Keywords
COVID-19, SARS-CoV-2, type 1 diabetes mellitus, diabetic ketoacidosis, pediatrics, child, systematic review, meta-analysis
This article is included in the Global Public Health gateway.
COVID-19, SARS-CoV-2, type 1 diabetes mellitus, diabetic ketoacidosis, pediatrics, child, systematic review, meta-analysis
In this new version of the manuscript, we introduced some changes to give clarity to the reader and improve the quality and methodological rigor of this systematic review, following the recommendations of reviewer 1.
Methods: We modified the eligibility, inclusion, and exclusion criteria. We changed the Population component of our PECO question and described with more detail the inclusion criteria. We included a small paragraph indicating that we considered a cutoff for excluding studies; we excluded papers in which more than 50% of patients were >18 years old or had other types of diabetes mellitus. This cutoff ensures that most participants in the included studies are children, which increases the likelihood that the results are relevant to them. We slightly changed the selection process performed by independent reviewers. We added a small paragraph detailing the statistical method used for obtaining mean difference (MD) and standard deviation (SD) when this information was not reported in the studies. Furthermore, we included the corresponding citations and references that support these statements.
Results: We modified "Table 2. Bias assessment of the included studies," presenting the complete Newcastle-Ottawa Scale (NOS) tool, not just the conclusion.
Citations and references. We have made the respective changes, including the citations and bibliographical references used.
See the authors' detailed response to the review by Carlos J. Toro-Huamanchumo
Type 1 diabetes (T1DM) is an autoimmune disease traditionally associated with viral infections.1,2 The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus shows a great affinity for the angiotensin-converting enzyme (ACE) 2 receptor and other receptors present in the islets of Langerhans in the pancreas. Therefore, the SARS-CoV-2 virus could induce insulin resistance, hyperglycemia, and diabetes mellitus (DM) decompensation. On the contrary, hyperglycemia could worsen the prognosis of the COVID-19 disease.3,4 There seems to be, in fact, a bidirectional relation between COVID-19 and DM.5,6
Some studies suggest that during the COVID-19 pandemic, the risk of T1DM has increased.7–9 Similarly, three systematic reviews and meta-analyses have shown an increase in the risk of developing DKA and severe DKA during the COVID-19 pandemic compared to the pre-pandemic era.10–12 Besides, people with DM are disproportionately affected by COVID-19. For example, they are more susceptible to be admitted to an intensive care unit (ICU) than those non-diabetic patients.13 In addition, patients with T1DM have 3.5 times higher mortality rates from COVID-19 than those without T1DM.14 However, data are still controversial. Some studies found no differences in the percentage of newly diagnosed T1DM complicating with DKA in COVID-19 and non-COVID-19 periods.15,16 Therefore, it is maybe due to more difficult access to healthcare systems.15 In fact, despite the advantages of telemedicine during the pandemic, several reports have shown that a considerable quantity of T1DM patients has presented complications, probably associated with fear and delay in seeking medical help.17 Another factor that could increase the incidence of T1DM, diabetic ketoacidosis (DKA), and severe DKA is the widespread use of steroids during the pandemic due to their ability to induce insulin resistance, hyperglycemia, and de novo DM.18,19
An international multicenter study in Europe and the USA aimed to examine the impact of the COVID-19 pandemic on the prevalence of DKA in pediatric type 1 diabetes. The researchers noted that the DKA prevalence at T1DM diagnosis during the pandemic years was 39%, significantly higher than the estimated prevalence of 33% for the two previous years. However, they did not find significant differences by sex or age.20
Although the evidence suggests that the SARS-CoV-2 pandemic has raised the incidence of T1DM, DKA, and severe DKA, information about the impact of COVID-19 on other clinical outcomes is scarce. Thus, we aimed to determine the impact of this pandemic on the probability of developing pediatric T1DM, DKA, severe DKA, DKA in newly diagnosed and established T1DM, ICU admissions, DKA complications, length of hospitalization stay, and mortality due to DKA.
We conducted this systematic review and meta-analysis following the recommendations of the Cochrane Handbook,21 the PRISMA,22 and the AMSTAR 223 guidelines. We previously registered the protocol in PROSPERO (CRD42021278821). We searched for observational (cohort, case-control, and cross-sectional) studies and randomized control trials published until 31 August 2022, in Medline (PubMed), Google Scholar, Scopus, ScienceDirect, EMBASE, and Web of Science. We combined different keywords, controlled vocabulary terms (e.g., MeSH and Emtree), and free terms following a predefined PECO framework (population: “children with type 1 diabetes mellitus” OR “children without type 1 diabetes mellitus”; exposure: “COVID-19” OR “SARS-CoV-2”; comparator: “NOT COVID-19” OR “NOT SARS-CoV-2”; outcome: “diabetic ketoacidosis” OR “DKA” OR “incidence” OR “hospital stay” OR “intensive care unit admission” OR “mortality” (Extended data). We did not limit searches by date or language.
The eligibility criteria followed the PECO question. We included studies that evaluated pediatrics’new-onset T1DM during the COVID-19 pandemic and during the same pre-pandemic period, which reported at least one of the following outcomes: children with new-onset and established T1DM, DKA among children with newly diagnosed and established T1DM, DKA, and severe DKA among newly diagnosed and established children with T1DM, ICU admissions, DKA complications, length of hospitalization stay, and mortality due to DKA. We excluded case reports, case series, duplicated publications, and papers in which more than 50% of patients were >18 years old or had other types of diabetes mellitus. This cutoff ensures that most participants in the included studies are children, which increases the likelihood that the results are relevant to them.21 Three reviewers (FEL-J, BADT-H, and GAV-T) independently. Three independent reviewers (FEL-J, BADT-H, and GAV-T) examined articles, and a fourth researcher (EDM-R) resolved discrepancies. We screened references from retrieved documents for additional articles. We reviewed the papers found and verified the compliance of the components of the PECO framework and the inclusion and exclusion criteria. In addition, we extracted and recorded the essential information from each article in a spreadsheet: authors' names, year and country of publication, type of study, number of patients, sex of the patients, number of events, the measure of association, and adjusted confounders if reported.
Initially, we planned to perform subgroup analyses according to DKA severity, mortality, length of hospitalization, and sex. However, as the protocol stipulated, these subgroup analyses would be executed if feasible regarding information and data. In addition, in concordance with the SAGER guidelines,24 we defined sex as the biological attributes associated with physical and physiological features separated as mutually exclusive and complementary categories (male or female); that is, the sum of both is equal to the total number of cases.
We pooled the number of patients and events of interest in the quantitative synthesis and calculated odds ratios (ORs) with 95% confidence intervals (95% CIs) using the Mantel-Haenszel method. We considered the risk ratio (RR) equivalent to OR if the incidence of the event evaluated was fewer than 10 percent.25 We used forest plots to represent the quantitative synthesis and assessed heterogeneity among studies with Cochran’s Q test and Higgins I2 statistic. We predefined that if heterogeneity was not significant (p > 0.05, I2 statistics < 40%), we would use a fixed-effects model. We carried out sensitivity and subgroup analyses and assessed the risk of bias with the Newcastle–Ottawa Scale (NOS) tool.26 Finally, we examined the publication bias using a funnel plot.
In addition, we compare the incidence per 105 children/year of T1DM between the pre-pandemic and pandemic period using medians and interquartile ranges (IQRs) and the Mann–Whitney test. We calculated the mean difference (MD) measures as the absolute difference between the means in the two (pre-pandemic and pandemic) groups. In the case of those studies that did not report the means or the standard deviation (SD) of the samples, we estimated it from the data provided by the authors. As long as the data are not significantly skewed away from normality,27 it is possible to estimate MD and SD if we know the size, the minimum, median, and maximum of the sample (scenario 1);28,29 the size, first quartile, median, third quartile (scenario 2);28,29 or the size, minimum, first quartile, median, third quartile, and maximum of the sample (scenario 3).28,30
We collected 112 studies, 98 in the primary screening and 14 in the secondary examination. Following the removal of duplicated articles, there were 87 articles left that we examined in title and abstract. Subsequently, we found and analyzed 46 papers in full text. We considered these 46 papers for qualitative and quantitative synthesis (Figure 1).
Of the 46 studies included in this review, six studies were cross-sectional studies (CSS), two papers were case-control studies (CCS), and thirty-eight documents were prospective or retrospective cohort studies (PCS, RCS). This review includes a total of 159,505 children with T1DM, 17,547 events of DKA—5,792 episodes of severe DKA, 15,600 episodes of DKA in de novo T1DM, and 521 episodes of DKA in established T1DM, 791 ICU admissions, 822 DKA related complications, and one death (Table 1).
| Study | Age (years) | DKA criteria | Group | Male sex (%) | Total (N) | DKA | Severe DKA | De novo T1DM | Established T1DM | ICU | Complications | Hospital stay | Dead |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kamrath C.17 2020. (MC) PCS. Germany. | ≤ 18 | Other | Pre-COVID | 517 (53.9) | 959 | 233 | 126 | 233 | ND | ND | ND | ND | ND |
| COVID | 327 (61.5) | 532 | 238 | 103 | 238 | ND | ND | ND | ND | ND | |||
| Al-Abdulrazzaq D.31 2021. (MC) PCS. Kuwait. | ≤ 12 | ISPAD | Pre-COVID | 150 (49.5) | 303 | 113 | 33 | 113 | ND | 33 | ND | ND | ND |
| COVID | 151 (46.6) | 324 | 166 | 60 | 166 | ND | 64 | ND | ND | ND | |||
| Alaqeel A.32 2021. (MC) RCS. Saudi Arabia. | 1–14 | ADA | Pre-COVID | 69 (44.8) | 154 | 112 | 24 | 15 | 97 | ND | ND | 2.9 ± 0.1 | ND |
| COVID | 51 (48.1) | 106 | 88 | 23 | 23 | 65 | ND | ND | 2.9 ± 0.2 | ND | |||
| Alassaf A.33 2022. (UC) RCS. Jordan. | ND | ISPAD | Pre-COVID | 37 (44.6) | 83 | 29 | 8 | 29 | ND | ND | ND | ND | ND |
| COVID | 28 (51.9) | 54 | 28 | 9 | 28 | ND | ND | ND | ND | ND | |||
| Atlas G.34 2020. (MC) RCS. Australia. | ND | Other | Pre-COVID | 118 (57.8) | 204 | 86 | 32 | 86 | ND | 25 | ND | ND | ND |
| COVID | 32 (55.2) | 58 | 30 | 13 | 30 | ND | 15 | ND | ND | ND | |||
| Boboc AA.35 2021. (UC) RCS. Romania. | < 18 | ISPAD | Pre-COVID | 170 (54.5) | 312 | 123 | 33 | 123 | ND | ND | ND | ND | ND |
| COVID | 75 (51.0) | 147 | 97 | 41 | 97 | ND | ND | ND | ND | ND | |||
| Bogale KT.36 2021. (UC) RCS. USA. | ≤ 18 | Other | Pre-COVID | 218 (58.9) | 370 | 172 | 123 | 172 | ND | ND | 20 | ND | ND |
| COVID | 23 (54.8) | 42 | 20 | 13 | 20 | ND | ND | 2 | ND | ND | |||
| Chambers MA.37 2022. (UC) RCS. USA. | < 18 | ISPAD | Pre-COVID | 167 (52.2) | 320 | 175 | 58 | 175 | ND | 157 | ND | 2.98 ± 0.17 | 0 |
| COVID | 83 (54.6) | 152 | 98 | 59 | 98 | ND | 116 | ND | 3.03 ± 0.18 | 0 | |||
| Cherubini V.38 2022. (MC) RCS. Italy. | ≤ 18 | Other | Pre-COVID | ND | 3068 | 1071 | 319 | 1071 | ND | ND | ND | ND | ND |
| COVID | ND | 1169 | 460 | 166 | 460 | ND | ND | ND | ND | ND | |||
| Danne T.39 2021. (MC) CCS. Germany. | ≤ 21 | ISPAD | Pre-COVID | 16254 (52.0) | 31258 | 280 | ND | ND | ND | ND | ND | ND | ND |
| COVID | 13178 (51.6) | 25543 | 228 | ND | ND | ND | ND | ND | ND | ND | |||
| Dilek SÖ.40 2021. (UC) CSS. Turkey. | < 18 | ISPAD | Pre-COVID | 21 (45.7) | 46 | 27 | 4 | 27 | ND | ND | 7 | ND | ND |
| COVID | 35 (47.3) | 74 | 68 | 15 | 68 | ND | ND | 17 | ND | ND | |||
| Donbaloğlu Z.41 2020. (UC) CSS. Turkey. | < 18 | ISPAD | Pre-COVID | ND | 78 | 43 | 20 | 43 | ND | ND | ND | ND | ND |
| COVID | 29 (51.8) | 56 | 30 | 13 | 30 | ND | ND | ND | ND | ND | |||
| Dżygało K.42 2020. (UC) RCS. Poland. | ≤ 18 | WHO | Pre-COVID | 26 (50.0) | 52 | 29 | 6 | 29 | ND | ND | ND | ND | ND |
| COVID | 22 (64.7) | 34 | 18 | 11 | 18 | ND | ND | ND | ND | ND | |||
| Fathi A.43 2022. (UC) RCS. USA. | ND | ND | Pre-COVID | 21 (42.0) | 50 | ND | ND | ND | ND | ND | 5 | 0.76 ± 0.07 | 0 |
| COVID | 16 (37.2) | 43 | ND | ND | ND | ND | ND | 3 | 1.02 ± 0.14 | 1 | |||
| Goldman S.44 2022. (MC) RCS. Israel. | < 18 | ISPAD | Pre-COVID | 181 (49.7) | 364 | 150 | 38 | 150 | ND | 71 | ND | 3.02 ± 0.51 | ND |
| COVID | 87 (59.6) | 146 | 85 | 29 | 85 | ND | 50 | ND | 4.00 ± 0.38 | ND | |||
| Gottesman BL.45 2022. (UC) CSS. USA. | < 19 | ND | Pre-COVID | ND | 641 | 261 | ND | 261 | ND | 41 | ND | ND | ND |
| COVID | 81 (43.3) | 187 | 93 | ND | 93 | ND | 16 | ND | ND | ND | |||
| Han MJ.46 2021. (MC) RCS. South Korea. | ≤ 18 | Other | Pre-COVID | 11 (91.7) | 12 | 8 | ND | 8 | 4 | ND | 10 | ND | ND |
| COVID | 1 (14.3) | 7 | 7 | ND | 7 | 0 | ND | 6 | ND | ND | |||
| Hawkes CP.47 2021. (UC) RCS. USA. | < 18 | ADA | Pre-COVID | ND | 93 | 35 | 11 | 35 | ND | ND | ND | ND | ND |
| COVID | ND | 73 | 33 | 11 | 33 | ND | ND | ND | ND | ND | |||
| Hernández HM.48 2022. (MC) RCS. Spain. | < 1 | ISPAD | Pre-COVID | 27 (51.9) | 52 | 22 | ND | 22 | ND | ND | ND | ND | ND |
| COVID | 20 (54.1) | 37 | 12 | ND | 12 | ND | ND | ND | ND | ND | |||
| Ho J.49 2021. (MC) RCS. Canada. | < 18 | DCCP | Pre-COVID | 47 (41.2) | 114 | 52 | 15 | 52 | ND | 9 | 3 | ND | ND |
| COVID | 46 (43.0) | 107 | 73 | 29 | 73 | ND | 19 | 13 | ND | ND | |||
| Jacob R.50 2021. (MC) CSS. Israel. | ≤ 18 | ADA | Pre-COVID | ND | 154 | 62 | 20 | 31 | 31 | 35 | ND | ND | ND |
| COVID | ND | 150 | 84 | 26 | 46 | 38 | 40 | ND | ND | ND | |||
| Kamrath C.51 2021. (MC) PCS. Germany. | ≤ 18 | Other | Pre-COVID | ND | 42417 | 7312 | 2101 | 7312 | ND | ND | ND | ND | ND |
| COVID | 1799 (55.6) | 3238 | 1094 | 401 | 1094 | ND | ND | ND | ND | ND | |||
| Kaya G.52 2022. (UC) RCS. Turkey. | <18 | ISPAD | Pre-COVID | 42 (53.2) | 79 | 32 | 12 | 32 | ND | ND | ND | 15.02 ± 5.53 | ND |
| COVID | 24 (54.5) | 44 | 30 | 14 | 30 | ND | ND | ND | 10.02 ± 3.89 | ND | |||
| Kiral E.53 2022. (MC) RCS. Turkey. | < 18 | ISPAD | Pre-COVID | 241 (46.6) | 517 | 517 | 292 | 165 | 127 | ND | 378 | ND | ND |
| COVID | 207 (43.1) | 480 | 480 | 337 | 226 | 111 | ND | 329 | ND | ND | |||
| Kostopoulou E.54 2021. (MC) PCS. Greece. | < 18 | ND | Pre-COVID | 12 (70.6) | 17 | 17 | 3 | 6 | ND | 1 | ND | ND | ND |
| COVID | 9 (42.9) | 21 | 18 | 14 | 14 | ND | 6 | ND | ND | ND | |||
| Lavik AR.55 2022. (MC) RCS. USA. | ≤ 19 | ISPAD | Pre-COVID | ND | 1041 | 547 | ND | ND | ND | ND | ND | ND | ND |
| COVID | ND | 1035 | 556 | ND | ND | ND | ND | ND | ND | ND | |||
| Lawrence C.56 2021. (UC) RCS. Australia. | < 18 | ISPAD | Pre-COVID | 21 (50.0) | 42 | 11 | 2 | 11 | ND | ND | ND | ND | ND |
| COVID | 3 (27.3) | 11 | 8 | 5 | 8 | ND | ND | ND | ND | ND | |||
| Lee MS.57 2022. (UC) CSS. South Korea. | < 19 | ISPAD | Pre-COVID | 1 (10.0) | 10 | 4 | 0 | 4 | ND | ND | ND | ND | ND |
| COVID | 6 (60.0) | 10 | 6 | 1 | 6 | ND | ND | ND | ND | ND | |||
| Lee Y.58 2022. (MC) RCS. South Korea. | < 18 | ISPAD | Pre-COVID | 51 (51.5) | 41 | 16 | 9 | 16 | ND | ND | 3 | ND | ND |
| COVID | 46 (54.8) | 51 | 31 | 9 | 31 | ND | ND | 6 | ND | ND | |||
| Loh C.59 2021. (UC) CCS. Germany. | ≤ 18 | ISPAD | Pre-COVID | 36 (49.3) | 73 | 15 | 6 | 9 | 6 | ND | ND | 9.86 ± 11.02 | ND |
| COVID | 21 (40.4) | 52 | 15 | 8 | 8 | 7 | ND | ND | 10.13 ± 0.67 | ND | |||
| Luciano TM.60 2022. (UC) PCS. Brazil. | < 18 | Other | Pre-COVID | 14 (56.0) | 25 | 9 | 3 | 9 | ND | ND | 9 | ND | ND |
| COVID | 9 (50.0) | 18 | 12 | 6 | 12 | ND | ND | 11 | ND | ND | |||
| Mameli C.61 2021. (MC) PCS. Italy. | < 18 | ISPAD | Pre-COVID | 293 (47.0) | 624 | 184 | 62 | 184 | ND | 25 | ND | ND | ND |
| COVID | 110 (43.0) | 256 | 91 | 39 | 91 | ND | 17 | ND | ND | ND | |||
| Marks BE.62 2021. (UC) RCS. USA. | ≤ 21 | ADA | Pre-COVID | 163 (52.6) | 310 | 145 | 52 | 145 | ND | ND | ND | ND | ND |
| COVID | 101 (55.5) | 182 | 105 | 51 | 105 | ND | ND | ND | ND | ND | |||
| Mastromauro C.63 2022. (UC) RCS. Italy. | ≤ 19 | ISPAD | Pre-COVID | 81 (61.4) | 132 | 48 | 11 | 48 | ND | ND | ND | ND | ND |
| COVID | 20 (50.0) | 40 | 22 | 9 | 22 | ND | ND | ND | ND | ND | |||
| McGlacken BSM.64 2021. (MC) CSS. UK. | < 18 | ISPAD | Pre-COVID | 15 (50.0) | 30 | 9 | 3 | 9 | ND | 2 | ND | ND | ND |
| COVID | 9 (52.9) | 17 | 13 | 8 | 13 | ND | 4 | ND | ND | ND | |||
| Mönkemöller K.65 2021. (MC) PCS. Germany. | < 18 | Other | Pre-COVID | 223 (23.2) | 959 | 231 | 125 | ND | ND | ND | ND | ND | ND |
| COVID | 233 (43.8) | 532 | 237 | 100 | ND | ND | ND | ND | ND | ND | |||
| Nóvoa MY.66 2022. (UC) RCS. Spain. | < 14 | ADA | Pre-COVID | ND | 28 | 12 | ND | 12 | ND | ND | ND | ND | ND |
| COVID | ND | 42 | 19 | ND | 19 | ND | ND | ND | ND | ND | |||
| Passanisi S.67 2022. (MC) RCS. Italy. | ≤14 | Other | Pre-COVID | 18 (41.9) | 43 | 19 | 8 | 19 | ND | ND | ND | ND | ND |
| COVID | 51 (45.9) | 111 | 52 | 20 | 52 | ND | ND | ND | ND | ND | |||
| Rabbone I.68 2020. (MC) PCS. Italy. | < 15 | ISPAD | Pre-COVID | ND | 208 | 86 | 31 | 86 | 22 | ND | ND | ND | ND |
| COVID | ND | 160 | 61 | 27 | 61 | 13 | ND | ND | ND | ND | |||
| Salmi H.69 2021. (MC) RCS. Finland. | ≤ 15 | ND | Pre-COVID | 128 (55.4) | 231 | 20 | 20 | 20 | ND | 25 | ND | ND | ND |
| COVID | 48 (57.1) | 84 | 13 | 13 | 13 | ND | 20 | ND | ND | ND | |||
| Sellers EAC.70 2021. (MC) RCS. Canada. | ND | ND | Pre-COVID | ND | 236 | 86 | 39 | 86 | ND | ND | ND | ND | ND |
| COVID | ND | 260 | 143 | 69 | 143 | ND | ND | ND | ND | ND | |||
| Tittel SR.71 2020. (MC) RCS. Germany. | < 18 | ND | Pre-COVID | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| COVID | ND | 532 | ND | ND | ND | ND | ND | ND | ND | ND | |||
| Vlad A.72 2021. (MC) RCS.Romania. | < 14 | ND | Pre-COVID | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| COVID | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |||
| Vorgučin I.73 2022. (MC) RCS. Serbia. | < 19 | ISPAD | Pre-COVID | 73 (55.3) | 132 | 45 | 16 | 45 | ND | ND | ND | ND | ND |
| COVID | 50 (50.5) | 99 | 42 | 15 | 42 | ND | ND | ND | ND | ND | |||
| Wolf RM.74 2022. (MC) RCS. USA. | ≤ 26 | ISPAD | Pre-COVID | 622 (48.7) | 17749 | 493 | 145 | 493 | ND | ND | ND | ND | ND |
| COVID | 648 (46.3) | 17597 | 599 | 215 | 599 | ND | ND | ND | ND | ND | |||
| Zubkiewicz A.75 2021. (MC) RCS. Poland. | < 18 | ISPAD | Pre-COVID | 1054 (53.7) | 1961 | ND | ND | ND | ND | ND | ND | ND | ND |
| COVID | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
T1DM: type 1 diabetes mellitus, DKA: diabetic ketoacidosis, RCS: Retrospective cohort study, PCS: Prospective cohort study, CSS: Cross-sectional study, ISPAD: International Society for Pediatric and Adolescent Diabetes, ADA: American Diabetes Association, DCCP: Diabetes Canada Clinical Practice, WHO: World Health Organization, ICU: admission to the intensive care unit, UC: unicenter, MC: multicenter, ND: not described.
Following the approach of most studies, we analyzed outcomes comparing the pre-pandemic and the pandemic periods—regardless of their COVID-19 status (positive or negative)—instead of reporting events in children with COVID-19 positive or negative. Consequently, we only included papers that reported both groups of children (a pre-pandemic and a pandemic cohort). The lack of a pre-pandemic group was the leading cause of the exclusion of most studies (Extended data).
We analyzed nine outcomes (pre and during the COVID-19 pandemic) in children: 1) the incidence of T1DM, 2) the incidence of DKA in T1DM, 3) the incidence of severe DKA in T1DM, 4) the incidence of DKA in de novo T1DM, 5) the incidence of DKA in established T1DM, 6) the incidence of ICU admissions due to DKA in T1DM, 7) the incidence of DKA complications in T1DM, 8) the length of hospitalization stay, and 9) the risk of mortality due to DKA.
T1DM: type 1 diabetes mellitus, DKA: diabetic ketoacidosis, RCS: Retrospective cohort study, PCS: Prospective cohort study, CSS: Cross-sectional study, ISPAD: International Society for Pediatric and Adolescent Diabetes, ADA: American Diabetes Association, DCCP: Diabetes Canada Clinical Practice, WHO: World Health Organization, ICU: admission to the intensive care unit, UC: unicenter, MC: multicenter, ND: not described.
During the pre-COVID-19 era, the median incidence of DKA among children with T1DM was 17.28 per 105 patients/year (IQR 10.87–26.9), and during the COVID-19 era, the incidence of DKA among children with T1DM was 19.35 per 105 patients/year (IQR 14.65–34.7). However, this difference was not statistically significantly (p = 0.41, Mann–Whitney test).
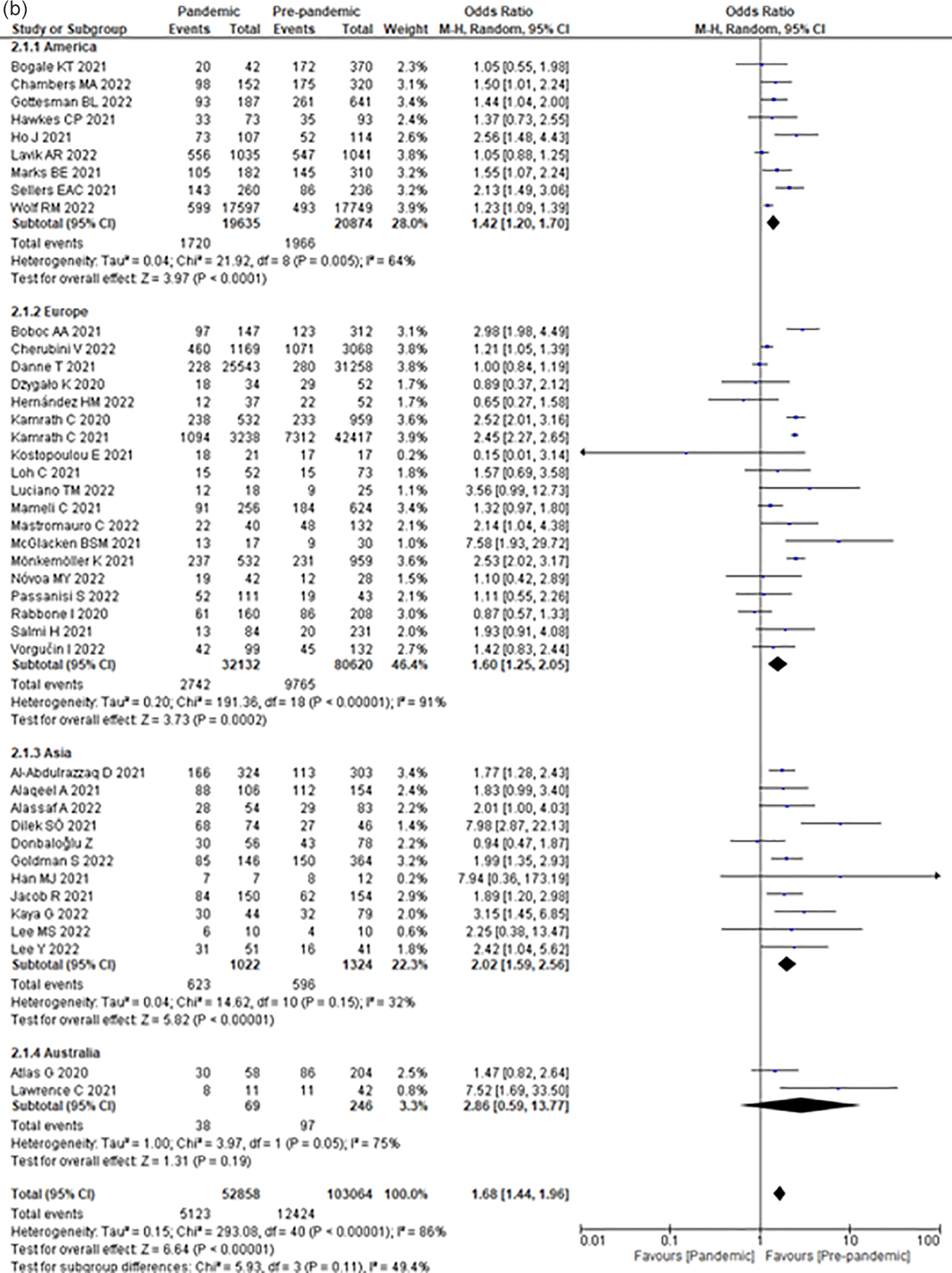
Compared to the pre-COVID-19 era, the COVID-19 era increased the odds of DKA by 68% (OR 1.68; 95% CI 1.44–1.96) (Figure 2a) among children with T1DM.
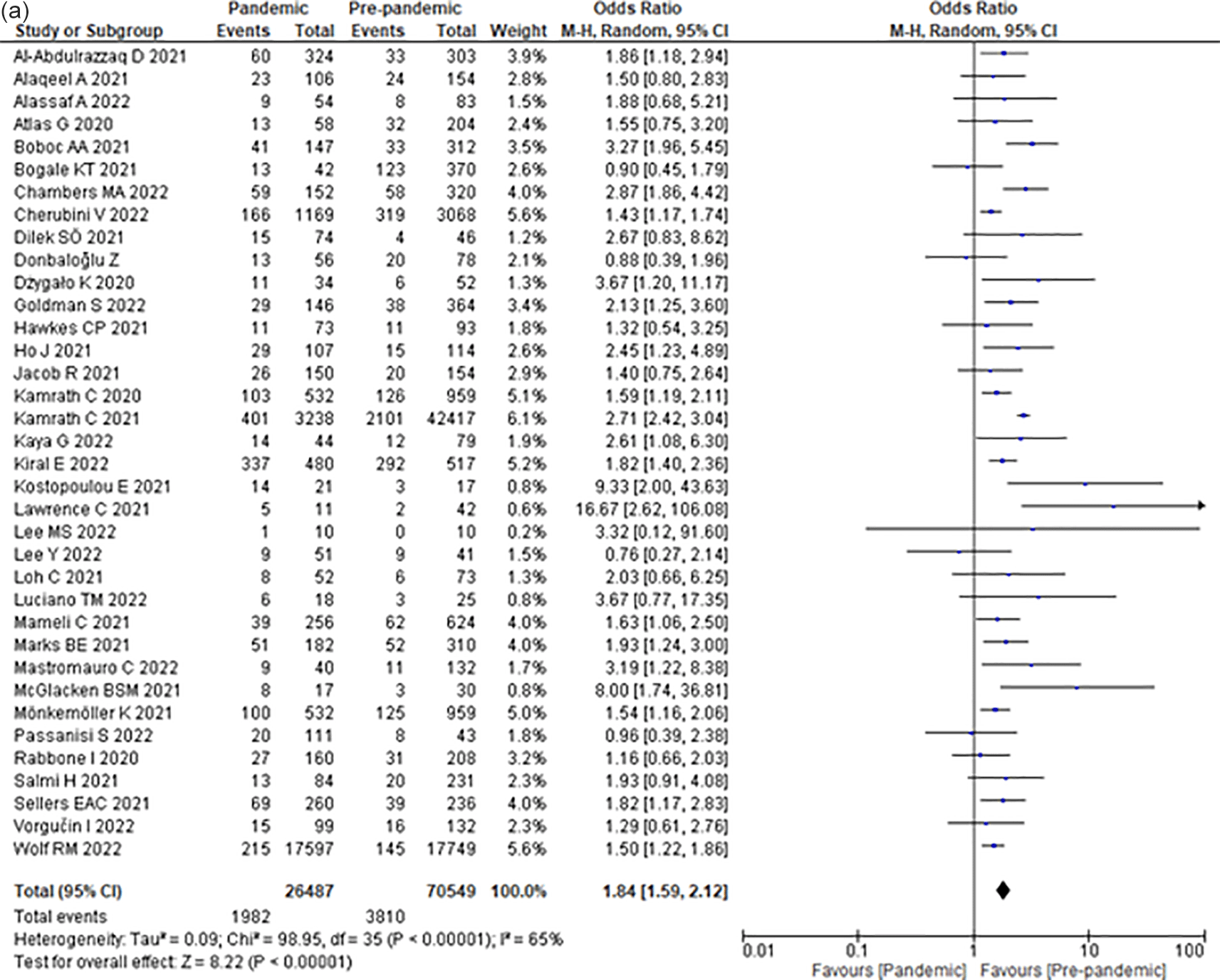
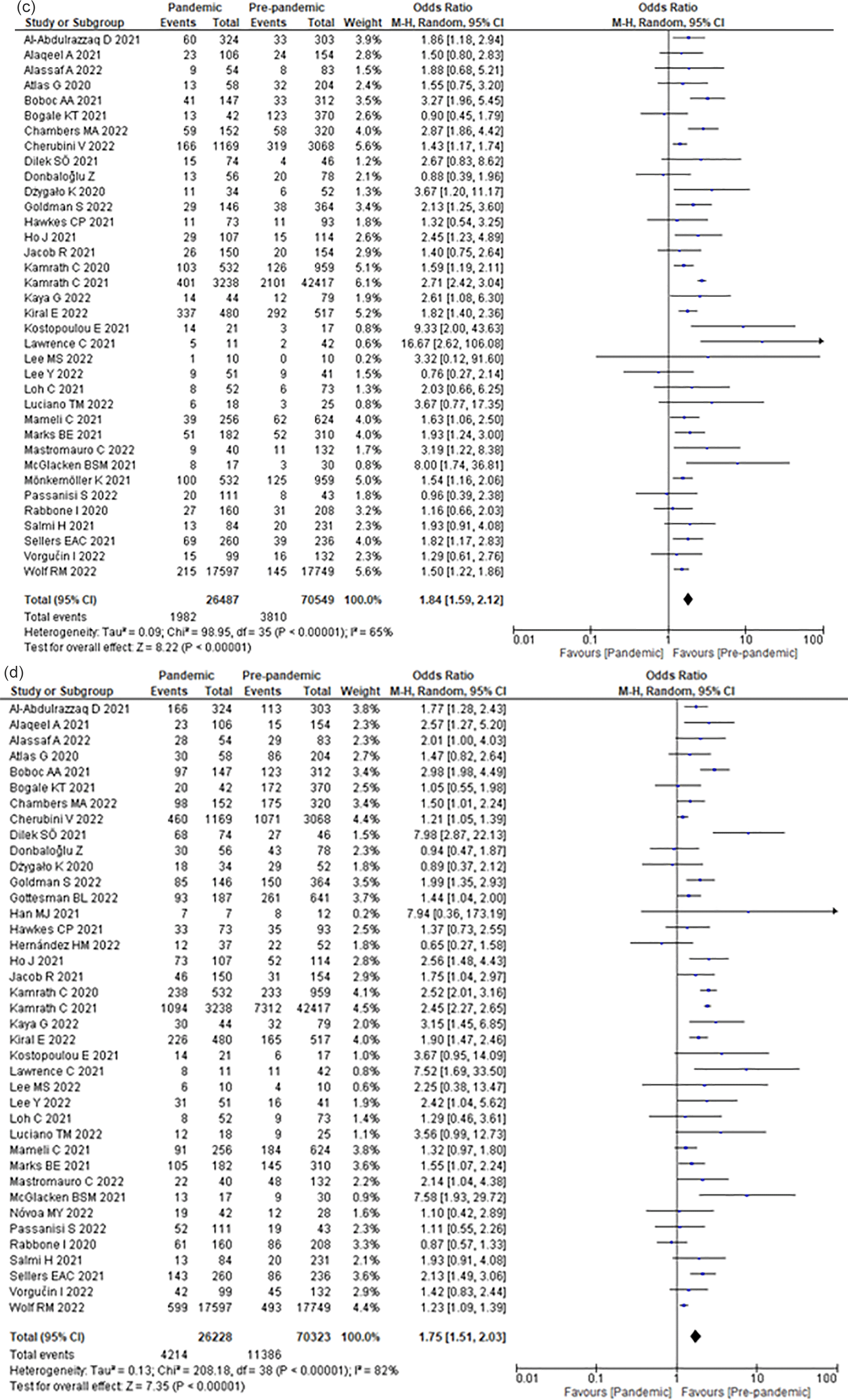
Compared to the pre-COVID-19 era, the COVID-19 era increased the odds of severe DKA by 84% (OR 1.84; 95% CI 1.59–2.12) (Figure 2b) among children with T1DM.
Compared with the pre-COVID-19 era, the COVID-19 era increased the odds of DKA by 75% (OR 1.75; 95% CI 1.51–2.03) (Figure 2c) among children with newly diagnosed T1DM.
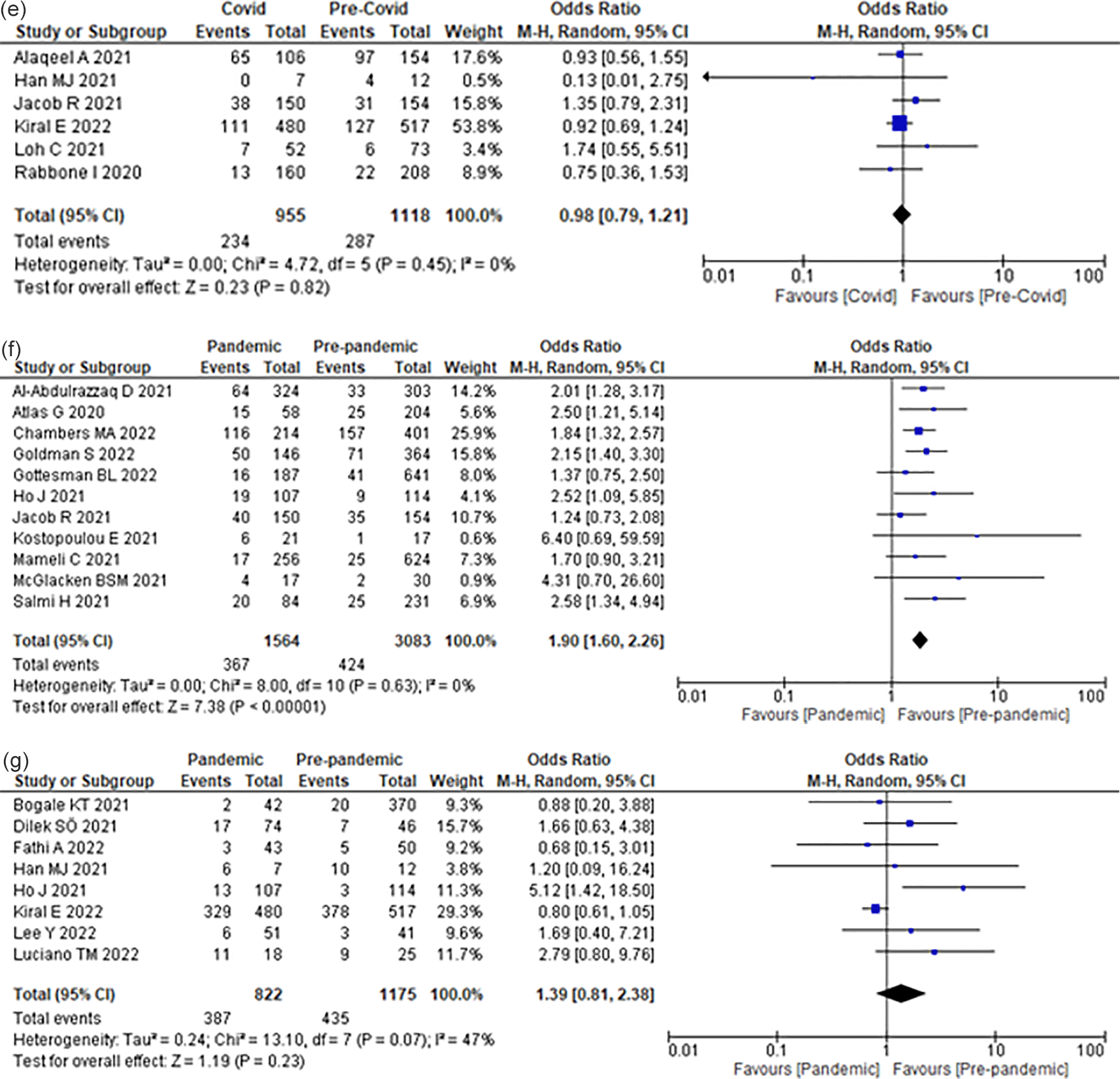
Compared to the pre-COVID-19 era, the COVID-19 era did not significantly increase the odds of developing DKA (OR 0.98; 95% CI 0.79–1.21) (Figure 2d) among children with established T1DM.
Compared with the pre-COVID-19 era, the COVID-19 era increased the odds of ICU admission due to DKA by 90% (OR 1.90; 95% CI 1.60–2.26) (Figure 2e) among children with T1DM.
Compared to the pre-COVID-19 era, the COVID-19 era did not significantly increase the odds of DKA complications (OR 1.39; 95% CI 0.81–2.38) (Figure 2f) among T1DM pediatric patients. Authors reported different definitions for “DKA complications”, including acute kidney injury,46 pulmonary edema,40 stroke,58 brain edema,40,43 altered mental status,36,46,49,58 treatment with hypertonic saline or mannitol,49 hypokalemia,40,60 hypocalcemia, or hypophosphatemia.40 On the other hand, a study did not define “DKA complication.”
Compared with the pre-COVID-19 era, the COVID-19 era did not significantly affect the duration of hospital stay due to DKA (MD 0.18; 95% CI -0.11–0.46) (Figure 2g) among children with T1DM.
We found two studies37,43 evaluating this outcome. Unfortunately, only one43 of these two studies reported events during the pandemic. Consequently, we decided not to conduct a meta-analysis for this clinical outcome.
Of the 46 studies included, 40 had a low risk of bias and six had a high risk of bias according to assessment with the Newcastle-Ottawa Scale (NOS) tool (Table 2).
| Author | Study design | Selection | Comparability | Outcome | Total | Conclusion |
|---|---|---|---|---|---|---|
| Al-Abdulrazzaq D.31 2021. | (MC) PCS. | **** | ** | *** | 9 | Low risk |
| Alaqeel A.32 2021. | (MC) RCS. | *** | ** | *** | 8 | Low risk |
| Alassaf A.33 2022. | (UC) RCS. | *** | ** | ** | 7 | Low risk |
| Atlas G.34 2020. | (MC) RCS. | ** | ** | * | 5 | High risk |
| Boboc AA.35 2021. | (UC) RCS. | *** | ** | ** | 7 | Low risk |
| Bogale KT.36 2021. | (UC) RCS. | *** | ** | *** | 8 | Low risk |
| Chambers MA.37 2022. | (UC) RCS. | *** | ** | ** | 7 | Low risk |
| Cherubini V.24 2022. | (MC) RCS. | *** | ** | ** | 7 | Low risk |
| Danne T.39 2021. | (MC) CCS. | *** | ** | *** | 8 | Low risk |
| Dilek SÖ.40 2021. | (UC) CSS. | *** | ** | ** | 7 | Low risk |
| Donbaloğlu Z.41 2020. | (UC) CSS. | *** | ** | ** | 7 | Low risk |
| Dżygało K.42 2020. | (UC) RCS. | *** | ** | *** | 8 | Low risk |
| Fathi A.43 2022. | (UC) RCS. | *** | ** | ** | 7 | Low risk |
| Goldman S.44 2022. | (MC) RCS. | ** | ** | * | 5 | High risk |
| Gottesman BL.45 2022. | (UC) CSS. | ** | ** | * | 5 | High risk |
| Han MJ.46 2021. | (MC) RCS. | *** | ** | ** | 7 | Low risk |
| Hawkes CP.47 2021. | (UC) RCS. | ** | ** | * | 5 | High risk |
| Hernández HM.48 2022. | (MC) RCS. | *** | ** | *** | 8 | Low risk |
| Ho J.49 2021. | (MC) RCS. | *** | ** | *** | 8 | Low risk |
| Jacob R.50 2021. | (MC) CSS. | *** | ** | ** | 7 | Low risk |
| Kamrath C.17 2020. | (MC) PCS. | **** | ** | *** | 9 | Low risk |
| Kamrath C.51 2021. | (MC) PCS. | **** | ** | *** | 9 | Low risk |
| Kaya G.52 2022. | (UC) RCS. | **** | ** | ** | 8 | Low risk |
| Kiral E.53 2022. | (MC) RCS. | *** | ** | *** | 8 | Low risk |
| Kostopoulou E.54 2021. | (MC) PCS. | **** | ** | *** | 9 | Low risk |
| Lavik AR.55 2022. | (MC) RCS. | *** | ** | *** | 8 | Low risk |
| Lawrence C.56 2021. | (UC) RCS. | *** | ** | *** | 8 | Low risk |
| Lee MS.57 2022. | (UC) CSS. | *** | ** | ** | 7 | Low risk |
| Lee Y.58 2022. | (MC) RCS. | *** | ** | *** | 8 | Low risk |
| Loh C.59 2021. | (UC) CCS. | *** | ** | ** | 7 | Low risk |
| Luciano TM.60 2022. | (UC) PCS. | **** | ** | *** | 9 | Low risk |
| Mameli C.61 2021. | (MC) PCS. | **** | ** | *** | 9 | Low risk |
| Marks BE.62 2021. | (UC) RCS. | *** | ** | *** | 8 | Low risk |
| Mastromauro C.63 2022. | (UC) RCS. | *** | ** | *** | 8 | Low risk |
| McGlacken BSM.64 2021. | (MC) CSS. | *** | ** | ** | 7 | Low risk |
| Mönkemöller K.65 2021. | (MC) PCS. | **** | ** | *** | 9 | Low risk |
| Nóvoa MY.66 2022. | (UC) RCS. | *** | ** | *** | 8 | Low risk |
| Passanisi S.67 2022. | (MC) RCS. | *** | ** | *** | 8 | Low risk |
| Rabbone I.68 2020. | (MC) PCS. | **** | ** | *** | 9 | Low risk |
| Salmi H.69 2021. | (MC) RCS. | *** | ** | *** | 8 | Low risk |
| Sellers EAC.70 2021. | (MC) RCS. | *** | ** | *** | 8 | High risk |
| Tittel SR.71 2020. | (MC) RCS. | *** | ** | *** | 8 | High risk |
| Vlad A.72 2021. | (MC) RCS. | *** | ** | *** | 8 | Low risk |
| Vorgučin I.73 2022. | (MC) RCS. | *** | ** | *** | 8 | Low risk |
| Wolf RM.74 2022. | (MC) RCS. | *** | ** | *** | 8 | Low risk |
| Zubkiewicz A.75 2021. | (MC) RCS. | *** | ** | *** | 8 | Low risk |
The funnel plot suggested publication bias (Figure 3).
To our knowledge, this is the first systematic review and meta-analysis that asses nine outcomes associated with the effect of COVID-19 on pediatric T1DM and DKA. According to our results, the COVID-19 pandemic significantly increased the incidence of DKA (OR 1.68; 95% CI 1.44–1.96), severe DKA (OR 1.84; 95% CI 1.59–2.12), DKA in newly diagnosed T1DM (OR 1.75; 95% CI 1.51–2.03), and ICU admissions (OR 1.90; 95% CI 1.60–2.26). Conversely, we found no association between the COVID-19 pandemic and the incidence of T1DM, DKA in established T1DM, DKA complications, the length of hospitalization stay, and mortality (Figure 2a-g). These findings are in agreement with other meta-analyses.
Nassar M et al.76 conducted a systematic review to identify the prevalence, clinical presentation, and outcomes of T1DM in patients with COVID-19. They searched for observational studies in four databases. The results evaluated were the duration of hospital stay, general ward admission, ICU admission, frequency of DKA, serious hypoglycemia, and mortality. They included 15 papers in the qualitative analysis. They had reports that included information from both children and adults with COVID-19. The frequency of T1DM among patients with COVID-19 varied between 0 and 30%, while the prevalence of COVID-19 among patients with T1DM varied between 0 and 17%. The assessed outcomes ranged widely among the studies. Furthermore, the study’s duration of hospital stay, general ward admission, ICU admission, frequency of DKA, and serious hypoglycemia varied significantly among the included studies.
The systematic review by Nassar M et al.76 has several limitations reported by the authors. First, they could not perform a meta-analysis because of the lack of studies with appropriate information. Besides, the characteristics of the participants differed widely among the studies. Therefore, in our meta-analysis, we excluded 13 of the 15 studies of the paper by Nassar M et al. because these studies combined adults with pediatric patients or did not have a control (pre-pandemic) group.
Rahmati M et al.12 systematically explored the occurrence of de novo T1DM in children and its complications, such as diabetic ketoacidosis, previously and in the times of the pandemic of COVID-19. First, they carried out a systematic search of four databases. Then, they performed a quantitative synthesis comparing the probabilities of developing T1DM and diabetic ketoacidosis in children with T1DM before (the year 2019) and during (the year 2020) the pandemic. They also examined glycemic and glycated hemoglobin levels in pediatric participants with de novo T1DM previously and at the time of this pandemic. They found that the overall incidence ratio of T1DM in 2019 was 19.73 per 105 children and 32.39 per 105 in 2020. During 2020 the cases of de novo T1DM, diabetic ketoacidosis, and serious diabetic ketoacidosis raised significantly. Similarly, in 2020, the median glycemic and glycated hemoglobin levels in pediatric participants with de novo T1DM during the COVID-19 era increased notably. They concluded that the pandemic raised the likelihood of developing de novo T1DM, diabetic ketoacidosis, and serious diabetic ketoacidosis in children.
Rahmati M et al.12 conducted heterogeneity and sensitivity analysis and assessed the possibility of publication bias. However, their paper only included studies covering the first wave of the SARS-CoV-2 pandemic. Conversely, our study includes more recent studies that covered subsequent waves. In addition, we excluded two studies of the review by Rahmati M et al., one because it did not report DKA cases nor a numerator to calculate an incidence ratio, and the other because there was no control (pre-pandemic) group.
Alfayez OM et al.10 conducted a systematic review aiming to study the characteristics of DKA before and during the pandemic of SARS-CoV-2 in children with T1DM. First, they searched for observational and found 20 documents on DKA. Then, they performed a random model analysis and reported that the pandemic, compared to the period before, significantly raised the probability of developing DKA and serious DKA. Similarly, pediatric patients with de novo T1DM presented a substantially greater risk of developing DKA during the pandemic than those patients during the pre-COVID-19 era. However, the heterogeneity was significant in all of these estimates (I2 = 44%–71%). Two papers mentioned the likelihood of DKA among children with previously diagnosed T1DM, and this probability was not statistically increased during the COVID-19 era. The authors concluded that their research evidenced that DKA likelihood, particularly the chance of developing serious DKA, raised significantly during the COVID-19 period.
Alfayez OM et al.10 reported subgroup and sensitivity analysis, and explored the possibility of publication bias. Although their systematic review collected fewer than half as many studies as ours, all included studies had an adequate control group. Consequently, their conclusions are closer to ours. Nevertheless, we highlight that of the studies included by these authors, one is a case-control study,39 and four follow a cross-sectional design.40,50,57,64 In studies of cross-sectional and case-control design, we only are able to assess odds, not risk. Therefore, a better measure of the effect size would have been to report odds ratios instead of risk ratios,77,78 as the authors estimated.
Elgenidy A et al.11 conducted a meta-analysis to investigate the increase of DKA in pediatrics at the time of the SARS-CoV-2 pandemic. In three databases, they looked for papers evaluating the frequency of diabetic ketoacidosis. The researchers reported 24 studies, including 124,597 pediatric patients with T1DM. Their main finding was that the pandemic raised statistically significantly the likelihood of developing DKA in children with de novo T1DM (RR 1.41; 95% CI 1.19–1.67; p < 0.01), particularly of those with serious DKA (RR 1.66: 95% CI 1.30–2.11) compared with the pre-COVID-19 era. Statistical heterogeneity was substantial (I2 = 86% and 59%, respectively). They found no important rise in the probability of developing DKA during the pandemic in pre-existing T1DM or combined—de novo and pre-existing T1DM children compared with the pre-COVID-19 era. They concluded that the likelihood of DKA in children with de novo T1DM had risen in the time of the SARS-CoV-2 pandemic and tended to present in more serious forms.
Elgenidy A et al. performed subgroup and sensitivity analysis and evaluated the risk of publication bias. However, in our meta-analysis, we excluded 5 of the 17 studies quantitatively analyzed by Elgenidy A et al. because they did not report any of the events of interest or the lack of a control group. Moreover, these authors also included two case-control studies39,59 and cross-sectional studies40,50,57,64 and reported relative risks instead of odds ratios. Therefore, the same considerations previously mentioned for the meta-analysis of Alfayez OM et al. should apply.
The heterogeneity was significant in this systematic review and meta-analysis (I2 > 90%, p < 0.05). According to the subgroup examination, the type of study design and the provenance region of the studies explained this lack of homogeneity among studies (test for subgroups difference I2 = 83.2%, p = 0.003; I2 > 49.4%, p = 0.11; respectively). Sensitivity analysis did not alter the global size estimate, showing good consistency. Because of the limited data among studies, we decided not to carry out subgroup analysis and meta-regression according to other variables. Unlike the study by Elgenidy A et al., because most studies do not provide complete information, we did not perform subgroup according to the type of onset of diabetes (de novo, established, or combined—de novo and established—T1DM) or the degree of diabetic ketoacidosis (serious, moderate, or mild). On the contrary, following Rahmati M et al. and Alfayez OM et al., we analyzed the diabetes onset and the degree of DKA as an independent outcome.
We highlight several strengths in our meta-analysis: 1) the strategy search was comprehensive and compiled a more significant number of papers than any other previous systematic review or meta-analysis, 2) all the studies that we included involved a control (pre-pandemic) group, 3) all the papers that we included examined clinical—not surrogate—outcomes, and 4) we carried out sensitivity and subgroup analysis and examined for possible publication bias. Then, our conclusions are stronger than those previously reported by any other meta-analysis.
This study has important limitations: 1) heterogeneity was significant, 2) we were not able to carry out subgroup analyses regarding other essential factors such as age or sex, 3) it is possible that there exists a publication bias, as was suggested by our funnel plot, and finally, 4) we could not establish definite conclusions on other important outcomes such as the likelihood of T1DM, DKA complications, the duration of hospitalization stay, and mortality due to DKA. In addition, although we initially planned to perform subgroup analyzes according to sex, due to the scarcity of data (most studies combined information for both sexes), it was not possible to achieve this sub-analysis. In fact, none of the previously cited systematic reviews could perform a subgroup analysis according to sex.
Our systematic review shows that the SARS-CoV-2 pandemic significantly impacted T1DM and DKA outcomes in pediatric patients. This pandemic increased 1) the risk of DKA, 2) the risk of serious DKA, 3) the risk of DKA in children with de novo T1DM, and 4) ICU admissions due to DKA. Conversely, the relation of the SARS-CoV-2 pandemic with other outcomes such as 1) the incidence of pediatric T1DM, 2) the incidence of DKA in established pediatric T1DM, 3) the incidence of complications due to DKA, 4) the length of hospitalization stay, and 5) the risk of mortality due to DKA, were not statistically significant. Nonetheless, clinicians should interpret these findings with caution due to several limitations. Consequently, more research is still necessary to improve knowledge of the relationship between SARS-CoV-2 and diabetic ketoacidosis. Nevertheless, our results imply that healthcare systems should be alert and prepared for a potential rise in diabetic ketoacidosis cases, especially severe DKA cases, in future waves of viral respiratory pandemics.
EDM-R: Conceptualization, Data Curation, Formal Analysis, Investigation, Methodology, Resources, Visualization, Writing, Original Draft Preparation; FEL-J: Data Curation, Formal Analysis, Investigation, Writing – Review & Editing; BADT-H: Investigation, Writing – Review & Editing; GAV-T: Conceptualization, Investigation, Supervision, Validation, Writing – Review & Editing
Figshare: Figshare3: Raw data, https://doi.org/10.6084/m9.figshare.21644723. 79
This project contains the following underlying data:
Figshare: Figshare4: Extended data, https://doi.org/10.6084/m9.figshare.21644786. 80
This project contains the following extended data:
Figshare: PRISMA checklist for “Impact of the Covid-19 pandemic on the incidence and clinical outcomes of diabetic ketoacidosis among children with type 1 diabetes: systematic review and meta-analysis”, https://doi.org/10.6084/m9.figshare.21625790. 81
Figshare: Figshare2: PRISMA Flowchart, https://doi.org/10.6084/m9.figshare.21625775. 82
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pediatric Endocrinology
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
References
1. Bombaci B, Passanisi S, Sorrenti L, Salzano G, et al.: Examining the associations between COVID-19 infection and pediatric type 1 diabetes.Expert Rev Clin Immunol. 2023; 19 (5): 489-497 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Pediatrics, diabetes, allergy
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Partly
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: I know two authors as they are researchers from universities in my home country; however, we have never worked together. I confirm I am able to review this article impartially.
Reviewer Expertise: Evidence-Based Medicine, Epidemiology & Public Health, Obesity & Metabolism
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 10 Aug 23 |
read | read | |
|
Version 1 18 Jan 23 |
read | ||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (1)