test used analysis of the variance. A p-value of < 0.05 was considered to indicate a statistically significant difference.
The carrageenan-induced paw edema is the most common method for evaluating anti-inflammatory activity, as previously shown in a rat model study of acute inflammation.17 The inflammation was induced by localized paw edema with carrageenan that immediately increased paw edema and severity up to 6 h after carrageen injection. After the carrageen injection, all rats were orally administered a single different dose and evaluated paw volume. All doses of oral Diclofenac (10 mg/kg) and CBD (5, 10, 20, and 40 mg/kg) significantly decreased paw edema compared with placebo (10 mg/kg) at two, three, four, and five hours after induced paw edema (Figure 1, panel A). CBD doses at 10 and 20 mg/kg significantly decreased paw edema compared with diclofenac (p < 0.01; p < 0.01) and CBD doses at 5 mg/kg (p < 0.05; p < 0.01) at two hours, respectively. Moreover, the highest dose of CBD (40 mg/kg) was greater and decreased paw edema more than the dose of 5 mg/kg at two, three, and four hours (p < 0.01; p < 0.05; p < 0.05), respectively. The anti-edema effect of CBD and diclofenac remained until five hours, while treatments did not significantly decrease paw edema at six hours (Figure 1, panel A). In addition, we found that the anti-inflammatory effect of 20 mg/kg for oral CBD five hours after the induced paw edema was correlated with the efficacy of diclofenac (r = 0.753, p < 0.005: Figure 2). Paw edema figures at six hours after the carrageenan induction in different doses and controls were shown in Figure 1, panel B.
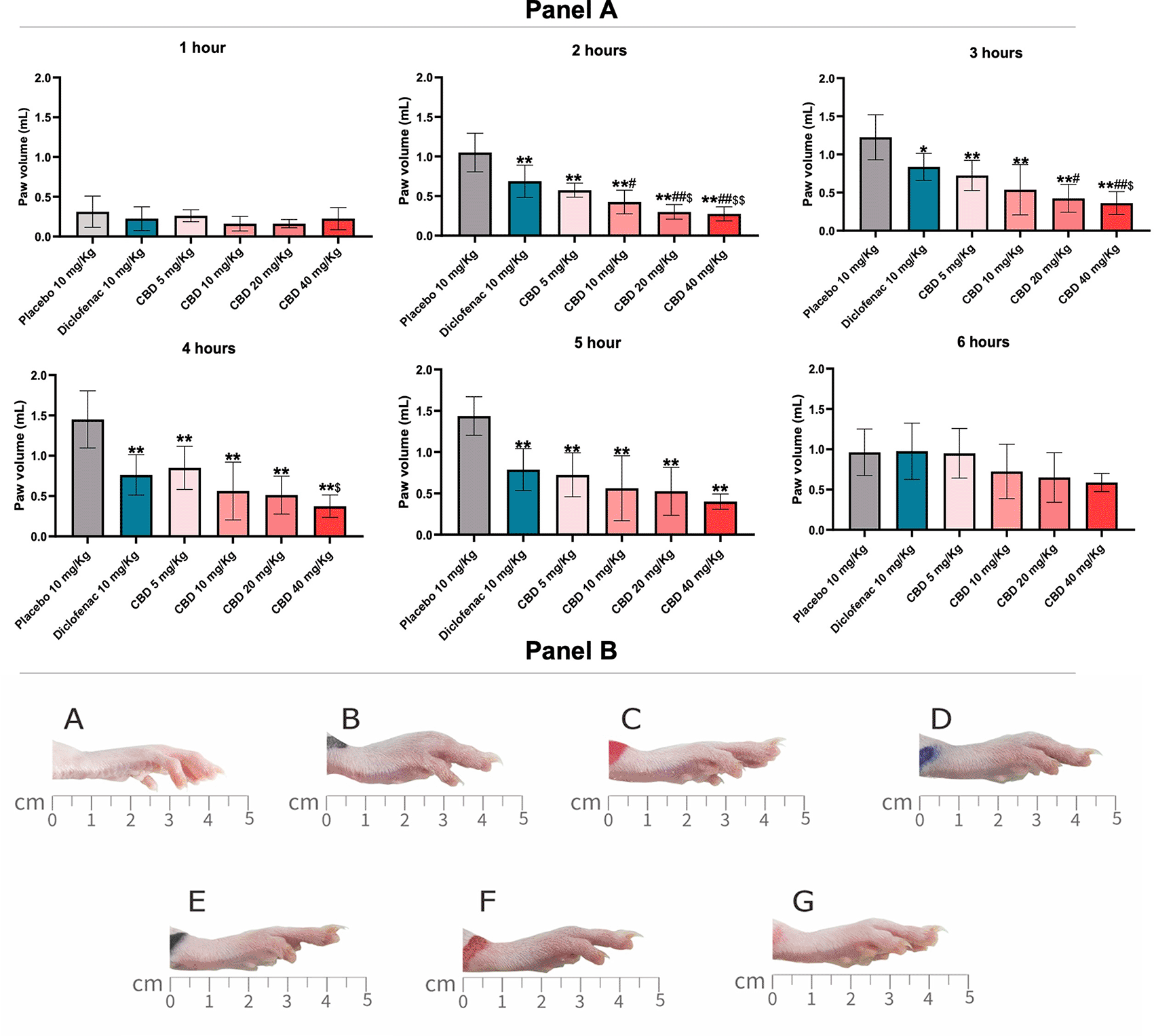
Panel A: Paw edema was measured with a plethysmometer and evaluated at 1, 2, 3, 4, 5, and 6 h after induced paw edema. Each bar represents the mean±SD and values obtained from eight animals per group. (one-way ANOVA followed by Tukey’s post-test). *p < 0.05 vs. control, **p < 0.01 vs. control, # p < 0.05 vs. diclofenac 10 mg/kg, ##p < 0.01 vs. diclofenac 10 mg/kg, $ p < 0.05 vs. CBD 5 mg/kg, and $$ p < 0.05 vs. CBD 5 mg/kg for all does of CBD. Panel B: The effect on paw edema six hours after carrageenan-induced paw edema. A is before inducing paw edema (baseline). Rats were treated with a Placebo of 10 mg/kg (B); Diclofenac 10 mg/kg (C); CBD 5 mg/kg (D); CBD 10 mg/kg (E); CBD 20 mg/kg (F), and CBD 40 mg/kg (G).
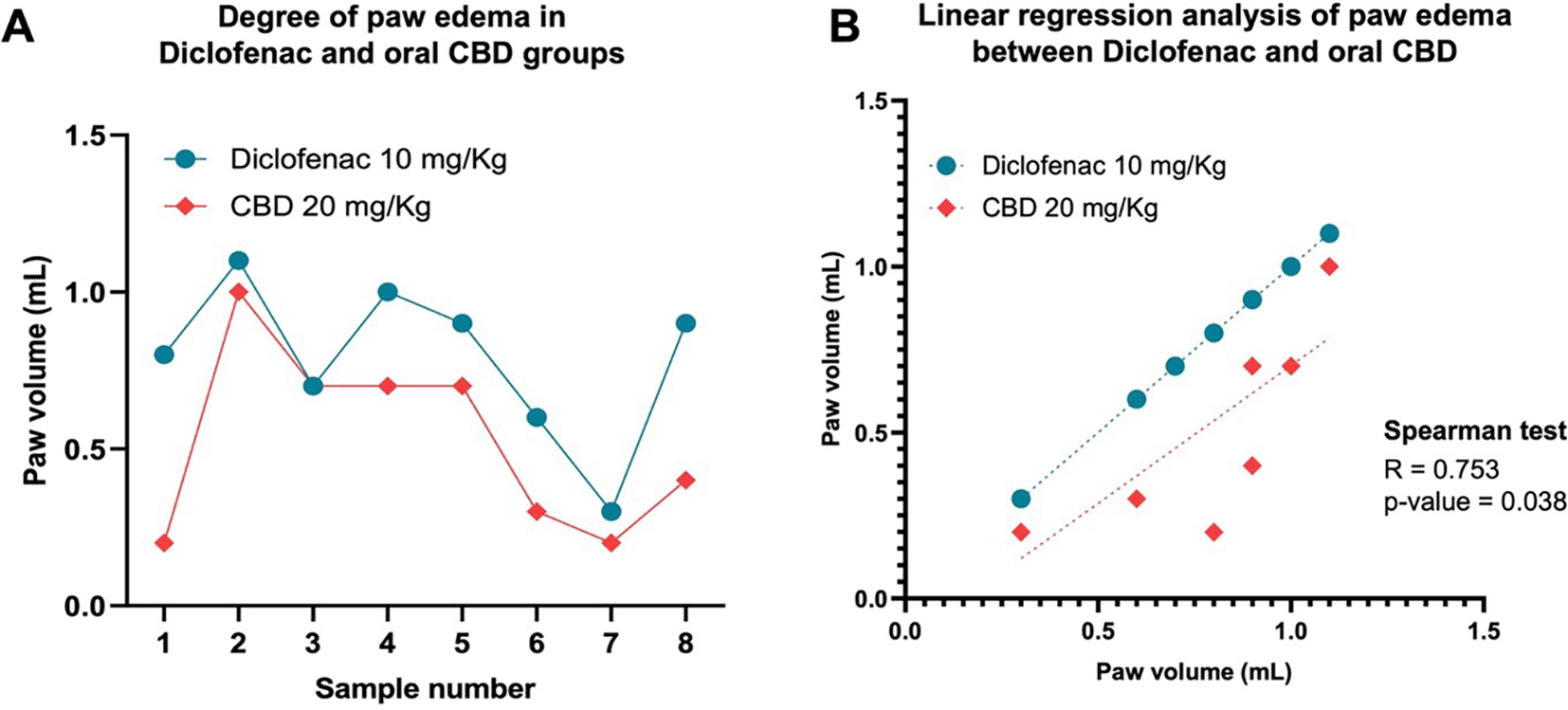
The degree of paw edema between both treatments is shown in each rat (A). Moreover, the correlation between both treatments is provided by the Spearman r- test and plotted by simple linear regression analysis (B).
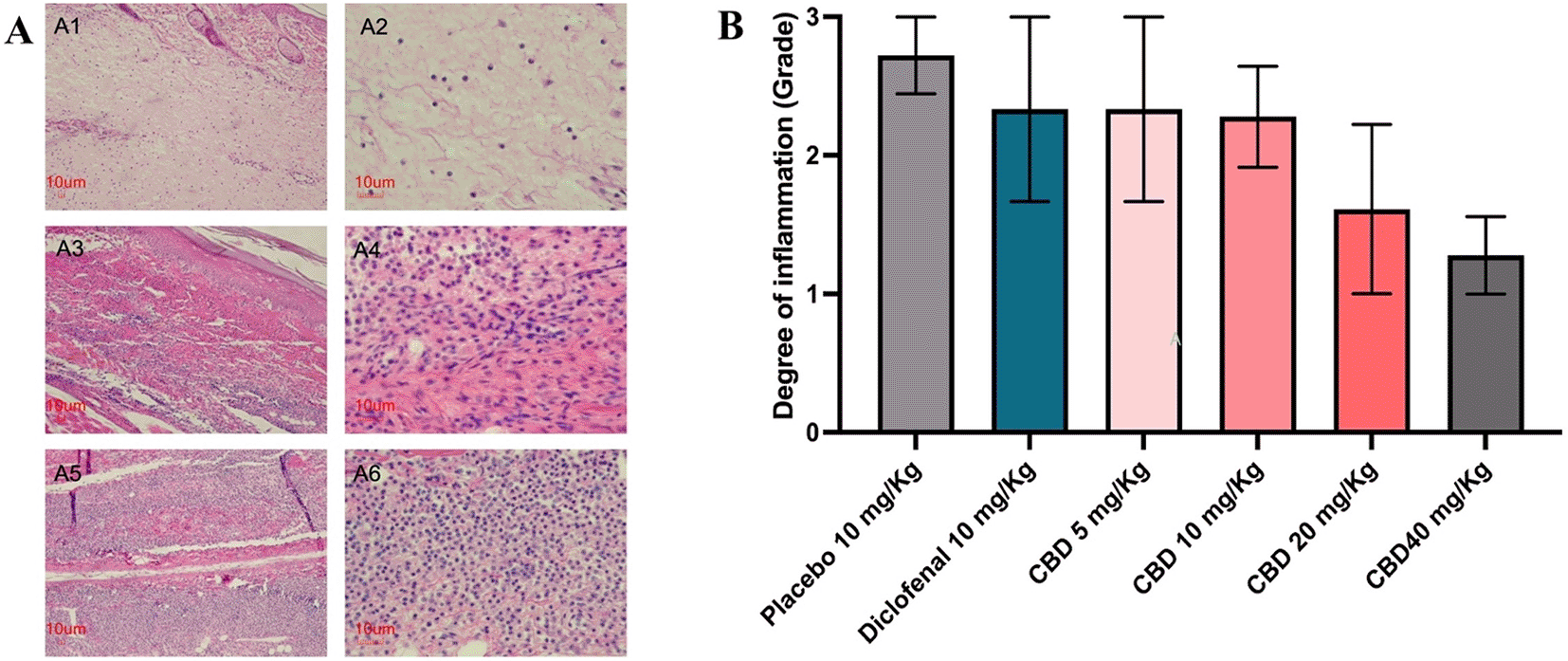
Histological examination consisted in an assessment of the hind paw tissues and revealed that sub-plantar injection of carrageenan. The site of carrageenan injection showed edema, congestion of vessels, and infiltration of inflammatory cells, such as neutrophils and lymphocytes, into the site of inflammation. The degree of inflammation graded the density of inflammatory cells into three tiers (Figure 3A). The degree of inflammation shows that all the treatments had a lower degree of inflammation than the placebo group. The degree of inflammation was highest in the placebo group (grading between 2 and 3) but also the lowest in the oral CBD (40 mg/kg) group (grading between 1 to 2). While diclofenac and oral CBD (5 and 10 mg/kg) groups had similar degrees of inflammation (grading between 2 to 3). The result of the histopathological study was in accordance with the paw edema results at six after carrageenan injection (Figure 3B).
The COX enzymes, a catalyzer for converting arachidonic acid to prostaglandin, have two isoforms: COX-1 and COX-2. The enzymes also are a common target for anti-inflammatory drugs such as nonsteroidal anti-inflammatory drugs (NSAIDs).18 To determine COX activity, after six hours of the carrageenan induction, tissue samples from oral CBD (40 mg/kg) and control groups were collected, and COX-1 and COX-2 levels were determined by the ELISA method. The results showed that COX-1 (Figure 4A) and COX-2 (Figure 4B) activity were not significantly different among placebo, diclofenac, and the treated-CBD groups. The COX-1 and COX-2 activity results agreed with the level of PGE2 (Figure 4C). Serotonin, a pro-inflammatory-like neurotransmitter,19 in serum isolated from the same group of samples and controls was also measured after six hours of carrageenan injection. Serotonin levels in the diclofenac and oral CBD (40 mg/kg) groups were significantly lower than the placebo (p < 0.01; p < 0.001), respectively. Moreover, the results between the diclofenac and oral CBD groups were not different, indicating that the anti-inflammatory efficacy of CBD is probably equal to that of diclofenac (Figure 4D).
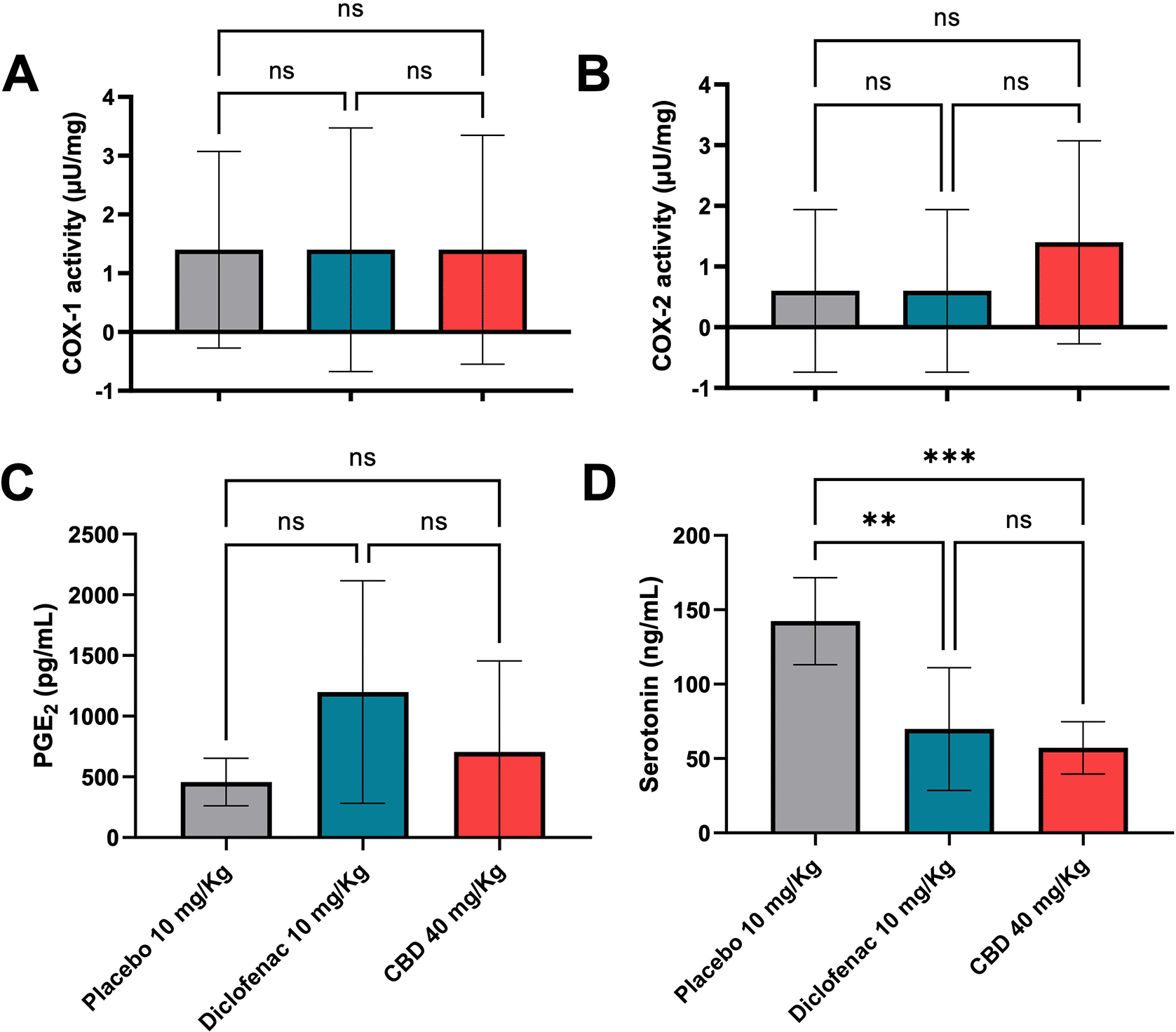
One-way ANOVA tested these analyses of variance following the Tukey post hoc test (**p < 0.01; ***p < 0.001, ns; not significant).
The level of serum cytokines and chemokines from rats that received different interventions, including placebo, diclofenac, and oral CBD (40 mg/kg), were determined using a bead array technique. As the results (Figure 5), four out of 18 cytokines and chemokines showed significantly different levels between CBD and either placebo or Diclofenac which were MCP-1 (Figure 5N), MCP-3 (Figure 5O), MIP-2 (Figure 5Q), and RANTES (Figure 5R). The others were not significantly different between the compared groups. However, the IL-17A level of the CBD group had a trend of lowered IL-17A when compared with placebo (Figure 5I).
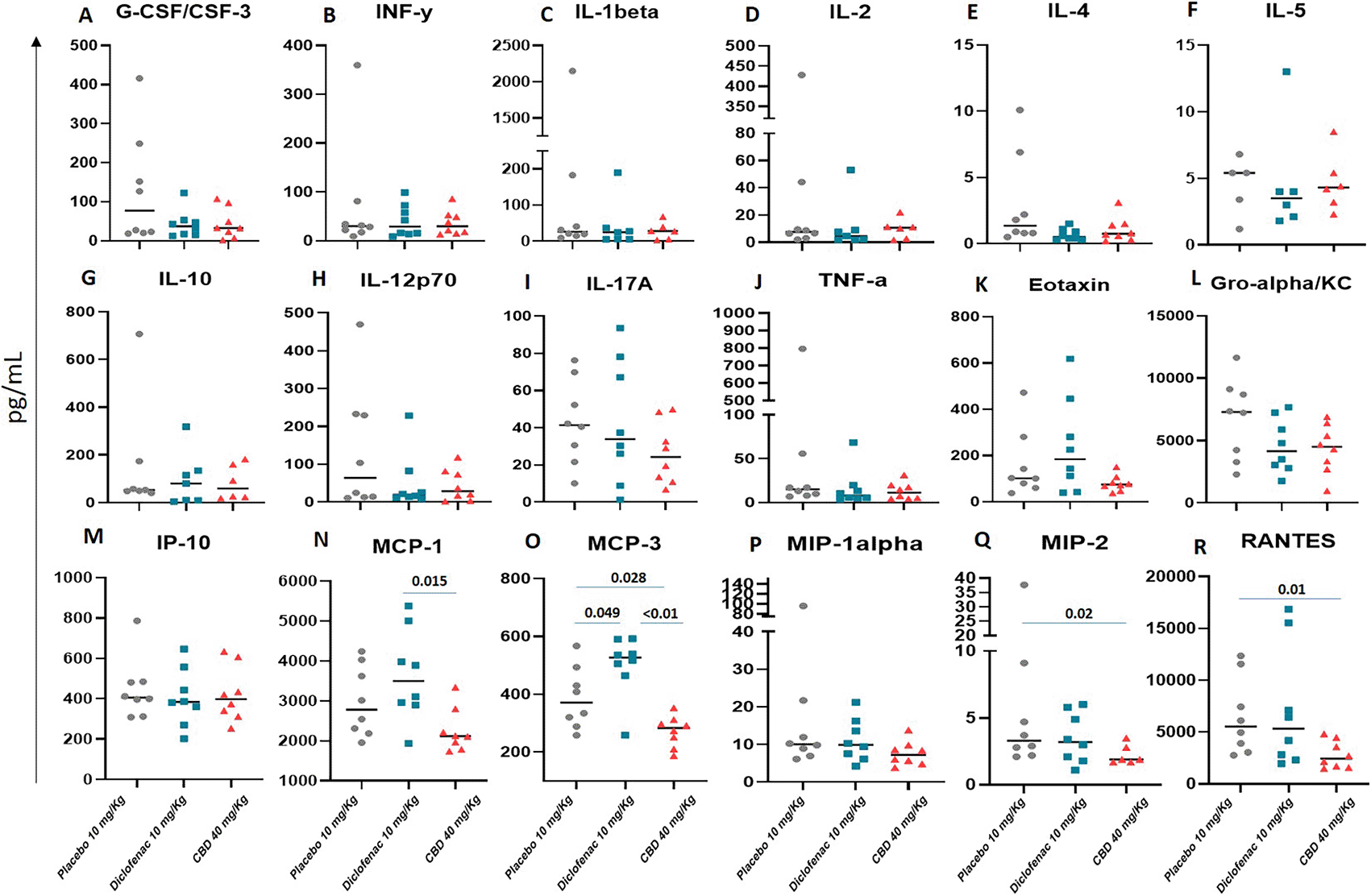
Those serum cytokines and chemokines were collected from the cardiac at six hours and detected by bead-based flow cytometry. The Mann performed comparisons between the groups–Whitney U test, p-value < 0.05 would be considered statistical significance.
Carrageenan-induced inflammation is a well-known model for screening anti-inflammatory activity. This model could predict the dose of anti-inflammatory activity in human inflammation diseases and correlates well with an effective dose in patients.20 In carrageenan-induced paw edema, the inflammation response is time-dependent and biphasic with various mediators.21,22 The first phase (0-2 h after carrageenan injection) increased vascular permeability and release chemical mediators such as COX, bradykinin, histamine, and serotonin products.23–26 The second phase (3-4 h after carrageenan injection) is attributed to the infiltration of leukocytes and released inflammatory mediators such as kinin, PGE2, leukotrienes, platelet-activating factor (PAF), free radicals of oxygen-derivatives and pro-inflammatory cytokines.27–29 Histopathological evaluation of the inflamed right hind paw also supported the anti-inflammatory effect of oral CBD. The oral CBD reduced paw edema and infiltration of inflammatory cells such as neutrophils and lymphocytes into the site of inflammation. This study demonstrated that oral CBD might be anti-inflammatory in both phases of carrageenan-induced inflammation.
Costa et al. (2004) also reported that the administration of oral CBD has a beneficial action that reduces carrageenan-induced paw edema. CBD had a time, and dose-dependent effect after a single carrageenan injection. Paw edema following the carrageenan injection had a maximum peak at three hours. The lower dose of oral CBD (5 mg/kg) had a significant anti-inflammatory activity. However, lower doses of oral CBD did not reduce lipoperoxide production or COX system overactivity. However, it significantly inhibited the increased expression of eNOS in paw tissue.29
Overall, cytokine and chemokine levels of the received oral CBD group were lower than the placebo group. For example, significantly lower levels of MCP-1, MCP-3, MIP-2, and RANTES were seen in those rats treated with CBD (Figure 4). Those MCP-1 (also known as CCL2), MCP-3 (CCL7), and MIP-2 (CCL4) promote inflammatory proteins and are mainly produced by monocyte and macrophage. These chemokines are also expressed in blood cells, fibroblasts, epithelial cells, and vascular smooth muscle cells. Also, they act as a chemoattractant of eosinophils, basophils, dendritic cells (DCs), neutrophils, NK cells, and activated T lymphocytes.30–32 RANTES (CCL5) is usually expressed and secreted by T cells and monocytes. However, CCL5 can also work as a chemoattractant for several leukocytes, but mainly involves T cell activation and regulation process.33 Together, those chemokines are crucial for immune responses and inflammation. In addition, the lowest IL-17A level was seen for CBD usage, and even the statistical comparison was not significantly different compared to the placebo and diclofenac groups (Figure 5I). However, the trend indicated that CBD probably decreased the IL-17A level. The IL-17A is well-known as a pro-inflammatory cytokine secreted by activated T helper cells, especially for Th17.34 This cytokine stimulates the transcriptional factor NF-kappa B and enhances the production of IL-6, which promotes more inflammation. Importantly, high levels of IL-17A are associated with various chronic inflammation and autoimmune diseases.35
This study demonstrated that oral CBD could reduce cytokine and chemokine levels in rats treated with CBD at 40 mg/kg compared to either placebo or diclofenac (10 mg/kg) group. This finding is consistent with other research groups, revealing that CBD decreases pro-inflammatory cytokines and suppresses immune responses by several mechanisms.36 A report demonstrated that CBD reduced CCL2, CCL5, eotaxin, IL-1ra, and IL-2.37 Another study also showed that CBD could reduce IL-4, IL-5, and eotaxin levels.38 We also demonstrated that the levels of those three cytokines in the CBD group were lower than for the placebo but not significantly different regarding the statistical analysis (Figure 5E, F, and K).
The possible mechanisms of CBD for immune suppression include CBD acting as an allosteric modulator of CB1 or CB2 receptors, which might work as a CB1 or CB2 antagonist. Also, CBD probably inhibits fatty acid amide hydrolase (FAAH), interrupting CB1 and CB2 receptors.37 Another mechanism of cytokine suppression might be related to the transient receptor potential V1 or the vanilloid receptor (TRPV1) because the TRPV1 antagonist reduces the cytokine-suppressing function of CBD in human tissue.39 According to the CBD intervention, the reduced serum cytokine and chemokine were seen in rats treated with oral CBD rather than placebo in this study. It is also related to localized edema (Figure 1), which decreased in rats treated with a high dose of CBD. This suggests that CBD is a potential immunosuppressant and has a potential role as an anti-inflammatory in the clinical setting.
In conclusion, the oral administration of CBD had a potentially beneficial anti-inflammatory effect on carrageenan-induced paw edema. However, the mechanism of the therapeutic effect of CBD has remained controversial. CBD may also be a good candidate for the treatment of inflammatory diseases. Further safety investigations of CBD in clinical use are required.
Zenodo: Raw data oral CBD, https://doi.org/10.5281/zenodo.7852520. 40
This project contains the following underlying data:
Cytokine Chemokinecsv
Zenodo: ARRIVE Essential 10 checklist for “Anti-inflammatory effects of oral cannabidiol in rat models”, https://doi.org/10.5281/zenodo.7960633. 41
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The research could not have been completed without assistance from a senior pathologist. The author would like to thank Professor Vorachai Sirikulchayanonta, MD, Faculty of Science, Rangsit University, Pathum Thani, Thailand, for his intensive guidance and advice in the part of histopathology analysis.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neurophysiology, Pain, Cannabinoids, Nitric oxide, Anti-inflammatory mechanism.
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacology of natural products and phytochemical analyses.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 15 Jun 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)