Keywords
Cyclooxygenase, molecular docking, paracetamol, pharmacokinetics, toxicity
This article is included in the Cheminformatics gateway.
Cyclooxygenase, molecular docking, paracetamol, pharmacokinetics, toxicity
Globally, chronic pain affects about 1.9 billion people with the most prominent pain being tension type headaches (Fayaz et al., 2016). In UK, 13%-50% of adults suffer from chronic pain (Mills et al., 2019). In sub-Saharan Africa Prevalence of Pain in seropositive patients is 59-89% (Mills et al., 2019) in Kenya, Uganda, and South Africa. 87.5% of cancer patients in South Africa and Uganda documented cancer-related pain (Wang et al., 2019). In Kenya, studies have shown that patients often pass on due to pain-related conditions (Nchako et al., 2018). Pain assessment in Paediatric patients is negligible and there is limited data supporting this (Rothemeyer & Enslin, 2016) in Sub-Saharan Africa.
Most outpatient cases suffer from chronic pain which is of more than one type of pain (Buhck et al., 2022) and the cost of pain management exceeds greatly the costs from cancer, diabetes and cancer management. 67% of patients with chronic pain suffer from comorbid maniac disorder (Annagür et al., 2014). Different types of pain exist and they include.
Neuropathic pain that includes Diabetic neuropathy pain, post-herpetic neuralgia and central pain associated with CVA, Nociceptive pain associated with injuries to the tissues and include bruises, burns or sprains, Inflammatory pain associated with RA or infection Psychogenic pain such as headaches or abdominal pain and Mechanical pain associated with metastatic malignancies.
Most used drug for pain and fever management. Both over-the-counter and prescription is Paracetamol (Freo et al., 2021). Paracetamol’s action involves both antipyretic and analgesic. Paracetamol has very little anti-inflammatory effects as compared to NSAIDs such as ibuprofen. Guidelines have so far recommended Paracetamol as a drug of choice in pain management (Freo et al., 2021). Paracetamol has good efficacy since its formulations are broad and range from rapid dissolving formulations to suppositories. Paracetamol has opioid-sparing effects and this is helpful in mitigating opioid-induced side effects.
Acetaminophen binds to Cox 3, Cox 2, and Cox 1 enzymes leading to the inhibition of the synthesis of prostaglandins such as prostaglandin E (Ayoub, 2021). Acetaminophen blocks the peripheral generation of the pain impulse and by blocking prostaglandin production in the CNS. Paracetamol also exerts its effect by affecting endocannabinoid, serotonergic, inhibition of voltage-gated calcium channels, and inhibition of T-type calcium channels (Przybyła et al., 2020).
Despite the admirable effects of paracetamol, its side effects when overdosed are detrimental. Paracetamols have long been used in alleviating pain in pregnancy however, studies have linked delay in the neurological development of infants to the use of paracetamol during pregnancy (Bauer et al., 2021). Other effects reported are reproductive, urology system as well as genital disorders. Pregnant mothers are required to take paracetamol cautiously and they should alert the pharmacist or their physician.
Acetaminophen metabolism by the liver to generate NAPQI that causes acetylation and formation of covalent bonds with liver proteins causing liver damage, which can lead to hepatic failure (Mazaleuskaya et al., 2015).
Acetaminophen (N-acetyl-p-aminophenol, APAP) is metabolized in the liver to its prodrug phenacetin through O-dealkylation which is hepatotoxic and carcinogenic. Acetaminophen is as well metabolized in the body to glucuronide conjugate as well as sulphate conjugates in the body indicated as PAP-gluc and APAP-sulphate respectively. In overdose, about 5% of acetaminophen is oxidized to NAPQI which binds covalently to sulfhydryl groups in the liver causing liver necrosis and eventually hepatotoxicity (PharmGKB, n.d.) (Figure 1).
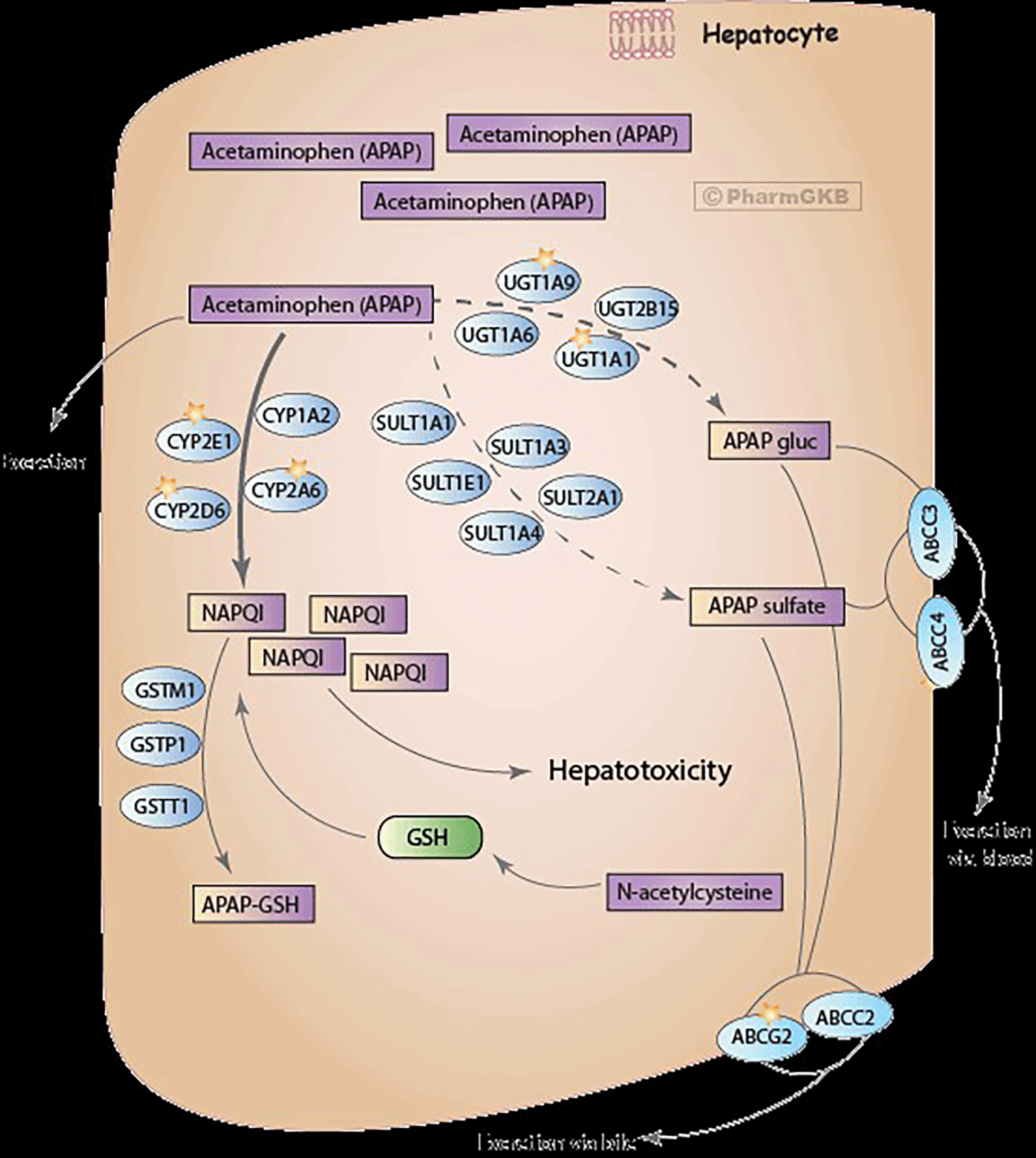
Note: Has been reproduced with permission from, PharmGKB summary: pathways of acetaminophen metabolism at the therapeutic versus toxic doses by Mazaleuskaya et al. (2015).
This study determined similar zinc compounds to Paracetamol. The compounds were docked to Cox 1, 2, and 3 enzymes, and the docking scores were compared to that of Paracetamol. Compounds with better docking scores than paracetamol were highlighted and pharmacokinetics and toxicity profiles were analyzed.
I. To identify zinc compounds similar to paracetamol.
II. To identify compounds with better docking scores than paracetamol.
III. To identify the pharmacokinetics properties of compounds with better docking scores than paracetamol.
IV. To identify toxicity profiles of the compounds with better docking scores than paracetamol
Paracetamol was searched through PubChem (RRID:SCR_004284) and canonical smiles were copied and pasted to SwissSimilarity (http://www.swisssimilarity.ch/index.php). Paracetamol was used as a query to generate compounds with similar structures using the Zinc database. Similar compounds were generated using Ligand-based Virtual screening. The generated compounds that had a similarity score to Paracetamol above 80% were randomly selected and 20 compounds were sampled in total.
Cyclooxygenase 1, 2, and 3 were downloaded from Protein Data Bank (RRID:SCR_012820) using unique PDB codes that are 5F19,6Y3C and 1EJF. Preparations for these receptors were done using Chimera (RRID: SCR_004097) where all non-standard residues were eliminated. Ligands were prepared for docking first by auto-optimization to make the ligands be at the minimum energy using Avogadro (RRID: SCR_011958) (Eberhardt et al., 2021). The optimized compounds were further minimized by the addition of hydrogen and charges using Chimera (RRID:SCR_004097).
Molecular docking of compounds to cyclooxygenase 1, 2 and 3 was done using Chimera (RRID: SCR_004097) and Autodock vina (RRID: SCR_011958). Compounds that had better docking scores to Paracetamol were further analyzed through Biovia Discovery studio to determine the interactions between the binding sites of these receptors with the compound’s pharmacophore.
Pharmacokinetics profiles of the promising compounds were analyzed using SwissADME online tool (http://www.swissadme.ch/). Effects of the compounds on CYP enzymes were analyzed. The ability of these compounds to pass through Blood Brain Barrier was determined. Gastrointestinal absorption of compounds and their conformation with Lipinski rule of five was further analyzed.
Toxicity profiles of the compounds were analyzed using ProTox II (RRID:SCR_018506 webserver. Oral toxicities were determined using LD50. Organ toxicities were analyzed such as hepatoxicity. Immunotoxicity, carcinogenicity, and Tox 21 stress receptors were further analyzed.
(https://doi.org/10.7910/DVN/SO4CEH)
ZINC00294715; 0.980; ZINC00394165; 0.987, ZINC00406627; 0.980, ZINC01557001; 0.987, ZINC01714506; 0.986, ZINC01714507; 0.986, ZINC01747085; 0.985; ZINC19281575; 0.992, ZINC34120167; 0.994 and ZINC71451975; 0.975 showed higher binding to Cox 3 than paracetamol. These compounds were further analyzed for their toxicities as well as pharmacokinetic profiles (Table 1).
| Serial no. | Similarity scores | compound | Binding to cox3 (1EJF) | Binding to cox2 (5F19) | Binding to cox1 (6Y3C) |
|---|---|---|---|---|---|
| 1. | Paracetamol | -5.3 | -6.6 | -6.2 | |
| 2. | 98.00% | ZINC00294715; 0.980; | -5.5 | -6.8 | -6.1* |
| 3. | 98.70% | ZINC00394165; 0.987; | -6.2 | -8 | -7.7 |
| 4. | 98.00% | ZINC00406627; 0.980; | -5.9 | -6.6 | -7.6 |
| 5. | 99.40% | ZINC00874201; 0.994; | -5.2 | -6.5 | -4.9* |
| 6. | 98.70% | ZINC01557001; 0.987; | -5.7 | -6.4 | -6.5 |
| 7. | 98.60% | ZINC01714506; 0.986; | -6.7 | -8.1 | -7.6 |
| 8. | 98.60% | ZINC01714507; 0.986; | -6.4 | -8.3 | -6.8 |
| 9. | 98.50% | ZINC01747085; 0.985; | -5.9 | -7.3 | -6.6 |
| 10. | 98.20% | ZINC02568449; 0.982; | -5.2 | -7.1 | -6.4 |
| 11. | 97.90% | ZINC04522243; 0.979; | -5.1 | -6.7 | -5.9 |
| 12. | 98.60% | ZINC14982950; 0.986; | -5.2 | -6.3 | -6.4 |
| 13. | 98.50% | ZINC18274777; 0.985; | -5.2 | -6.9 | -6.2 |
| 14. | 97.50% | ZINC19093853; 0.975; | -5.3 | -6.8 | -6.6 |
| 15. | 99.20% | ZINC19281575; 0.992; | -5.4 | -6.6 | -6.3 |
| 16. | 98.10% | ZINC19898944; 0.981; | -5.0 | -6.4 | -6.5 |
| 17. | 99.40% | ZINC34120167; 0.994; | -5.5 | -6.3 | -6.6 |
| 18. | 99.10% | ZINC39650276; 0.991; | -5.1 | -5 | -5.5* |
| 19. | 99.40% | ZINC41699382; 0.994; | -5.1 | -6.3 | -5.6* |
| 20. | 98.20% | ZINC41723295; 0.982; | -5.2 | -7.1 | -6.8 |
| 21. | 97.50% | ZINC71451975; 0.975; | -5.4 | -6.3 | -6.3 |
ZINC00874201; 0.994; ZINC01557001; 0.987; ZINC14982950; 0.986; ZINC19898944; 0.981; ZINC34120167; 0.994; ZINC39650276; 0.991; ZINC41699382; 0.994; and ZINC71451975; 0.975; showed lower docking scores to Cox II than Paracetamol.
ZINC00294715; 0.980; has a higher binding score to Cox 3 and Cox 2 than paracetamol. The zinc compound also showed a lower docking score to Cox 1 therefore, its pharmacokinetic properties were analyzed by using SwissADME online tool (Table 1).
Paracetamol and ZINC00294715; 0.980; showed high GIT absorption and lack of Cytochrome inhibition. They lack inhibition of CYP1A2, CYP3A4, CYP2C9, and CYP2C19. They are not substrates to P-glycoprotein. Paracetamol showed BBB permeation while ZINC00294715; 0.980; showed a lack of BBB permeation. They both showed no violation of Lipinski’s rule of five.
Oral toxicity
ZINC01714506; 0.986 had the highest oral LD50 of 2000 mg/kg as compared to Paracetamol which had an oral LD50 of 338 mg/kg. ZINC01557001; 0.987; and ZINC34120167; 0.994; had similar oral LD50 of 1100 mg/kg. None of the compounds had lower LD50 than Paracetamol and these compounds, are, therefore, safer than paracetamol when taken orally (Table 2).
ZINC01714506; 0.986, ZINC01714507; 0.986; ZINC34120167; 0.994; and ZINC71451975; 0.975; were considered inactive in causing hepatotoxicity as compared to Paracetamol that was predicted Hepatoactive. None of the compounds were predicted immunoactive (Table 3).
Carcinogenic, mutagenic, and cytogenic toxicity (Table 4)
Tox 21 toxicity (Table 5)
| Compounds | Ahr | P |
|---|---|---|
| Paracetamol | I | 0.9 |
| ZINC01747085; 0.985; | A | 0.55 |
| ZINC00394165; 0.987; | A | 0.72 |
| ZINC00406627; 0.980; | A | 0.81 |
| ZINC01557001; 0.987; | A | 0.59 |
| ZINC71451975; 0.975; | A | 0.56 |
| ER | P | |
|---|---|---|
| Paracetamol | I | 0.94 |
| ZINC00394165; 0.987; | A | 0.57 |
| ZINC00406627; 0.980; | A | 0.67 |
| MMP | P | |
|---|---|---|
| Paracetamol | I | 0.96 |
| ZINC00406627; 0.980; | A | 0.74 |
| ZINC00394165; 0.987; | A | 0.67 |
Paracetamol’s pharmacophore binds at Cox 1 receptor by van der Waals forces as well as by conventional hydrogen bonding. Additional bonding in cox-2 is involved which is amide pi stacking as well as pi bonds with alkyl groups. ZINC01557001; 0.987; pharmacophore bonded with Cox-1 binding sites using van der Waals forces, donor–donor bonds as well as pi-alkyl bonding. ZINC01557001; 0.987; pharmacophore bonded with Cox 2 bindings sites using additional conventional hydrogen bonds as well as pi-sigma bonds. ZINC34120167; 0.994 pharmacophore bonded with Cox-1 using carbon–hydrogen bonding, pi-alkyl bonding, and van der Waals forces. ZINC34120167; 0.994 bonding in Cox-2 involved additional conventional hydrogen bonds (Figure 2).
Paracetamol has been hypothesized to inhibit the production of prostaglandin E2 primarily in the brain and has weaker effects on tissues at the periphery of the body (Ayoub & Flower, 2019). Prostaglandin E2 is involved in the generation of hypothermia which is often more dependent on the Cox-2 enzyme than Cox-1. Paracetamol-induced hypothermia is, therefore, majorly due to the inhibition of Cox-2. Cox-3 enzyme is a variant of Cox-1 produced through modifications on mRNA sequence (Ayoub & Flower, 2019).
The analgesic action of Paracetamol has been linked to the inhibition of central Cox due to its lipid solubility as well as unionized at the physiological state and prevention of the production of peripheral prostaglandins (Ayoub, 2021). Paracetamol has also been researched to inhibit connectivity of brain regions such as grey matter and this is mediated by its metabolite, N-(4-hydroxyphenyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (Barrière et al., 2020).
ZINC01714506; 0.986; was predicted to have the highest binding score to Cox-3 as compared to Paracetamol. ZINC01714507; 0.986 showed the highest docking score -8.3 kj/mol for cox 2 receptor as compared to paracetamol.
Paracetamol and ZINC00394165; 0.987 showed LD50 of 338 mg/kg making these compounds to be most lethal than the rest of the compounds. ZINC01714507; 0.986 was predicted to have LD50 of 500 mg/kg, ZINC00406627; 0.980 showed LD50 of 565 mg/kg, ZINC71451975; 0.975 was predicted to having LD50 of 600 mg/kg and ZINC19281575; 0.992 showed LD50 of 700 mg/kg. ZINC01747085; 0.985 was predicted to have LD50 of 1030 mg/kg. ZINC01557001; 0.987, and ZINC34120167; 0.994 showed LD50 of 1100 mg/kg. ZINC01714506; 0.986 showed LD50 of 2000 mg/kg and therefore, was considered the safest than the rest of the zinc compounds. ZINC01714506; 0.986 had the highest Oral LD50 of 2000 mg/kg as compared to Paracetamol oral LD50 of 338 mg/kg. ZINC01557001; 0.987; and ZINC34120167; 0.994; had similar oral LD50 of 1100 mg/kg and therefore, they are relatively safe compounds.
Paracetamol and its zinc compounds ZINC00294715; 0.980, ZINC01747085; 0.985, ZINC00394165; 0.987, ZINC00406627; 0.980, ZINC01557001; 0.987 and ZINC19281575; 0.992 were predicted to be active in causing hepatotoxicity with the probability of 0.74, 0.71, 0.65, 0.68, 0.65, 0.62 and 0.58 respectively. Paracetamol hepatotoxicity profile has been studied widely and the results from this study conform to the studies done by other researchers (Rotundo & Pyrsopoulos, 2020).
Three zinc compounds were predicted carcinogenic. These compounds are ZINC01747085; 0.985; ZINC00394165; 0.987; and ZINC00406627; 0.980; with probability scores of 0.52, 0.54, and 0.53 respectively. ZINC00394165; 0.987; ZINC00406627; 0.980; ZINC01714506; 0.986; and ZINC01714507; 0.986 were predicted active in causing mutagenicity with probability scores of 0.98, 0.79, 0.62 and 0.61 respectively.
Aryl hydrocarbon receptor is associated with metabolism and in causing toxic effects through the metabolism of compounds such as xenobiotics, tryptophan-based compounds, microorganism derived compounds amongst other effects. Aryl hydrocarbon receptor mediates cell cycle processes in the cells, metabolism, and reproduction and mediates immune reactions. Ahr is also associated with skin regulation by regulating oxidative stress as well as toxins from the environment and other sources (Napolitano et al., 2021). ZINC01747085; 0.985; ZINC00394165; 0.987; ZINC00406627; 0.980; ZINC01557001; 0.987; and ZINC71451975; 0.975; were predicted active for Ahr receptor with the probability scores of 0.84,0.55,0.72,0.81 and 0.59 respectively. Paracetamol was predicted inactive for the Ahr receptor. ZINC00394165; 0.987; and ZINC00406627; 0.980; were also predicted active for ER with probability scores of 0.57 and 0.67 respectively. ZINC00406627; 0.980; and ZINC00394165; 0.987; were predicted active for MMP with probability scores of 0.67 and 0.74 respectively which has an important role in oxidative stress and in acute Kidney injury (Su et al., 2022).
ZINC00294715; 0.980; ZINC01557001; 0.987; and ZINC19281575; 0.992; were predicted to lack Blood Brain Barrier permeation as compared to paracetamol and therefore, their use as antipyretics could be limited without further chemical modifications to enhance lipid solubility. ZINC00394165; 0.987, ZINC01714506; 0.986; and ZINC01714507; 0.986 were predicted to inhibit CYP1A2 with ZINC01714507; 0.986 with capacity to inhibit CYP 2C9. All compounds were predicted to have high GIT absorption and all conformed to Lipinski’s rule of five.
The aims of the study were achieved. Compounds with better docking scores to Cox 1, 2, and 3, pharmacokinetics as well as toxicity profiles were highlighted. 16 compounds showed higher docking scores to Cox 1 than paracetamol and therefore, they are promising in the development of new analgesic compounds. Nine out of 20 compounds had better docking scores to Cox -2 than paracetamol and therefore, they could be analyzed further as hypothermic compounds. ZINC01714506; 0.986; ZINC01714507; 0.986; and ZINC00394165; 0.987 showed the highest docking scores to cox 3 receptors with probability scores of -6.7kcal/mol, -6.4 and -6.2 kcal/mol. ZINC01714506; 0.986 was predicted to be the safest although it showed CYP1A2 inhibition compared to paracetamol and its zinc compounds.
In summary, the following compounds ZINC01557001; 0.987; ZINC01714506; 0.986; ZINC34120167; 0.994; ZINC00394165; 0.987, ZINC01714507; 0.986; and ZINC01747085; 0.985; are promising in drug discovery for new analgesic and antipyretic drugs, based on better docking scores and better oral LD50. Although these compounds are promising they have unwanted predicted activities such as ZINC01557001; 0.987; and ZINC01747085; 0.985 were, however, predicted hepatoactive. ZINC01747085; 0.985; was predicted carcinogenic. ZINC01714506; 0.986 was predicted mutagenic.
1. Quantitative structure-activity relationship of ZINC01557001; 0.987; ZINC01714506; 0.986; ZINC34120167; 0.994; ZINC00394165; 0.987, ZINC01714507; 0.986; and ZINC01747085; 0.985; should be done in order to modify these compounds for better aqueous and lipid solubility and improve on oral toxicities, and decrease side effects associated with Cox 1 as well as untoward effects such as carcinogenic and effects of Ahr receptor.
2. In silico studies of ZINC01557001; 0.987; ZINC01714506; 0.986; ZINC34120167; 0.994; ZINC00394165; 0.987, ZINC01714507; 0.986; and ZINC01747085; 0.985; are as well recommended.
Harvard Dataverse: Underlying data for In silico analysis of Paracetamol. DOI: https://doi.org/10.7910/DVN/SO4CEH (Faith et al., 2023).
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacology, Toxicology, fetal origin of diseases, Developmental toxicity
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 21 Jun 23 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)