Keywords
SVEP1, Polydom, sepsis, vascular endothelial cell, lymphatic endothelial cell
This article is included in the Cell & Molecular Biology gateway.
SVEP1, Polydom, sepsis, vascular endothelial cell, lymphatic endothelial cell
Sepsis remains the leading cause of death despite the development of acute care and the widespread use of guidelines.1–3 Among the various factors related to the severity of sepsis,4–6 a recent genome-wide association study has revealed that sushi, von Willebrand factor type A, EGF, and pentraxin domain-containing 1 (SVEP1) gene polymorphism is associated with altered mortality and organ dysfunction in septic shock.7,8 The negative impact of genetic polymorphisms of SVEP1 has also been reported in coronary artery disease with promoted inflammation and atherosclerosis.8–10 These findings suggest that SVEP1 plays a critical role in the progression of systemic inflammation. Therefore, investigating SVEP1 following surgical invasion and sepsis may contribute to further elucidation of the pathophysiology and development of novel treatments.
SVEP1 is an extracellular matrix protein containing a signal peptide followed by multiple different protein domains: a pentraxin domain, a von Willebrand factor type A domain, ephrin2-like cysteine-rich repeats, a hyalin domain, domain similar to thyroglobulin type 2 repeats, 10 epidermal growth factor domains, and 34 complement control protein modules.11 SVEP1, with a size greater than 300 kDa, is present in the cytoplasm and is degraded into N-terminal SVEP1 (90 kDa) after secretion into the extracellular space.12 This cell adhesion molecule appears to play a key role in regulating intercellular adhesion and embryonic lymphatic development via the angiopoietin-2 and Tie1/Tie2 receptor systems.12–15
Cell adhesion and vascular permeability have been recognized as important elements that alter the pathophysiology of sepsis.16,17 An interaction between angiopoietin and Tie, which modulates endothelial permeability, has an impact on the progression of organ dysfunction and mortality in septic shock.18–23 A previous study showed that inhibition of SVEP1 expression resulted in an increase in the levels of soluble intracellular adhesion molecules (sICAM) and soluble E-selectin in an in vitro model of endotoxemia, implicating that SVEP1 is responsible for regulating vascular permeability in sepsis.24 Despite the potentially strong associations between SVEP1 and sepsis, the role of SVEP1 during systemic inflammation is yet to be determined.
Therefore, in this study, we tested the hypothesis that surgical invasion and sepsis alter SVEP1 gene expression and protein production in a murine model.
C57BL/6 mice were purchased from Japan CLEA (Tokyo, Japan). All mice were housed in a controlled environment with a 12-h day and night cycle under specific pathogen-free conditions. Eight- to 12-week-old male and female mice were used for the experiments. The experimental procedures were approved by the Institutional Animal Care and Use Committee of Chiba University (approval number; 2-88; 3/13/2020). This study was carried out in accordance with the recommendations of the Chiba University Resolution on the Use of Animals in Research. The protocol was approved by the Institutional Animal Care and Use Committee of the Chiba University, School of Medicine. The mice were maintained under specific pathogen-free conditions at the Animal Center of the Chiba University Graduate School of Medicine. All efforts were made to ameliorate harm to animals. If necessary, we sacrificed mice under anesthesia to minimize any suffering of animals.
The cecal ligation and puncture (CLP) procedure was used to create an intra-abdominal sepsis model. Briefly, a midline incision was made on the abdomen, and the cecum was exposed following anesthesia with 2% isoflurane. The cecum was ligated with a 15 mm length from the tip of the cecum and punctured once with a 20-gauge needle after squeezing feces into the cecum. The abdomen was closed in layers. Next, 1 mL of saline was injected subcutaneously for fluid resuscitation. The same procedures were used in the sham operation model, except for CLP. After the surgery, the mice were sacrificed by cervical dislocation at the time of sample collection under anesthesia. In the control (no surgery), sham operation model, and CLP model, samples were harvested at 2, 6, and 24 h after the surgery without perfusion.
We compared the gene expression and protein levels of SVEP1 between the control (no surgery), sham operation model, and sepsis model with cecal ligation and puncture in mice. Samples were collected at 2, 6, and 24 h after surgery. We used eight mice in each model for reverse transcription-quantitative polymerase chain reaction and western-blotting, and four mice in each model for flow cytometry. We used a total of 92 mice in all experiments. We did not conduct priori sample size calculation. As we had no pre-defined exclusion criteria, no mice were excluded in each experiment.
Total RNA was isolated using a RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Single-stranded cDNA was transcribed from total RNA using a SuperScript III™ First-Strand Synthesis System for RT-PCR and random hexamers (Invitrogen, USA). RT-qPCR was performed with a Power SYBR® Green PCR Master Mix (including Dual-Lock™ Taq DNA Polymerase) (Thermo Fisher Scientific, USA) and analyzed using an ABI PRISM® 7000 Sequence Detection System (Applied Biosystems, USA). We used GAPDH as a housekeeping gene. Primers are shown in Table 1. The thermal cycling were run on 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds and 60°C for 1 minute, 95°C for 15 seconds, 60°C for 1 minute, and 95°C for 15 seconds.
RT-qPCR, reverse transcription-quantitative polymerase chain reaction; F, forward; R, reverse.
The rabbit anti-N-terminal region of mouse SVEP1 (polyclonal N antibody) developed at the Institute for Protein Research, Osaka University12 was used to detect proteins. The proteins collected from the homogenized organs were run on polyvinylidene difluoride gels and transferred onto silver nitrate or polyvinylidene difluoride membranes. For immunoblotting, the membranes were probed with the poly N antibody and peroxidase-conjugated affinity pure donkey anti-rabbit IgG (Jackson Immuno Research, USA) and then reacted with ECL Prime Western Blotting Detection Reagent (GE Healthcare, USA). The signal intensity of SVEP1 in the organs was analyzed using ImageJ software (RRID:SCR_003070) (Bethesda, MD, USA). We showed representative densitometric images of western blotting that were cropped as protein bands corresponding to the size of SVEP1 and GAPDH. We did not splice the images.
Whole lung tissue was collected without perfusion and treated with collagenase to isolate single cells. After labeling with cell-surface antibodies, including anti-mouse CD45.2, anti-mouse CD31 (BD Pharmingen, USA), and anti-mouse lymphatic vessel endothelial receptor 1 (LYVE-1) (LifeSpan Biosciences, USA), the cells were stained with poly N antibody as the primary antibody and goat anti-rabbit IgG-FITC (Santa Cruz Biotechnology, USA) as the secondary antibody. This was followed by permeabilization and fixation using the Foxp3/Transcription Factor Staining Buffer Set® (eBioscience, USA). Samples were run on a FACSCalibur® (BD Bioscience, USA) and analyzed using FlowJo® (RRID:SCR_008520) (BD Bioscience). A freely available alternative for the analysis is R version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria, https://www.r-project.org/) using the flowCore package (RRID:SCR_002205).
We compared the control with the sham operation model and CLP model using the Kruskal-Wallis test with Dunnett’s multiple comparison test. We compared the sham operation model with the CLP model at each point in time using the Student’s t-test or Mann-Whitney test according to normality. We used GraphPad Prism 8 (RRID:SCR_002798) (GraphPad software, USA) for statistical analysis. R version 4.1.2 (R Foundation for Statistical Computing) is a freely available alternative for statistical analysis. Results were considered statistically significant at p < 0.05.
We first evaluated SVEP1 gene expression and protein production in vital organs, including the heart, lungs, liver, spleen, kidneys, and colon, in C57BL/6 mice at baseline. The lungs and spleen had 29.6- and 11.2-fold higher SVEP1 gene expression, respectively, compared with the average expression in the other four organs (Figure 1A).36
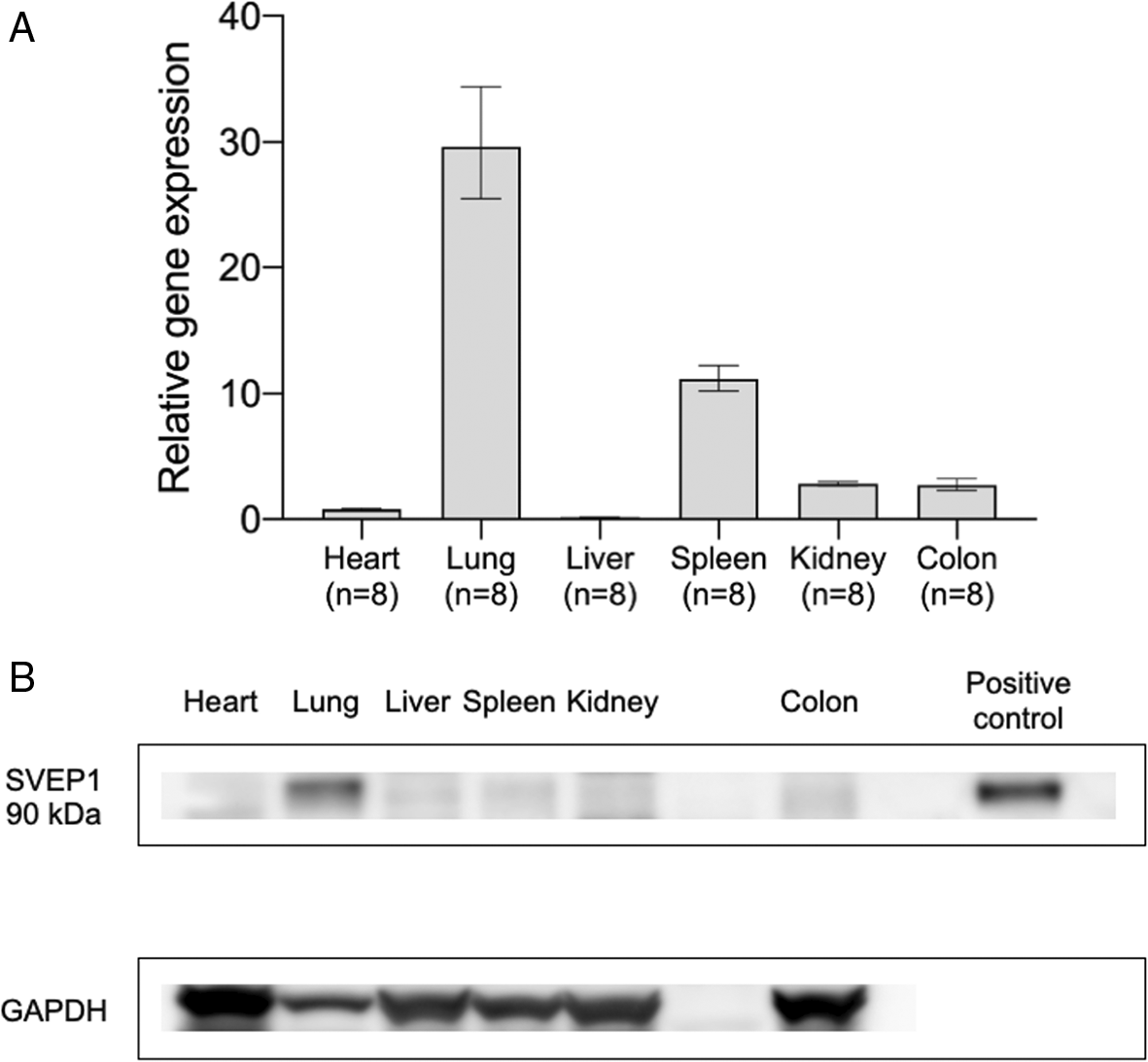
(A) Relative SVEP1 gene expression levels in various organs (normalized by the average of four organs: heart, liver, kidneys, and colon) are shown. Data are shown as the mean ± SEM; n = 8 mice in each group. (B) Representative densitometric images of western blotting in various organs at baseline are shown. GAPDH was used for normalization. SVEP1, sushi, von Willebrand factor type A, EGF, and pentraxin domain-containing 1.
Subsequently, SVEP1 protein expression in the organs was evaluated using the poly N antibody. The target band for the lung tissue was approximately 90 kDa, which is the size of the N-terminal SVEP1 secreted into the extracellular space. No target bands were clearly detected for the heart, liver, spleen, kidneys, and colon (Figure 1B). These results demonstrated that the gene expression and protein levels of SVEP1 were relatively high in the lungs at baseline.
Since SVEP1 gene expression and protein levels were higher in the lungs at baseline than in other organs, we focused on the lung and examined the time course of SVEP1 gene and protein expression in the lungs after sham operation and CLP.
In the lungs, there was a significant decrease in SVEP1 gene expression 2 h after sham operation and 2/6/24 h after CLP surgery. SVEP1 gene expression returned to baseline levels at 6 and 24 h after sham operation. Furthermore, sepsis decreased SVEP1 gene expression until 24 h after CLP surgery. We found that SVEP1 gene expression was significantly reduced in the CLP model at all three time points compared with the sham operation model (Figure 2). These results indicate transiently decreased gene expression of SVEP1 in surgical invasion and persistent suppression of gene expression after sepsis induction.
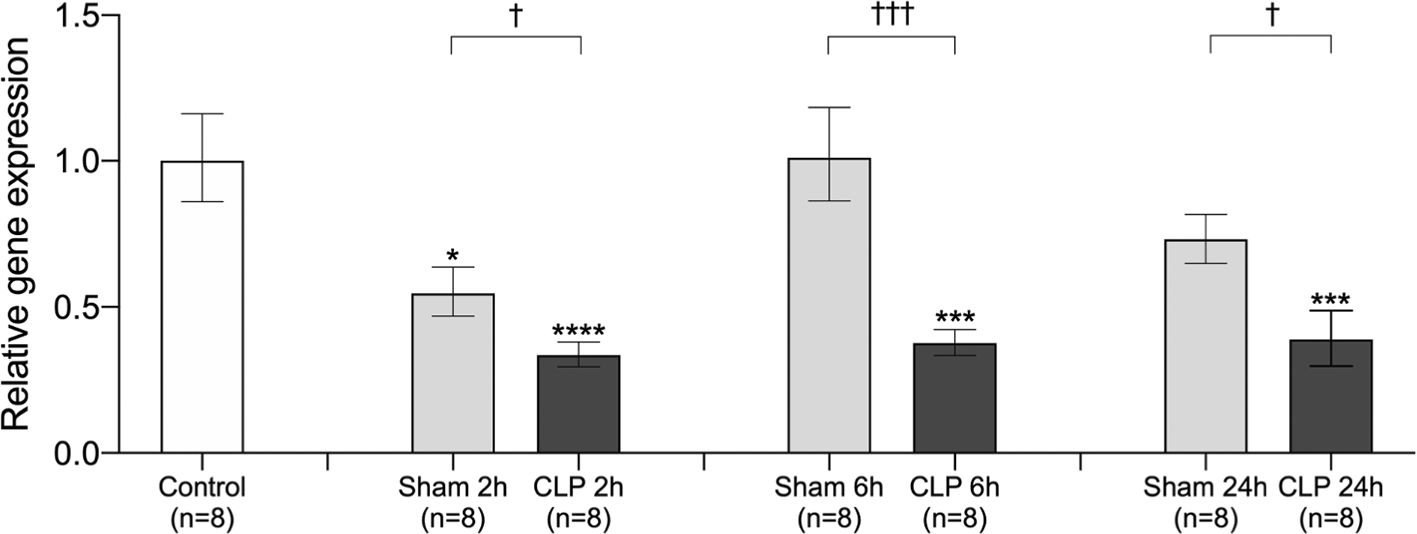
Relative SVEP1 gene expression in the lungs was compared between control (no surgery), 2 h after sham operation (Sham 2h), 6 h after sham operation (Sham 6h), 24 h after sham operation (Sham 24h), 2 h after CLP procedure (CLP 2h), 6 h after CLP procedure (CLP 6h), and 24 h after CLP procedure (CLP 24h). The data were normalized using the control. Error bars represent the mean ± SEM; n = 8 mice in each group. We compared the control to the Sham and CLP models using the Kruskal-Wallis test with Dunnett’s multiple comparison test (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001). We compared the sham operation model with the CLP model at each time point using the Student’s t-test or Mann-Whitney test according to the normality (†, p < 0.05; ††, p < 0.01; †††, p < 0.001; ††††, p < 0.0001). SVEP1, sushi, von Willebrand factor type A, EGF, and pentraxin domain-containing 1; CLP, cecal ligation and puncture.
We quantitatively evaluated the SVEP1 90 kDa protein in the lungs by western blotting (Figure 3A). There was no change in the SVEP1 90 kDa protein level 2/24 h after sham operation and 2/6/24 h after CLP surgery. The SVEP1 90 kDa protein level was slightly elevated only 6 h after sham operation, and there was a significant difference between the Sham 6 h and CLP 6 h group (Figure 3B).
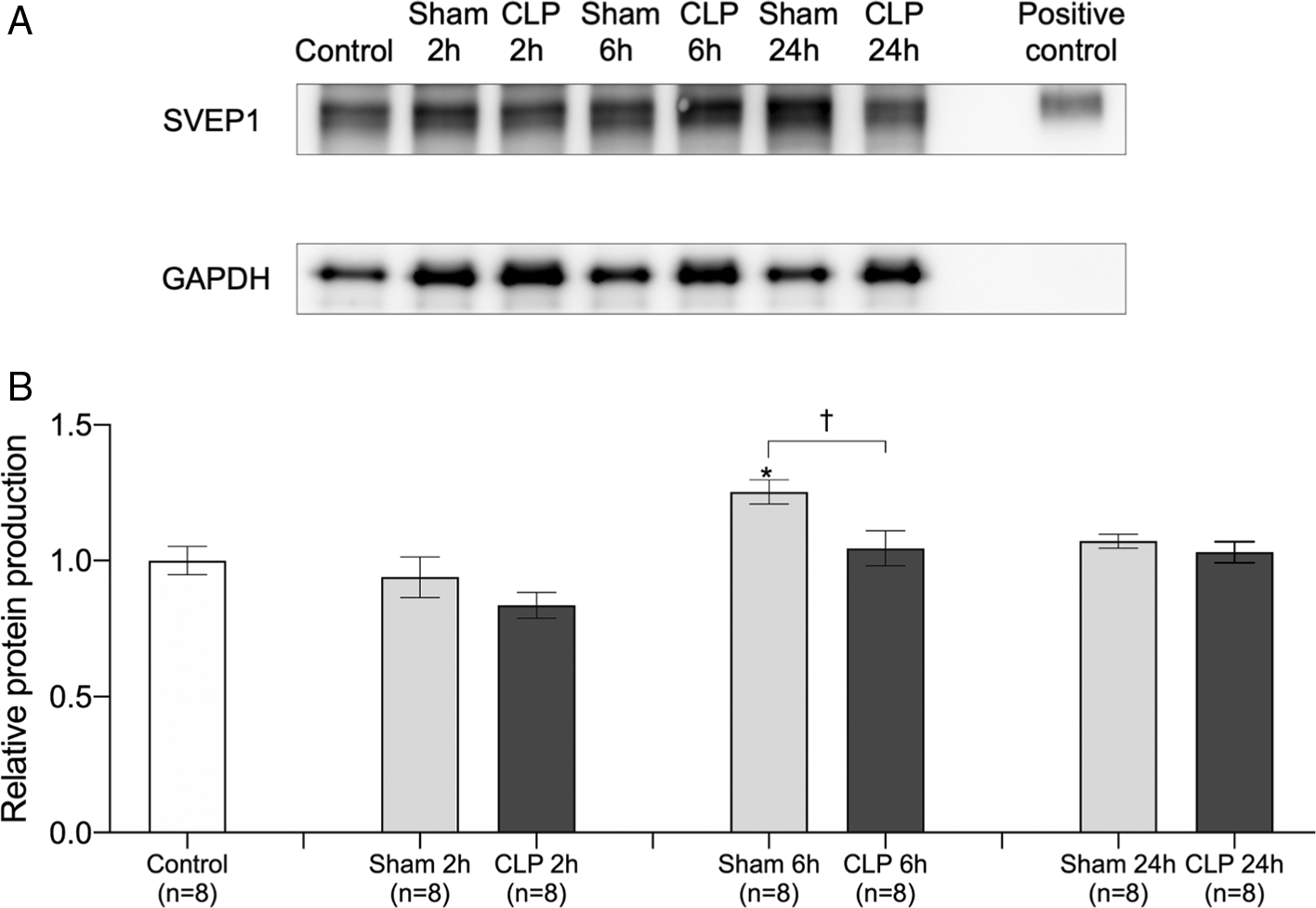
(A) Representative densitometric images of western blotting in the lungs are shown. GAPDH was used for normalization. (B) Relative expression of 90 kDa SVEP1 protein was compared between control (no surgery), 2 h after sham operation (Sham 2h), 6 h after sham operation (Sham 6h), 24 h after sham operation (Sham 24h), 2 h after CLP procedure (CLP 2h), 6 h after CLP procedure (CLP 6h), and 24 h after CLP procedure (CLP 24h). The data were normalized using the control. Error bars represent the mean ± SEM; n = 8 mice in each group. We compared the control with the Sham and CLP models using the Kruskal-Wallis test with Dunnett’s multiple comparison test (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001). We compared the sham operation model with the CLP model at each time point using the Student’s t-test or Mann-Whitney test according to the normality (†, p < 0.05; ††, p < 0.01; †††, p < 0.001; ††††, p < 0.0001). SVEP1, sushi, von Willebrand factor type A, EGF, and pentraxin domain-containing 1; CLP, cecal ligation and puncture.
Since SVEP1 is an extracellular matrix protein,12 we examined the time course of gene expression of other extracellular matrix proteins, such as collagen1 and fibronectin, in the lungs after sham operation and CLP surgery. The gene expression of collagen1 was significantly decreased after sham operation and CLP surgery. At 6 and 24 h after the insult, gene expression was significantly lower in the CLP model than in the sham operation model (Figure 4A). By contrast, the gene expression of fibronectin significantly increased after sham operation and CLP surgery. In the sham operation model, fibronectin gene expression returned to baseline levels after 6 h, whereas gene expression in the CLP model remained significantly higher than that in the sham operation model (Figure 4B). The time course of collagen1, but not fibronectin, showed a similar trend with that of SVEP1 gene expression in sepsis.
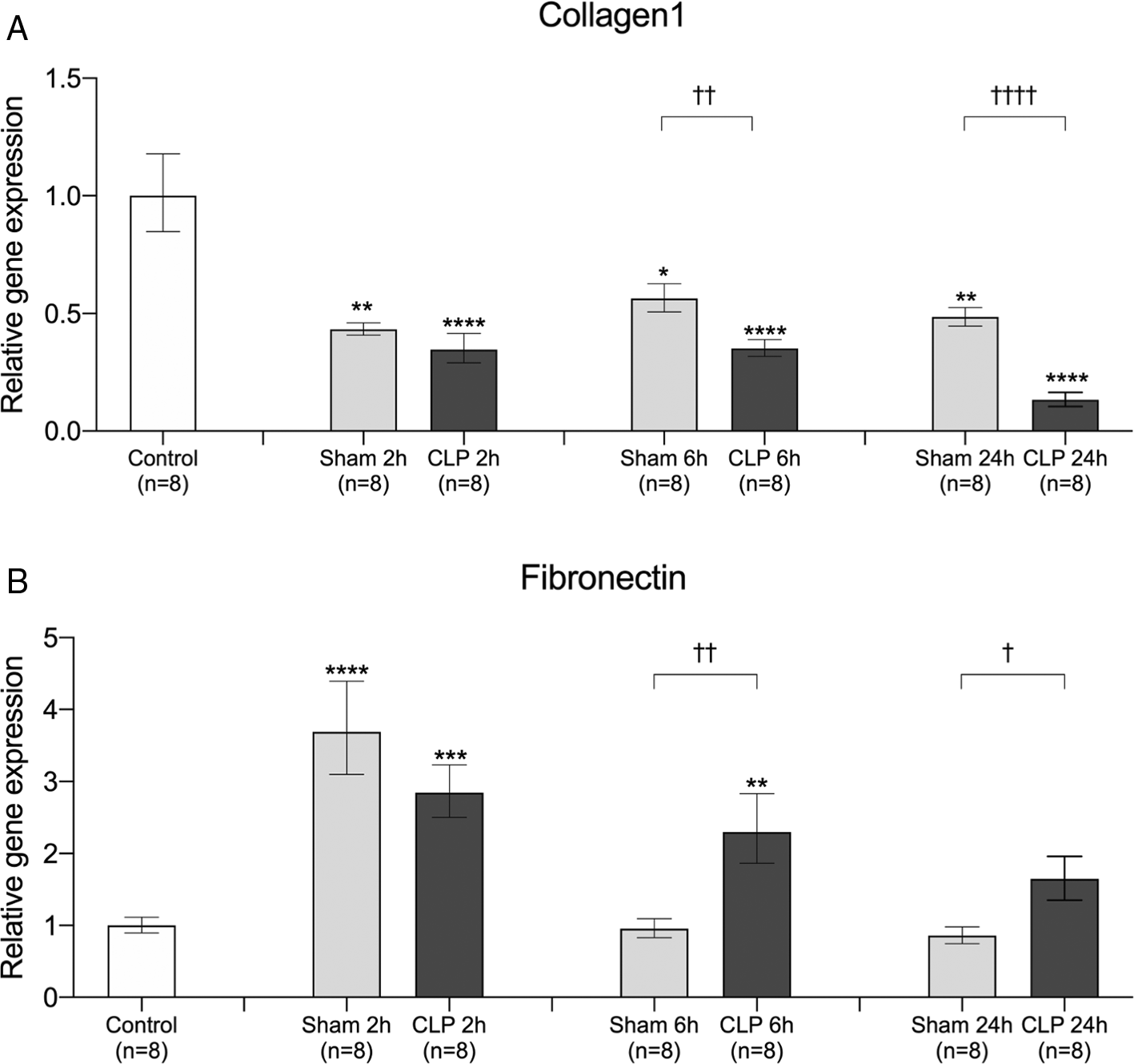
Relative gene expression of (A) collagen1 and (B) fibronectin in the lungs was compared between control (no surgery), 2 h after sham operation (Sham 2h), 6 h after sham operation (Sham 6h), 24 h after sham operation (Sham 24h), 2 h after CLP procedure (CLP 2h), 6 h after CLP procedure (CLP 6h), and 24 h after CLP procedure (CLP 24h). The data were normalized using the control. Error bars represent the mean ± SEM; n = 8 mice in each group. We compared the control to the Sham and CLP models using the Kruskal-Wallis test with Dunnett’s multiple comparison test (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001). We compared the sham operation model with the CLP model at each time point using the Student’s t-test or Mann-Whitney test according to the normality (†, p < 0.05; ††, p < 0.01; †††, p < 0.001; ††††, p < 0.0001). CLP, cecal ligation and puncture.
Next, we performed flow cytometric analysis to identify the dynamics of SVEP1-expressing cells at the single-cell level in the lungs after surgery. At baseline, SVEP1 was expressed on CD31-positive vascular endothelial cells, LYVE-1-positive lymphatic endothelial cells, and CD45.2-positive hematopoietic cells (Figure 5B, D and F; Control).

The percentage of (A) CD31high/SVEP1high, (C) LYVE-1high/SVEP1high, and (E) CD45.2high/SVEP1high cells was compared between control (no surgery), 2 h after sham operation (Sham 2h), 6 h after sham operation (Sham 6h), 24 h after sham operation (Sham 24h), 2 h after CLP procedure (CLP 2h), 6 h after CLP procedure (CLP 6h), and 24 h after CLP procedure (CLP 24h). Representative flow plots of (B) CD31high/SVEP1high, (D) LYVE-1high/SVEP1high, and (F) CD45.2high/SVEP1high are shown. The data were normalized using the control. Error bars represent the mean ± SEM; n = 8 mice in each group. We compared the control with the Sham and CLP models using the Kruskal-Wallis test with Dunnett’s multiple comparison test (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001). We compared the sham operation model with the CLP model at each time point using the Student’s t-test or Mann-Whitney test according to the normality (†, p < 0.05; ††, p < 0.01; †††, p < 0.001; ††††, p < 0.0001). SVEP1, sushi, von Willebrand factor type A, EGF, and pentraxin domain-containing 1; CLP, cecal ligation and puncture; LYVE-1, lymphatic vessel endothelial receptor 1.
The percentage of CD31high/SVEP1high vascular endothelial cells was significantly lower at 24 h after sham operation, whereas CLP surgery decreased the percentage at 2 and 6 h after the operation compared with the baseline. We found that the percentage of CD31high/SVEP1high cells was significantly lower at 2 and 6 h after CLP surgery compared with the sham operation (Figure 5A and B).
The percentage of LYVE-1high/SVEP1high lymphatic endothelial cells tended to decrease over 24 h after sham operation without significance. By contrast, the CLP model had a decreased percentage of LYVE-1high/SVEP1high compared with the control at 6 and 24 h and at all time points compared with the sham operation model (Figure 5C and D).
Although the percentage of CD45.2high/SVEP1high lung hematopoietic cells demonstrated no significant differences in the sham operation or CLP model compared with the control, the CLP model had a significantly higher percentage than the sham operation model at 2 and 6 h, which was followed by a return to baseline levels 24 h after the surgery (Figure 5E and F).
In this study, we demonstrated that SVEP1 is highly expressed in the lungs at baseline. SVEP1 expression was decreased over the course of sepsis, with a decreased percentage of SVEP1high vascular endothelial cells and lymphatic endothelial cells and an increased percentage of SVEP1high hematopoietic cells.
Since the 300 kDa full-length SVEP1 protein is secreted into the extracellular matrix and degraded into a 90 kDa protein, we examined the dynamics of 90 kDa SVEP1 protein expression in the lungs after CLP. Because there were no significant changes in the amount of the 90 kDa protein, the expression was potentially regulated at the transcriptional level rather than active degradation or consumption of the extracellularly distributed SVEP1. The present study revealed that SVEP1 gene expression in the lungs decreased not only after the CLP procedure, but also after sham operation. These findings suggest that the gene expression of SVEP1 responds not only to sepsis induction, but also to surgical invasion in the early phase after surgery. In addition, the decreased gene expression of collagen1, resembling the dynamics of SVEP1, was induced by surgical invasion and sepsis. The notable downregulation of SVEP1 by surgical stimulation without sepsis is intriguing. It has been reported that cytokines, such as TNF-α, are activated after surgical invasion and induce biological responses similar to sepsis.25–28 Based on our results, we believe that SVEP1 gene expression may be associated with these cytokines. Although estrogen reportedly regulates the expression of SVEP1,29 the exact mechanisms underlying the regulation of SVEP1 remain to be determined. Our findings that SVEP1 shows similar dynamics to collagen1 following the insult could be a critical clue to clarify the mechanisms underlying the regulation of SVEP1 expression.
Since SVEP1 is highly expressed in lung tissue consisting of heterogeneous cell types, we analyzed the expression of SVEP1 at the single-cell level by flow cytometry. In accordance with the finding that SVEP1 deficiency in coronary artery disease increases endothelial CXCL1 expression and promotes plaque formation,30 the present study suggests that SVEP1 is secreted from endothelial cells. Since SVEP1 is supplied by vascular and lymphatic endothelial cells, the decreased percentage of cells with CD31high or LYVE-1high/SVEP1high after CLP surgery indicates that sepsis reduces intracellular SVEP1 protein production in the vascular and lymphatic endothelial cells or promotes the extracellular release of the protein. In terms of the reduced percentage of cells with CD31high and LYVE-1high after sepsis induction, the endothelial-mesenchymal transition might contribute to decreasing the overall percentage of CD31-positive cells after sepsis induction. The decreased percentage of CD31-positive cells in septic shock patients because of endothelial-mesenchymal transition supports our speculation of the altered specific population.31
In the time course of SVEP1 expression after surgery in lung cells of mice with sepsis, an increased percentage of CD45.2high/SVEP1high cells indicates that hematopoietic cells containing SVEP1 migrate to the lungs during sepsis. Although blood cells reportedly express less SVEP1,15 our results demonstrated a different pathology of SVEP1 following sepsis. Further analysis is needed to identify the localization of SVEP1 and the cells responsible for producing SVEP1.
In sepsis, endothelial hyperpermeability because of disruption of the glycocalyx layer16,19 and tight junctions17,32 exacerbates the pathophysiology of sepsis. Angiopoietin-1 and angiopoietin-2 act antagonistically with each other and regulate the function of tight junctions.18–21,33–35 An association between decreased expression of SVEP1 and increased expression of sICAM and E-selectin in a model of endotoxemia has been reported.24 These findings suggest that SVEP1 may regulate vascular permeability in cooperation with angiopoietin and cell adhesion molecules. Although we have not demonstrated the exact role of SVEP1 in sepsis, the time course of SVEP1 expression in vascular endothelial cells and lymphatic endothelial cells indicates that SVEP1 potentially regulates vascular permeability.
This study has several limitations. First, since we did not perform perfusion fixation prior to tissue collection, blood cells in the tissue potentially affected the whole analysis. Second, we used both male and female mice in this study; therefore, sex-associated differences might have affected the results. Third, the detailed functions of SVEP1 were not investigated in this study. Future research should clarify the significant contribution of SVEP1 in sepsis pathophysiology.
SVEP1 expression was highly upregulated in the lungs compared with other organs at baseline. Sepsis suppressed SVEP1 gene expression with a decreased percentage of SVEP1high vascular endothelial cells and lymphatic endothelial cells and an increased percentage of SVEP1high hematopoietic cells.
BioStudies: Sepsis decreases lung SVEP1 expression in a murine model. Accession number S-BSST942, https://identifiers.org/biostudies:S-BSST942. 36
This project contains the following underlying data:
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular biology, cardiovascular sciences
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: I have experience in working with SVEP1 and flow cytometry.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Cummings M, Sarveswaran J, Homer-Vanniasinkam S, Burke D, et al.: Glyceraldehyde-3-phosphate dehydrogenase is an inappropriate housekeeping gene for normalising gene expression in sepsis.Inflammation. 2014; 37 (5): 1889-94 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: SVEP1, animal models, translational research.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 19 Jan 23 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)