Keywords
metachronous cancer, radical cystectomy, upper tract urothelial carcinoma, urothelial carcinoma of the bladder.
This article is included in the Oncology gateway.
metachronous cancer, radical cystectomy, upper tract urothelial carcinoma, urothelial carcinoma of the bladder.
The most prevalent tumor of the urinary system is bladder carcinoma (BC). The most prevalent (90%) histologic type of BC is urothelial carcinoma of the bladder (UCB). The invasion of the basement membrane, lamina propria, or deeper by neoplastic cells of urothelial origin is defined as urothelial carcinoma (UC). The term “transitional cell carcinoma” has been replaced by “urothelial carcinoma” by the World Health Organization.1 Bladder cancer is the tenth most often diagnosed cancer worldwide, according to GLOBOCAN 2020 data, with roughly 573,000 new cases and 213,000 deaths. Men are more likely than women to acquire it, with global incidence and mortality rates of 9.5 and 3.3 per 100,000 for men and nearly 4-times those for women.2 Metachronous upper urinary tract carcinoma (UUTC) typically appears in 2 to 9% of bladder cancer patients within 3 years after their cystectomy. Metachronous carcinomas are those that are discovered six months after the main lesion’s surgery and are found in a different location than the primary lesion.3 As a result, when monitoring bladder cancer, it’s appropriate to consider this possibility.3 We reported a rare case of UCB that develops to UUTC after radical cystectomy. The work is documented following the CARE guidelines.4
We reported a case of 52-year-old male with urothelial carcinoma of the renal pelvis with a previous history of UCB. The patient was diagnosed with UCB in 2019. At first, he was experiencing intermittent hematuria. This was followed by frequent micturition and dysuria. The patient was a frequent smoker, with 2 packs of cigarette consumption a day. The patient denied any history of chronic disease. The patient also denied any history of neoplasm in the family. The patient was a teacher, and he denied any contact with industrial chemical substances or hair dye during his working day. The patient was then referred to the Local General Hospital and was performed transurethral resection of bladder tumor (TURBT) and was diagnosed with muscle invasive bladder cancer (MIBC). After that, the patient was referred to our center and underwent radical cystectomy (RC) with an ileal conduit. The histopathological report revealed a high-grade invasive urothelial carcinoma of the bladder. Two weeks following RC, the patient also underwent below-the-knee amputation of the left leg due to acute limb ischemia. In January 2020, the patient underwent a computed tomography (CT)-scan examination, and there was no sign of malignancy in the urinary tract.
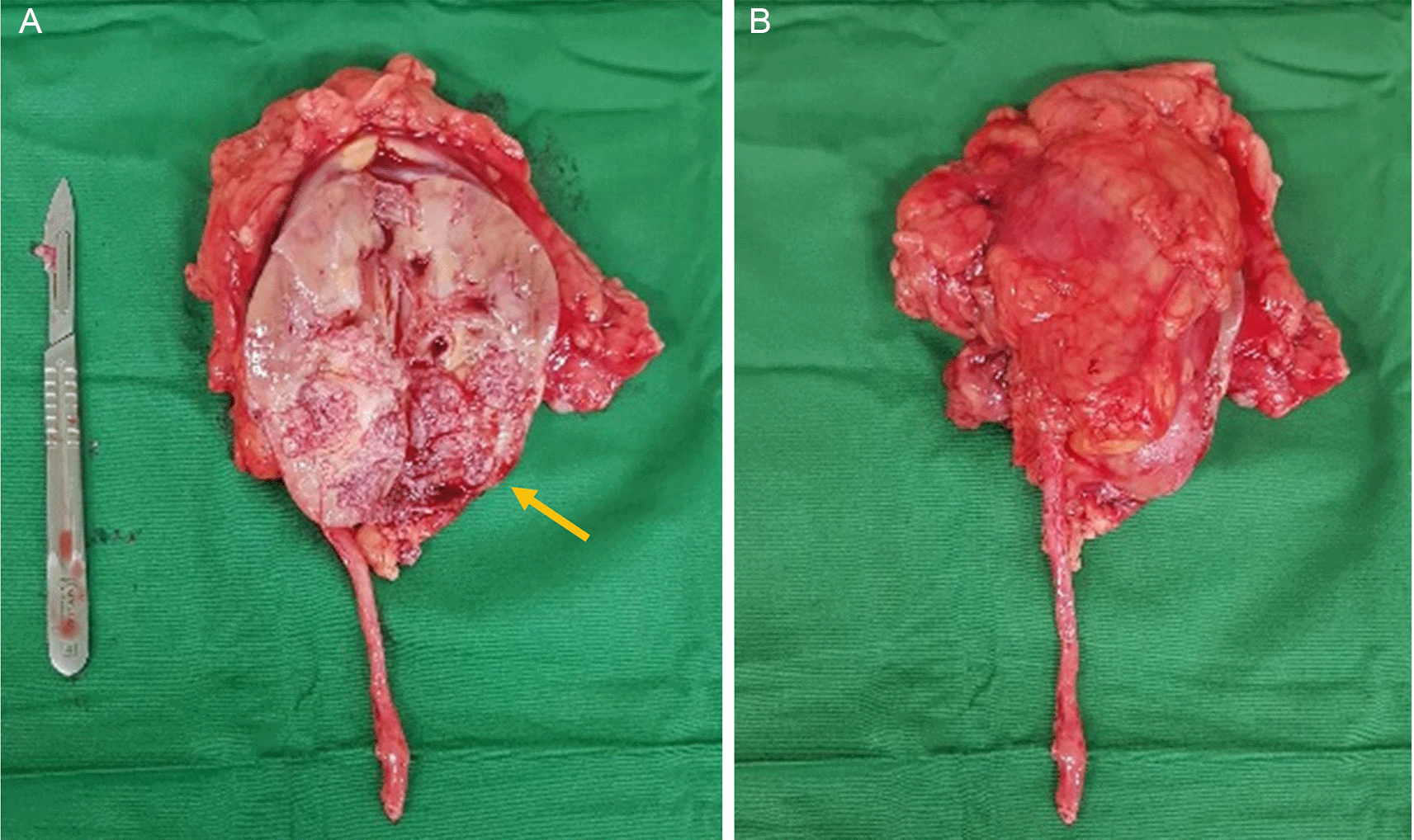
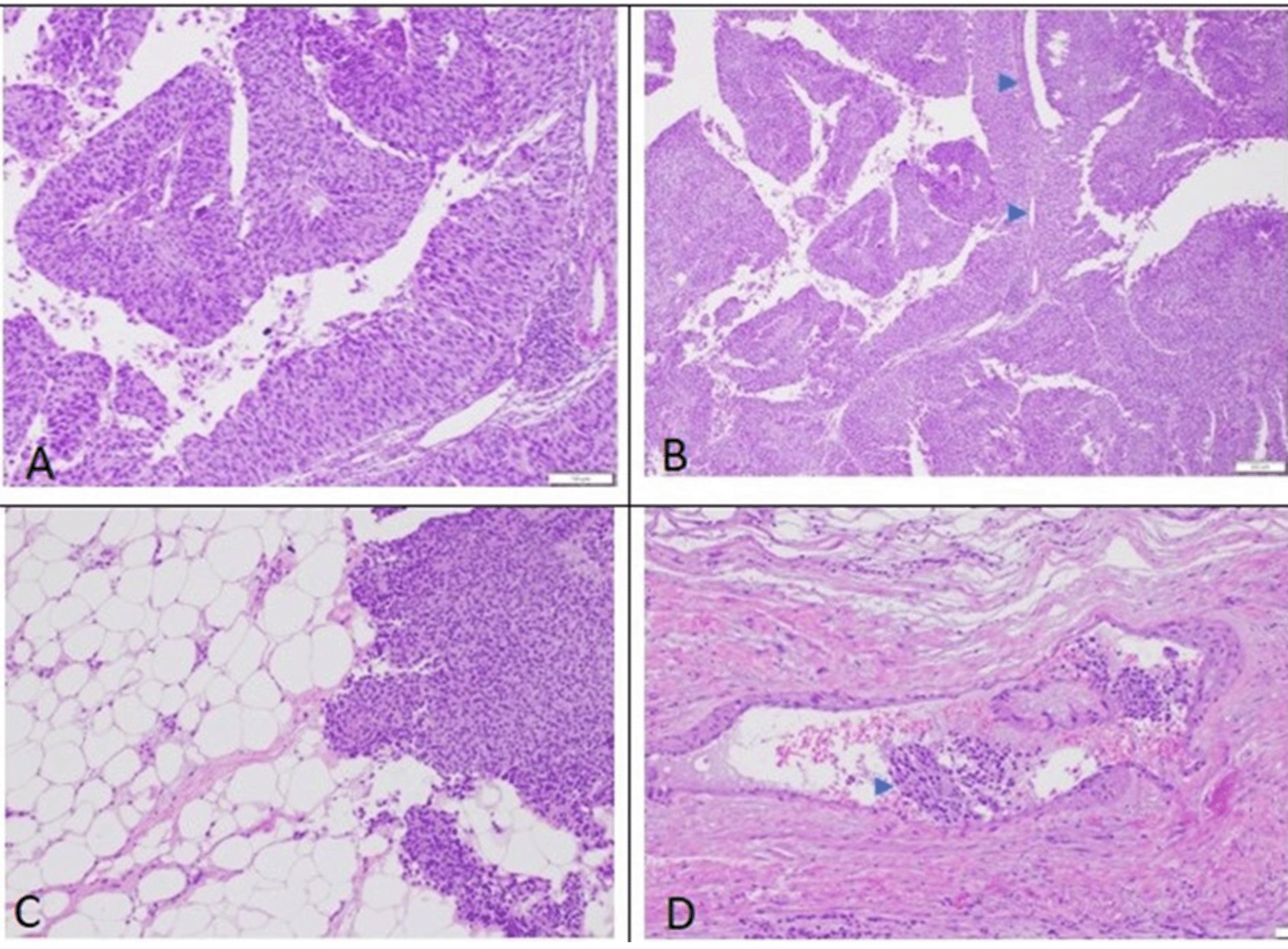
In early 2021, two years after the radical cystectomy, the patient experienced intermittent hematuria again. However, due to the COVID pandemic, the patient refuses to seek medical treatment. In late December 2021, the hematuria worsened and the patient had to receive a blood transfusion due to severe anemia. A computed tomography scan on January 2022, revealed a mass on the right renal pelvis and upper ureter with suspicion of a newly formed tumor (Figure 1). After the patient’s condition improved, he was referred again to our center and underwent a right radical nephrectomy on February 2022. A macroscopic examination revealed a right kidney with a size of 10×7.5×6 cm. Upon cleavage, a grey-white tumor mass with a size of 5×3×4 cm (Figure 2) was observed. The specimen was fixed in 10% buffered formalin. Microscopic examination of right kidney surgery section showed ureteral and renal parenchyma tissue (Figure 3). The tumor had invaded beyond the muscularis into the peripelvic fat or the renal parenchyma without lymphovascular invasion. The distal margin of the ureter showed no tumor invasion. This finding showed a tumor with histopathology characteristic of high-grade invasive urothelial carcinoma of the upper urinary tract. The patient also experienced hemorrhagic stroke 2 weeks following the radical nephrectomy. The patient was due to receive adjuvant therapy after his stroke-related condition was stable. Recently, the patent has had a routine check-up for urology, oncology, neurosurgery, and palliative care, no progression related to the malignancy was found.
UUTC is a cancer of the urothelial cells that line the upper urinary tract, from the renal calyces to the pelvises and the ureter to the ureteral orifice. UUTC is uncommon cancer, accounting for about 5% of all urothelial cancers and fewer than 10% of all renal malignancies. Meanwhile, bladder cancer is common urinary tract carcinoma. The incidence of bladder carcinoma was roughly 500,000 cases with an estimated death of more than 200,000 every year.2 The terms UCB and UUTC are interchangeable. As a result, investigations on UCB are frequently generalized to UUTC. Although UCB and UUTC show some histological similarities and share some risk factors, the most notable of which is tobacco use, there are significant clinical and molecular distinctions between the two entities.5 Within two to three years following their cystectomy, 2 to 9% of bladder cancer patients commonly develop metachronous upper urinary tract carcinoma (UUTC). When a metachronous carcinoma is identified in a different area than the primary lesion, it is usually found six months following the main lesion’s surgery.3
The molecular pathogenetic framework of UUTC and UCB is the same. As a result, patients with UUTC may have a history of UCB in up to 41% of cases or have contemporaneous UCB in about 20% of instances, which is caused by an epigenetic pan-urothelial “field deficiency”. A small percentage of UCB patients have synchronous UUTC (1.8%) or develop metachronous UUTC (0.7% to 4%). The total risk of metachronous UUTC following RC ranges from 2% to 7%, with the majority of tumors developing within the first 2 to 4 years after RC.6 In our case, the patient developed UUTC 2 years following RC for urothelial bladder carcinoma. This rare finding is relatively in line with what a previous study found in terms of the period it needs to develop metachronous UUTC following UCB.
A study by Doeveren et al. showed that patients who have been diagnosed with urothelial carcinoma of the urinary tract are more likely to develop a tumor throughout the urinary tract.7 Following the first diagnosis of urothelial carcinoma, two explanations have been proposed to explain the increased risk of recurrence in the urinary system. Carcinogenic impacts affect the whole urinary system, resulting in multifocal cancers that form independently of one another. As a result, these tumors aren’t assumed to have the same progenitor cell. In this case report, the patient was an active smoker which can be assumed that the development of metachronous was due to carcinogenic. This, however, does not explain the disparity in UUTC and UCB incidences in general, nor the disparity in the incidence of tumors in the contralateral urinary tract or the bladder after the first diagnosis of UUTC. The other hypothesis proposed is that tumor cells can proliferate and spread through the intraluminal or intraepithelial pathway. Intraluminal seeding occurred due to the implantation of malignant cells on other sites in the urinary tract. Whereas intraepithelial spreading happened due to continuous migration followed by the proliferation of transformed cells on the urinary tract epithelium.8,9
Our patient was a former smoker, hence one of the risks for developing UCB was cigarette smoking. Smoking intensity (cigarettes/day) and duration of smoking had a strong association with BC development. Former smokers had a reduced risk of bladder cancer than current smokers. Unfortunately, former smoker still have a higher risk of BC development than non-smokers.10,11
There was no clear explanation of the risk of developing UUTC following UCB therapy. However, several studies found patients who develop UUTC after UCB treatment including TURBT, intravesical chemotherapy, and radical cystectomy.12 A study by Lin et al. showed upper tract recurrences in 60 patients who underwent local excision of BC. Compared to Ta lesions, T1 bladder cancer had a 2.5-fold greater probability of recurrence in the upper tract.13 Faba et al. assessed the link between upper tract recurrence and TURBT resection of the intramural area of the distal ureter. UCB in the intramural area of the distal ureter was found in 112 out of 2317 patients who underwent TURBT for NMIBC.14
Nishyama et al. conducted a multi-institutional retrospective assessment of 402 patients with bladder cancer who received BCG following TURBT. The researchers discovered 7.5% of recurrences in the upper tract. Upper tract recurrence was predicted by intravesical recurrence and tumor characteristics at TURBT.15 In our case, the patient was diagnosed with muscle-invasive bladder cancer (MIBC). A study by Nuhn et al. showed that MIBC was associated with a high risk of developing metachronous carcinoma. The pathophysiology underlying this occurrence was thought to be similar to NMIBC.16 However studies that emphasize metachronous recurrence is limited and relatively fewer than the NMIBC.
Merrill et al. conducted oncologic monitoring after RC in 1797 patients stratified by pathologic stage. Over a median follow-up of 10.6 years, postoperative monitoring was not standardized. Urine cytology and chest/abdomen/pelvis imaging were performed every 3 months for the first 2 years after surgery, then at 6-month intervals for the next 2 years, followed by yearly imaging. Upper tract recurrence after radical cystectomy was seen in 87 individuals, with an overall recurrence incidence of 8.1%.17
Our patient was also in accordance with the previous studies found about UUTC development following RC. The survival rate for a patient with metachronous urothelial carcinoma is poor. Based on the previous study, the median survival rate was 27 months. At death, the average age was 77 (range 59 to 88).18 This suggested the need for long-term strict surveillance of the patient with bladder cancer regardless of the treatment they received, in order to detect and give an early aggressive treatment of metachronous events. Further studies are needed to get a comprehensive mechanism of UUTC and the development of metachronous UUTC after RC.
Studies on genetic factor contributing to the development of metachronous urothelial carcinoma are also limited. The mechanisms underlying multifocal bladder cancer are still unclear. A previous study observed the loss of heterozygosity (LOH) at 10 microsatellite loci and methylation of the p16INK4 CpG island in numerous tumors and pathologically normal mucosa in bladder cancer patients to see if normal mucosa had already undergone genetic or epigenetic alterations. In 77% of samples of normal epithelium, LOH or methylation was found, and LOH found in normal epithelium samples was typically found in tumor samples. This finding suggested that a population of cells in morphologically normal epithelium shared genetic or epigenetic abnormalities with bladder cancer, which may serve as a basis for the development of numerous tumors.19 Further study about genetic change is needed.
Our study also has limitations, first, the inability to generalize because the data was only available for one patient case and one gender. Second, there is currently no way to demonstrate a cause-and-effect connection. While these limitations are common in case report studies, however, we also hope that this case can add up the available data regarding Metachronous urothelial carcinoma, considering a rare occurrence.
Metachronous UCB to the upper urinary tract following RC was a rare case. Strict surveillance of patients with UCB is needed to detect the development of metachronous carcinoma events in the urinary tract. Early detection and aggressive treatment are always the best policy in managing malignancy cases. In the future, we hope there will be more studies that can explain comprehensively the mechanism, risk factors, and genetic study of metachronous UUTC following BC surgery.
Written informed consent was received from the patient. The patient and his family agreed to the publication of this article.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Yes
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the case presented with sufficient detail to be useful for other practitioners?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular Biologist
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 23 Jun 23 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)