Keywords
quality improvement initiative, IV antihypertensive medications, hospital setting, length of stay, patient safety, healthcare costs
This article is included in the Health Services gateway.
quality improvement initiative, IV antihypertensive medications, hospital setting, length of stay, patient safety, healthcare costs
Hypertension is a common condition seen in hospitalized patients. Aggressive management with intravenous (IV) antihypertensive medications is often inappropriately utilized for asymptomatic patients with severe hypertension.1 However, there is a lack of evidence to support the routine use of IV antihypertensive medications in this population.2 Studies have shown an increased risk of complications, such as acute kidney injury, cerebral vascular accident, myocardial infarction, dizziness, and falls, associated with this practice.3–5 In addition to the potential harm to patients, this approach also leads to increased healthcare costs.
To address this issue, an algorithm was developed (Figure 1) to guide nursing and medical staff in the management of asymptomatic patients with severe hypertension with the goal of reducing the blood pressure moderately and slowly using oral medications when appropriate. The primary objective of this project was to evaluate the effect of this intervention on the utilization rates of IV antihypertensive medications after providing education on the flowchart pathway to nursing and medical staff. The secondary objective was to assess the impact of the intervention on length of stay.
The project involved reviewing aggregate pharmacy data that were de-identified. No patient records were accessed and the project involved minimal risks. Quality improvement initiatives do not require IRB approval per our institutional guidelines and the US Department of Health Services Regulations. An informed consent form was used to obtain consent from the participants of this project.
This project was conducted at a 500-bed community hospital, College Station, Texas. Patients in the Emergency Department, Intensive Care Unit and Stroke Unit were excluded based on the appropriate need for IV antihypertensive medications in these areas.
Aggregated hospital inpatient pharmacy data regarding the utilization of intravenous (IV) antihypertensive medications was extracted for the months of July 2022 through March 2023.6 This data identified the types of IV antihypertensive medications utilized during the project period. Hydralazine and labetalol represented over 90% of the IV antihypertensive doses given during the project period. Therefore, this project focused on these two drugs. The data were collected and recorded in a secure electronic database for analysis. Overall length of stay data were obtained from case management department data.
The primary outcome measure for this project was the difference in average monthly utilization of IV antihypertensive medications between the four-month period before and the four-month period after the intervention. The utilization was measured using the standardized “days of therapy per 1000 patient days (DOT/1000)” to account for the potential differences in patient volume and length of stay during the project period. The secondary outcome was the effect of this intervention on the average length of stay of the patient population studied.
An algorithm that outlined the appropriate management of severe asymptomatic hypertension in the hospital setting was created based on current evidence-based guidelines and local hospital protocols (Figure 1). This was reviewed and approved by the hospital’s Pharmacy and Therapeutics Committee.
Several educational sessions were conducted with the medical staff, nursing staff, and pharmacists to discuss the management of severe asymptomatic hypertension and utilization of the suggested algorithm. These educational sessions included presentations, case discussions, and interactive discussions to promote understanding and adherence to the recommended pathway. These sessions were facilitated by a multidisciplinary team which included clinical pharmacists, clinical nurse educators, and physician champions.
After the completion of the educational sessions, the utilization of IV antihypertensive medications was monitored over the subsequent four-month period to assess the impact of the intervention. The utilization data were collected from the pharmacy records and recorded in the electronic database for analysis.
Descriptive statistics, including mean, median, standard deviation, and frequency distributions, were used to summarize the utilization of IV antihypertensive medications during the project period. The changes in utilization and length of stay before and after the intervention were compared using paired t-tests. A p-value of less than 0.05 was considered statistically significant. The Microsoft Excel 365 data analysis tool was used to conduct statistical analyses.
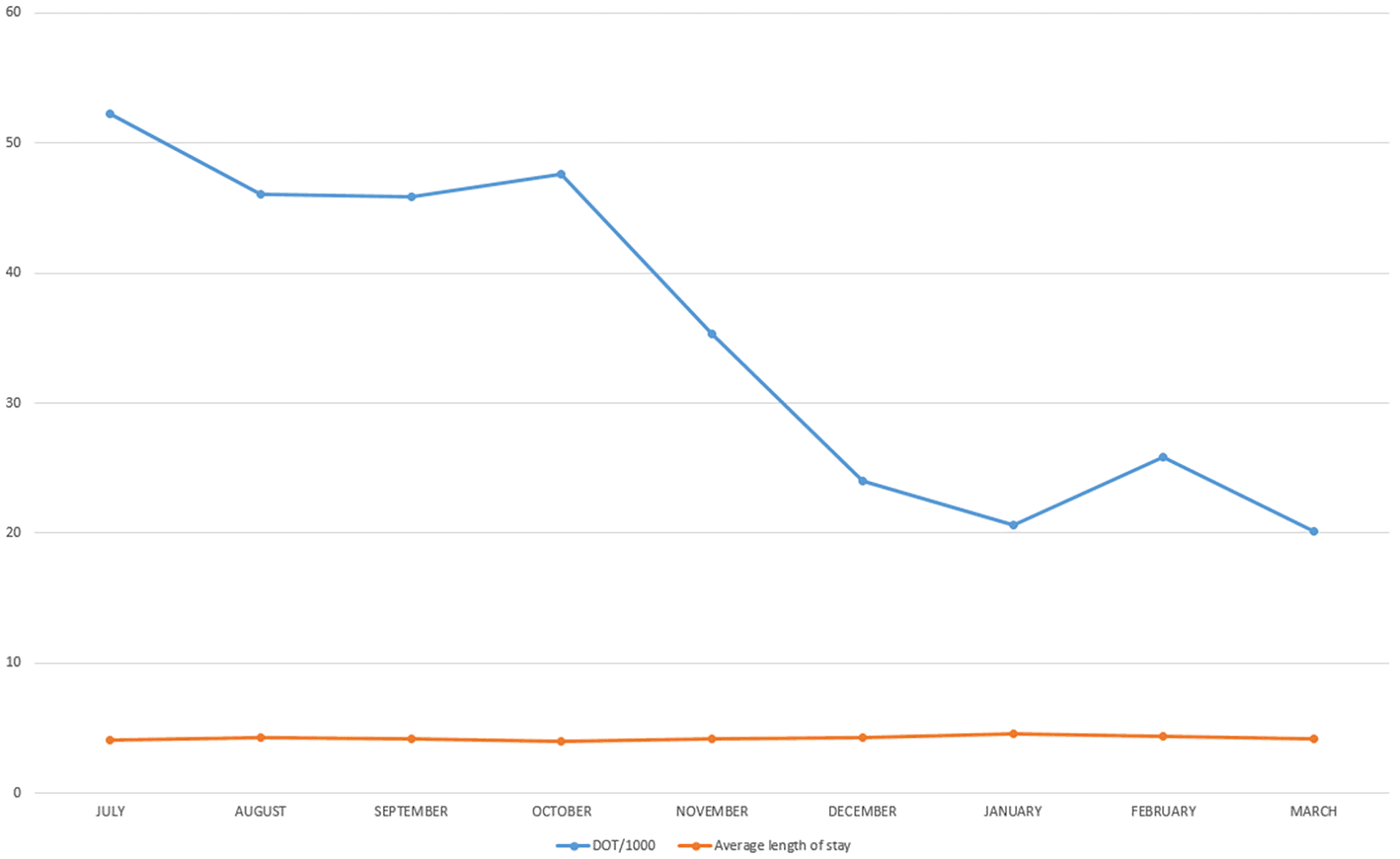
Aggregate pharmacy data on the usage of IV antihypertensive medications were reviewed for the duration of July 2022 to March 2023 (Table 1). The primary outcome was the impact on utilization of IV antihypertensives using DOT/1000. This measure showed a significant decrease in the four-month period after the intervention 25.24 (SD = 3.05) compared to the four-month interval before the intervention 48.21 (SD = 3.06). After the intervention, the utilization rate decrease was maintained for four consecutive months measured. For the secondary outcome, the average length of stay in days for patients hospitalized did not demonstrate any statistically significant change. The average length of stay before the intervention was 4.15 (SD = 0.12), whereas after the intervention, the average length of stay was 4.375 (SD = 0.12). The average length of stay before the intervention in July, August, September, and October was 4.1, 4.3, 4.2, and 4, respectively. After the intervention, the average length of stay increased in December (4.3) and January (4.6) and decreased in February (4.4) and March (4.2) (Figure 2).
| Month | DOT | Days present | DOT/1000 |
|---|---|---|---|
| July | 300 | 5740 | 52.26 |
| August | 259 | 5625 | 46.04 |
| September | 263 | 5732 | 45.88 |
| October | 247 | 5751 | 47.64 |
| November | 181 | 5118 | 35.37 |
| December | 143 | 5960 | 23.99 |
| January | 125 | 6053 | 20.65 |
| February | 139 | 5386 | 25.81 |
| March | 117 | 5797 | 20.18 |
The data were analyzed using a paired t-test to compare the mean utilization rate before and after the intervention. The results showed a significant difference between the means (t(6) = 8.65, p < 0.001). The effect size was large (Cohen’s d = 3.54). For the average length of stay in days for patients hospitalized, the data were analyzed using a paired t-test to compare the mean length of stay before and after the intervention. The results showed no significant difference between the means (t(6) = -1.99, p = 0.094). The effect size was small (Cohen’s d = 0.61).
In this project, we implemented a quality improvement initiative to reduce the utilization of non-indicated intravenous (IV) antihypertensive medications in the hospital setting. Our results showed that the implementation of an algorithm and educational sessions successfully decreased the utilization of IV antihypertensives, as measured by days of therapy per 1000 patient days. The reduction in IV antihypertensive utilization is important for patient safety and cost saving. By shifting the management approach towards oral medications, we can minimize the potential harm and increased healthcare costs associated with unnecessary IV antihypertensive use. While the intervention significantly reduced IV antihypertensive utilization, there was no significant difference in the average length of hospital stay before and after the intervention. This indicates that slowly lowering blood pressure with oral medications does not necessarily increase hospital length of stay.
There are several limitations to this project. First, the project was conducted in a single community hospital, which may limit the generalizability of the findings to other settings. Second, the project relied on pharmacy records for data collection, which may be subject to inaccuracies or missing data. Third, the project was conducted over a relatively short time frame of four months. This may not capture long-term changes in utilization patterns. Finally, the project did not assess the impact of the intervention on patient outcomes, such as blood pressure control or adverse events, which may provide further insights into the effectiveness of the intervention.
A quality improvement initiative introducing an algorithm with education for nursing and medical staff, significantly reduced the utilization of IV antihypertensives using DOT/1000 but did not significantly affect the average length of stay in days for patients hospitalized. This project has shown the potential to improve patient safety by reducing the risk of complications associated with unnecessary IV antihypertensive medication use and also has the potential to reduce healthcare costs. Further research is warranted to explore the potential clinical implications of these findings and to evaluate the long-term effectiveness of the intervention in optimizing antihypertensive medication usage in this patient population.
Written informed consent for publication of the participants’ details was obtained from the participants.
Figshare: Underlying data for ‘Implementing a quality improvement initiative for reducing intravenous antihypertensive utilization in the hospital setting improving patient safety without prolonging hospital stay’. https://doi.org/10.6084/m9.figshare.23523708.v1. 6
This project contains the following underlying data:
• Implementing a Quality Improvement Initiative for Reducing Intravenous Antihypertensive Utilization in the Hospital Setting Improving Patient Safety Without Prolonging Hospital Stay.pdf.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Grassi D, O'Flaherty M, Pellizzari M, Bendersky M, et al.: Hypertensive urgencies in the emergency department: evaluating blood pressure response to rest and to antihypertensive drugs with different profiles.J Clin Hypertens (Greenwich). 2008; 10 (9): 662-7 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Arterial Hypertension, Chronic Kidney Disease, Emergency Medicine, Nephrology
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Salman J, Salman A, Kumar S, Gjeka R, et al.: Improving the use of intravenous antihypertensive medications in the hospital setting: a quality improvement initiative for patient safety.BMJ Open Qual. 2019; 8 (4): e000626 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Inpatient hypertension
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 18 Jul 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)