Keywords
Coronary artery disease, Peripheral artery disease, Abdominal Aortic Aneurysm, Carotid Artery Stenosis, targeted screening, predictive model, clinical risk score
Coronary artery disease, Peripheral artery disease, Abdominal Aortic Aneurysm, Carotid Artery Stenosis, targeted screening, predictive model, clinical risk score
I have made revisions as per the suggestions of the reviewers, mainly to shorten the literature in Discussion. The prevalence of metabolic syndrome in our study was revised after reviewing the existing data. However, these revisions do not change the results of this study.
See the authors' detailed response to the review by Johanes Nugroho Ekoputranto
See the authors' detailed response to the review by Marc Vuylsteke
Atherosclerosis underlines some of the major cardiovascular diseases which are the leading cause of mortality worldwide. Due to its systemic nature, atherosclerosis in the coronary arteries may indicate occurrences in other arteries.1 Coronary arterial disease (CAD) is associated with peripheral artery disease (PAD), carotid artery stenosis (CAS), and abdominal aortic aneurysm (AAA). There is an increasing prevalence of vascular diseases in patients with a more severe CAD, especially high-risk CAD with metabolic syndromes and three-vessel disease.2–4 Therefore, the screening in patients with CAD is justifiable in assessing the risk of atherosclerosis occurrences in other arteries.
CAD is one of the leading causes of mortality worldwide with a substantial incidence rate. According to the Centers for Disease Control and Prevention (CDC) in the United States, a total of 20.1 million adults aged 20 years old and older have CAD.5 Screening for PAD, carotid artery stenosis, and AAA enable early detection, risk stratification, and early cardiovascular treatment; all in favor of reducing morbidity and mortality.6,7 However, screening program targeting all CAD patients is highly ineffective, costly, and requires multiple resources such as specific instruments and trained technicians. On the other hand, screening for early detection of some vascular diseases, e.g., PAD, in patients with significant CAD was not shown to be more beneficial compared to a routine medical checkup.8 Therefore, targeted screening in patients with CAD requires careful consideration based on risk factors for atherosclerosis in other arteries.
Targeted screening contributes to early detection while being more time and cost-effective than general screening; it can be beneficial for diseases previously deemed not necessary to screen. A study specifically assessed targeted screening for AAA was already done involving a small subset of indigenous people in Borneo.9 No research has been conducted to assess the predictors for developing multi-vascular diseases, including PAD, CAS, and AAA in patients with CAD. Furthermore, no studies assessed the number needed to screen (NNS) of screening for multi-vascular diseases in CAD patients and the impact of the clinical risk scoring system for said NNS. We hypothesize that a risk-scoring tool to measure the risk of other vascular diseases in CAD patients is feasible to construct and can be applied for targeted screening; in return, the risk-scoring tool can reduce the NNS for asymptomatic multi-vascular diseases. Therefore, this study aims to investigate factors predicting the occurrence of multi-vascular diseases in patients with CAD while constructing a predictive model and scoring system to enable targeted screening for future uses.
This study was conducted based on the STROBE guideline for observational studies.10 The study protocol was approved by the National Cardiovascular Center Harapan Kita Hospital committee of ethics (ethics approval number: LB.02.01/VII/509/KEP005/2021). All patients gave written informed consent prior to the recruitment of the study and were free to decline participation.
This cross-sectional study was conducted at the National Cardiovascular Center of Harapan Kita Hospital from March 2021 to December 2021. All patients who underwent elective coronary angiography or percutaneous coronary intervention from March 2021 to December 2021 were initially included. Patients diagnosed with coronary artery disease (CAD) were eligible for inclusion in our study. We excluded patients with previously diagnosed CAD that were hospitalized for other reason than elective coronary angiography or coronary angioplasty. We also excluded patients with connective tissue disorder. All included patients were concomitantly screened for vascular disease. The primary outcome of interest was vascular diseases in other vascular territories. For all patients, we examined the list of variables relating to sociodemographic characteristics, cardiovascular risk factors, and other related diseases.
The diagnosis of hypertension was based on documented medical history, the use of antihypertensive drugs, and the presence of elevated systolic and/or diastolic blood pressure according to European Society of Cardiology guidelines.11 Diabetes mellitus was diagnosed based on documented medical history through the use of hypoglycemic agents, and/or laboratory criteria according to the American Diabetes Association (ADA) 2021.12 Dyslipidemia was defined based on documented medical history and the use of lowering lipid agents or laboratory criteria according to the National Cholesterol Education Program’s Adult Treatment Panel III (NCEP-ATP III).13 Metabolic Syndrome was also defined according to the NCEP-ATP III. Information on the history of cerebrovascular disease (transient ischemic attack or stroke) was collected from the patient’s reports or medical records.13 CAD was diagnosed using angiography. The presence of coronary lesions was determined using visual estimation. Coronary artery lesions were considered as CAD if 1) at least one major epicardial artery or its major branches have significant stenosis (70% for left anterior descending artery, left circumflex artery, right coronary artery, or 50% for left main trunk) or 2) the patient was previously hospitalied for treatment of coronary artery lesions (balloon, stent, or coronary artery bypass grafting).
Lower extremity peripheral arterial disease (PAD) was defined with 1) ankle-brachial index of < 0.9 or 2) the patient was previously treated for PAD.14 Evaluation of carotid artery stenosis (CAS) and AAA was conducted using bedside ultrasound Affiniti 70 (Philips, Amsterdam, Netherlands) by a cardiovascular technician blinded to other data. Peak systolic velocity, end-diastolic velocity, and intima-media thickness of the common carotid artery and internal carotid artery were calculated to evaluate CAS. The degree of CAS was classified according to Grant et al.15 CAS was considered significant if 1) the presence of stenosis ≥50% from ultrasound examination or 2) the patient was previously treated for CAS (carotid stenting or carotid endarterectomy). AAA was defined as an enlargement of the abdominal aorta with a diameter of ≥3 cm or a previously treated AAA lesion (Endovascular aortic repair/EVAR or open surgical repair).16 The maximum and minimum abdominal aortic diameter (anteroposterior or transverse axis) were obtained.
Potential bias for each diagnosis of vascular disease were minimized by involving third-party examiners who were not aware of the existence of this study.
Categorical variables were expressed in the form of numbers and percentages, whereas continuous variables were expressed as their mean value±SD in data with normal distribution or their median (interquartile range) value in data without normal distribution. We compared categorical variables using Pearson’s chi-square or Fisher’s exact test while continuous variables were compared using the Student t-test or Mann-Whitney U test. We conducted multivariable stepwise logistic regression to generate prediction models with the primary endpoint of multivascular disease incorporating clinical variables. All variables with p value of <0.25 by univariate analysis were included in the multivariable model. The selection of variables for retention was based on p value of <0.05. We additionally performed the Hosmer-Lemeshow test to assess the goodness of fit of the model and plot the observed versus predicted data graph.
For each significant variable from multivariate analysis, a regression β coefficient was obtained, and a scoring system was created to predict the incidence of coexisting vascular diseases. Points for the scoring prediction rule were assigned by weighing each significant variable compared to the total β coefficient. Then, points were made using the weighted coefficients with rounding to the nearest whole number. We created the cutoff points to classify patients with low, moderate, and high-risk probability, respectively. To test the model discrimination, C-statistic was also conducted to calculate the area under the curve. We also conducted internal validation by bootstrap using the same amount of included samples. All statistical analyses were performed using SPSS version 23 (IBM, New York, USA) and STATA version 16 MP (StataCorp, Texas, USA).
A total of 1314 patients with CAD were identified; 203 (15.4%) patients have multi-vascular disease. All patients had complete medical record data and, therefore, no missing data in this study. Sociodemographic and clinical data are shown in Table 1. Amongst the variables in patient’s demographics, there was a significant difference in the proportion of patients with cerebrovascular disease, CAD three-vessel disease (CAD3VD), and CAD left main disease (CAD-LM). The prevalence of PAD, CAS, and AAA in patients with CAD were 143 (10.9%), 59 (4.5%), and 19 (1.4%), respectively. The overlap between vascular diseases is shown in Figure 1.
There is a difference in prevalence of vascular diseases in CAD patients with one, two, and three-vessel disease (Table 2).
Univariate analysis demonstrated individuals with older age, diabetes mellitus, cerebrovascular disease, CAD3VD, and left main disease were more likely to have multivascular disease (Table 3). After a multivariate analysis, four variables were retained to form the final clinical model: ages of ≥60 years (OR: 1.579; 95% CI: 1.153-2.164), diabetes mellitus (OR: 1.412; 95% CI: 1.036-1.924), cerebrovascular disease (OR: 3.656; 95% CI: 2.326-5.747), and CAD3VD (OR: 1.960; 95% CI: 1.250-3.073). All of the predictors remain significant after bootstrap internal validation (Table 3).
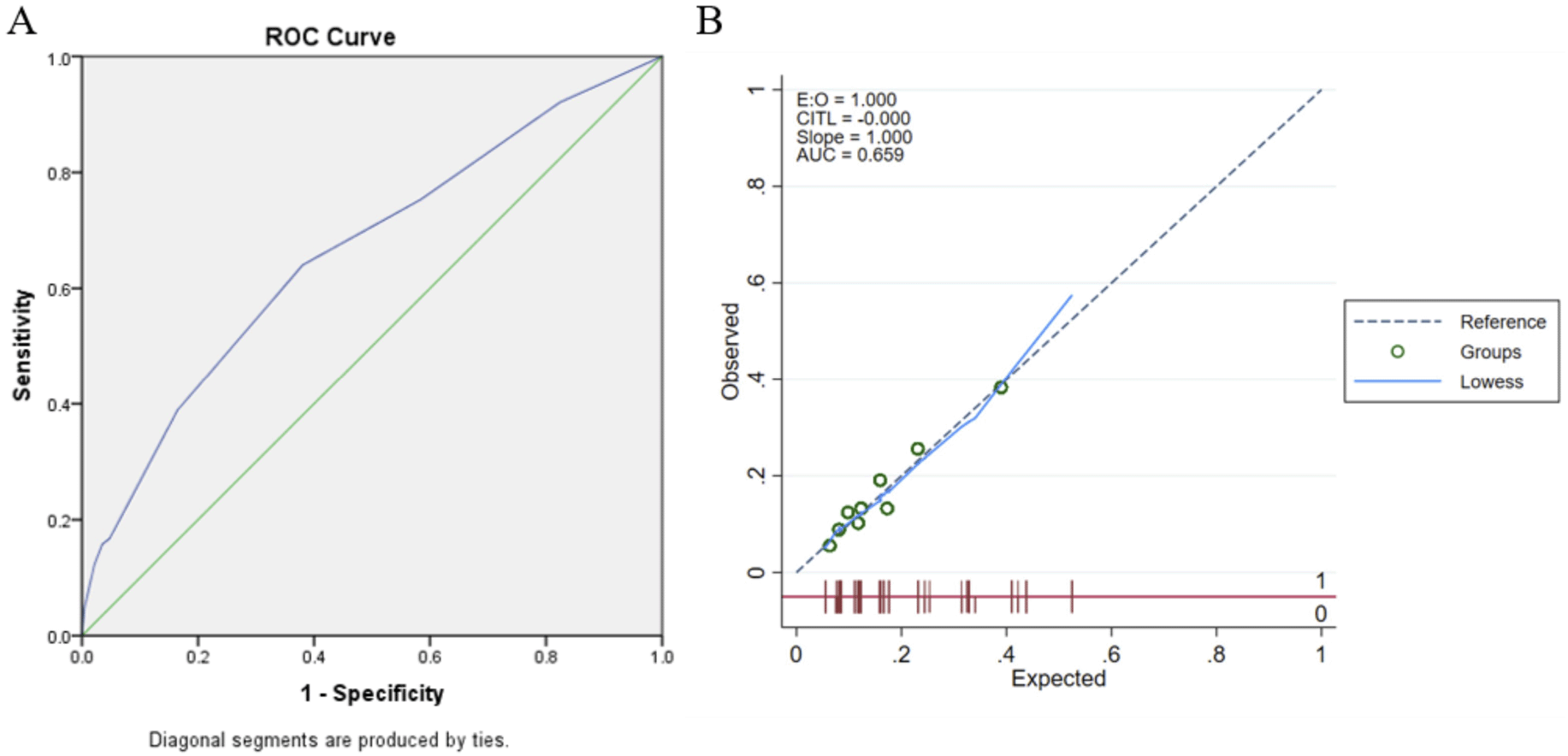
The area under the curve of the model was 0.659 (95% CI: 0.617-0.700) and was well calibrated (Hosmer-Lemeshow test p=0.379;). Using bootstrap validation, the optimism-corrected area under the curve was 0.653 (95% CI: 0.610-0.695), which represents the predictive ability of the model (Figure 2).
Those four variables were assigned weighted points scores for the final clinical prediction of multi-vascular disease based on the magnitude of the β coefficient (Table 4). We designated a score of 0–2 as low probability (9% of chance), 3–5 as moderate probability (21%), and 6–8 as high probability (42%). The discriminatory ability of the scoring system was moderate (AUC: 0.649; 95% CI: 0.610-0.687).
| Variable | Score |
|---|---|
| Age ≥ 60 | 1 point |
| Diabetes Mellitus | 1 point |
| Cerebrovascular Disease | 4 points |
| Coronary Artery Disease 3 Vessel Disease | 2 points |
Based on our data, targeted screening for patients with moderate risk or higher decreases the number needed to screen (NNS) from six to five while targeted screening for patients with high risk reduces the NNS from six to three. Targeted screening for patients with moderate risk or higher had the most balanced results of diagnostic values with positive predictive value (PPV), negative predictive value (NPV), sensitivity, and specificity of 45,1%, 86,2%, 15,8%, and 96,5% respectively.
This is the first study in Indonesia, to investigate factors predicting the occurrence of multi-vascular diseases in patients with CAD. Our findings show that three vascular diseases were all encountered in CAD patients namely; PAD with prevalence of 10.9%, CAS with prevalence of 4.5% and lastly AAA with prevalence of 1.4%. Our final model to predict multi-vascular disease were as the following: older age (≥ 60 years) [OR = 1.58 (1.15 – 2.16; p = 0.04], diabetes mellitus [OR = 1.41 (1.04 – 1.92); p = 0.029], cerebrovascular disease [OR = 3.66 (2.32 – 5.74); p < 0.001] and CAD3VD [OR = 1.96 (1.25 – 3.07; p = 003]. Furthermore, we constructed the scoring system as well, consisting of age ≥ 60 years old (1 point), diabetes mellitus (1 point), cerebrovascular disease (4 point) and CAD-3 vessels (2 points). Remarkably, the Hosmer-Lemeshow test with a p value of 0.38 demonstrated that the calibration of this final model was highly accepted, and in addition, utilising bootstrap validation, the AUC was 0.65 (95% CI: 0.61 – 0.69) reflecting the model’s capacity to make a sufficiently accurate discrimination.
The aforementioned predictors seem to be less prevalent than that in other studies, carried out elsewhere. In a study by Poredos et al. (2007), screening with the ankle-brachial index (ABI) revealed that 42% of CAD patients also suffered PAD.2 Tanimoto et al. (2015) found that CAS prevalence in patients with CAD ranged from 7.0% to 36.0%.3 Durieux et al. (2014) demonstrated that 2.5% to 14.4% of all CAD also had asymptomatic AAA, hence confirming the presence of AAA in CAD.4 The lower prevalence of the aforementioned predictors in this findings might be due to the younger patients recruited in our study, compared to others. It is acknowledged that the incidence of PAD, CAS and AAA increases along with age.17–19 The shift of peak prevalence towards younger subjects in Indonesia might be caused by the higher frequency of CAD risk factors encountered in younger generation. This is supported by the study of Hussain et al. (2016), which demonstrated a higher population attributable risk proportion (PAR) percentages of smoking habits, hypertension, excess body weight, diabetes mellitus and hypercholesterolemia in CAD subjects less than 55 years old compared to older population.20
The association between the severity of CAD and the extent of atherosclerosis was also observed in other vasculatures. The extent of fat deposit along coronary vasculatures is proportional to the severity of atherosclerosis, as demonstrated by the increased expression of interleukin-6 (IL-6) and leptin and the decreased expression of serum adiponectin.21 Our data showed no significant increased AAA prevalence in relation with CAD severities. This might be due to a small prevalence of AAA, in which affected to no statistical significance.
Given the final model in our study, Honda T, et al in the Hisayama study (2022) conducted a prospective observational study and constructed a risk prediction model for the development of ASCVD in Japanese adults. They found that age, sex, systolic blood pressure, diabetes mellitus, proteinuria, smoking habit and regular exercise are all predictors of ASCVD occurrence.22 The Hisayama study, in contrast to ours, used Japanese people who had no prior history of CVD. Consequently, they conducted a prospective observational study and followed the subjects for 24 years.
In order to construct risk prediction model, risk scoring was made from β-coefficient obtained from the multivariate analysis. The calibration of the final model fulfilled the goodness of fit, utilizing the Hosmer-Lemeshow test. Determinant capability of risk scoring was shown to be moderately good with AUC of 65.9%. Likewise, this determinant was quite comparable with another risk prediction model in those with stable CAD, carried out by Badheka et al (2011). They established the predictors such as: history of hypertension, smoking, age and history of diabetes with AUC of 68.6%.23 Additionally, our study demonstrated that general screening of all CAD patients resulted in 15.4% cases with multi-vascular disease, and number needed to screen (NNS) was 6. Meanwhile, targeted screening for patients with high-risk based on our own risk scoring would reduce the NNS from 6 to 3 identifying 45% CAD patients suffered other vascular disease. Consequently, our clinical risk scoring proved to have a very high specificity of 96.5%. Although choosing an NNS of 6 is arbitrary, but the decrement of NNS value from 6 to 3 was quite impressive.
The limitation of this study is the cross-sectional design, which does not measure the potential for future vascular disease incidents and enabled bias. Potential bias in this study primarily stems from assessor’s confirmatory bias, when diagnosing the incident of asymptomatic vascular disease. However, this confirmatory bias was lessened by involving third party examinators. Temporal and external validation is actually needed to confirm the study results.
Patients with CAD who have diabetes mellitus, cerebrovascular disease, CAD3VD, and above 60 years old are associated with increased odds of multi-vascular disease. By using risk scoring tool made from these risk factors, targeted screening in high-risk patients decrease the number needed to screen in half with high specificity.
Suko Adiarto: Conceptualization, Methodology, Writing – Original Draft, Writing – Review & Editing, Data Curation, Visualization. Luthfian Aby Nurachman: Formal Analysis, Writing – Original Draft. Raditya Dewangga: Formal Analysis, Investigation, Suci Indriani: Investigation, Methodology Taofan: Investigation, Methodology Amir Aziz Alkatiri: Investigation, Methodology Doni Firman: Investigation, Methodology Anwar Santoso: Conceptualization, Validation, Writing – Review & Editing, Supervision, Funding Acquisition All authors read and approved the final paper.
Figshare: Predicting Multi-Vascular Diseases_Dataset. DOI: http://doi.org/10.6084/m9.figshare.22881431. 24
This study contains the following underlying data: Predicting Multi-Vascular Diseases_Dataset.xlsx (data used for analysis).
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Vascular surgery
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cardiology and vascular medicine
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Vascular surgery
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 12 Sep 23 |
read | |
|
Version 1 26 Jun 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)