Keywords
Biogeography, decomposer fungi, invasive fungi, invasion biology, fungi, geographic distribution, taxonomy, introduced species, integrative species
This article is included in the Genomics and Genetics gateway.
This article is included in the Ecology and Global Change gateway.
This article is included in the From genes to genomes: Investigating the population species boundary in non-model Fungi collection.
Biogeography, decomposer fungi, invasive fungi, invasion biology, fungi, geographic distribution, taxonomy, introduced species, integrative species
Humans often move organisms across continents, either deliberately or inadvertently, and by doing so facilitate long distance dispersal. Introductions may lead to invasions, and invasive species are one of the top five threats to Earth’s biodiversity (Butchart et al., 2010; Pyšek et al., 2020). We define an invasive species as a species outside its natural range which becomes established in local habitats and threatens native biodiversity (Desprez-Loustau et al., 2007). Invasive species can cause severe, often unpredictable problems. For example, the chestnut blight fungal pathogen, Cryphonectria parasitica, caused devastation to Chestnut tree populations in North America, which in turn had cascading, ecosystem-wide effects (Desprez-Loustau & Rizzo, 2011). Invasive nonpathogenic fungi have not received as much attention as invasive vertebrates and plants, but because decomposer and symbiotic fungi shape biodiversity (because they themselves are diverse, and through interactions with animals and plants) and because decomposer fungi drive biogeochemical cycles, their potential impacts on native species and ecosystem services are tremendous (DAISIE, 2012).
Fungi are ubiquitous, but only a fraction of Earth’s total species have been described (Blackwell, 2011). Moreover, the past and present ranges of most fungal species remain unmapped, in part because the native habitats of many fungi are not documented: often, species are only known from the one or two specimens used to describe them (Pringle & Vellinga, 2006). In fact many fungi are described from places where they are introduced, for example, botanical gardens (Pringle & Vellinga, 2006). Finding a fungus somewhere does not necessarily mean it is native there (Golan & Pringle, 2017). Often, invasive fungi are identified as invasive only because they are charismatic or dramatically affect humans, for example the invasive Death Cap, which is deadly poisonous (Wang et al., 2023). Unsurprisingly, invasions by plant pathogens like C. parasitica are more studied than invasions by decomposers or symbionts (Fisher et al., 2012; Pringle & Vellinga, 2006). For example, while the story of the saprotrophic Clathrus archeri’s spread throughout Europe is more than a century old, its potential impacts on native fungal communities remain unknown (Brännhage et al., 2021; Desprez-Loustau et al., 2007). Nonetheless, the scarce data available confirm invasive nonpathogenic fungi matter: for example, the beetle symbiont Flavodon subulatus, which was introduced alongside its invasive beetle, suppresses native fungal species in the invasive range (Hulcr et al., 2021; Jusino et al., 2020).
The striking lawn mushroom Amanita thiersii is a saprotroph within the asymbiotic clade of the genus Amanita (Cui et al., 2018; Tulloss et al., 2016; Wolfe, Tulloss et al., 2012). Originally described from College Station, Texas, U.S.A. in 1957 (Thiers, 1957), herbarium records from the 1960’s onward document the dramatic spread of A. thiersii out of Texas and across the southern and midwestern United States (Wolfe, Kuo et al., 2012). An expanding range is a hallmark of an invasive species and has been documented for other symbiotic species in the genus, not only for the Death Cap Amanita phalloides (Pringle et al., 2009), but also the Fly Agaric A. muscaria (Vargas et al., 2019). North American populations of A. thiersii appear to be genetically homogeneous (Wolfe, Kuo et al., 2012). The lack of genetic diversity across its entire known distribution suggests an introduction associated with a genetic bottleneck. Although A. thiersii possesses characteristics of an invasive species, if it is native to Texas, then by definition it is not invasive. It may still be undergoing a range expansion, perhaps in response to climate change (Hobbie et al., 2017).
But is A. thiersii truly native to North America? Morphological similarities between A. thiersii and another white decomposer, the Argentinian A. foetens (Singer, 1953), raise two questions: are the two species the same species? Was the species introduced to North America from Argentina? If A. thiersii was introduced to the U.S.A. from South America, its rapid geographic spread and the dramatic increase in its population size over recent decades would define it as an invasive species. As conservation biology slowly begins to focus on fungi, as well as animals and plants (Gonçalves et al., 2021; May et al., 2018), efforts to record and stop the spread of introduced and invasive nonpathogenic fungi are ramping up (Dickie et al., 2016; Pyšek et al., 2020). An essential prerequisite is the ability to differentiate between native and invasive fungi.
Using an integrative species concept (Barrett & Freudenstein, 2011; Wiens, 2007), we hypothesized the two species are the same; A. thiersii is simply an A. foetens introduced from Argentina. To test our hypothesis, we first revisited and compared the original species descriptions of A. thiersii and A. foetens, focusing on morphological similarities and differences. Next, we investigated their current global ranges using the biodiversity databases iNaturalist and Mushroom Observer. Finally, we sequenced three genomes and compared the sequence data of a U.S.A. A. thiersii, an isotype of A. foetens, and a recently collected Argentinian mushroom initially identified as A. thiersii. Our data provide a unique opportunity to document the history of an Amanita species currently spreading in North America.
The species Amanita thiersii was first described by Harry D. Thiers from College Station, Brazos County, Texas, U.S.A. in 1957 (using the invalid name A. alba Thiers; Thiers, 1957), and it was later validly named for Thiers (Bas, 1969). The 1969 text is the protologue of A. thiersii. The specimens used to describe the species were collected in September 1952 from a lawn. The species Amanita foetens was first described from Pié del Periquillo in Tucumán Province, Argentina by Rolf Singer (Singer, 1953). The specimens used to describe A. foetens were collected in December 1951 from a semiarid pasture. Amanita foetens was revised again at length by Bas (Bas, 1969). We used all descriptions in our comparisons.
We used two public databases to establish the current known distributions of A. thiersii and A. foetens: iNaturalist (iNat) and Mushroom Observer (MO). While MO uses the name Amanita thiersii, iNat uses the name “Saproamanita thiersii.” The generic name “Saproamanita” Redhead, Vizzini, Drehmel & Contu was proposed in 2016 for use with asymbiotic Amanita species (Redhead et al., 2016), but it is controversial (Hawksworth, 2016; Tulloss et al., 2016). Confusingly, iNat uses the generic name “Amanita” for A. foetens, even though it is also asymbiotic. Both iNat and MO are populated with observations of mushrooms submitted by the public. Names for observations are determined by popular vote on iNat and by a different, more complex community voting system on MO. To search in each database, we used the search terms “Amanita thiersii” and “Amanita foetens”. Searching for “Amanita thiersii” in iNat leads to the page for “Saproamanita thiersii,” and using “Amanita foetens” leads to the page for “Amanita foetens”. Data from iNat were downloaded between June 14 and 15, 2022, and MO data were downloaded on June 7, 2022. We used iNat data with a data quality assessment of “research grade” and additional observations with photos clearly resembling white Amanita. Next, we manually checked each individual observation in both datasets to confirm species identifications using the gross morphology visible in pictures, authors’ descriptions, and/or DNA sequence data, as available. Observations without latitude and longitude were almost always excluded, as were observations not strongly resembling one of our target species. However, observations from South America were relatively rare (as compared to observations in North America), and in a few instances we estimated exact latitude and longitude coordinates from observer’s location descriptions, especially for MO observations from South America. In these cases, coordinates are not exact. Eventually, all observations made outside of North and South America were removed because none matched the descriptions for either A. thiersii or A. foetens. Data from each of the databases were compiled into a single dataset (dataset on Dryad) and mapped. The locations of specimens used in genome sequencing were manually added to the dataset.
We sequenced the genomes of three mushrooms: AmanitaBASE 10801, 10802 and 10175. AmanitaBASE 10801 (Elmore, 2020) is an isotype of A. foetens sent from the University of Michigan herbarium (voucher: MICH4948) originally collected in Pié del Periquillo, Tucumán Province, Argentina by R. Singer and H. Helberger in December 1951 (Singer original voucher: T1672). AmanitaBASE 10802 is an A. thiersii mushroom collected by S. Kay from a lawn in Baldwin City, Kansas, U.S.A. in 2009 (Kay voucher: SKay4041). A single spore of mushroom SKay4041 was cultured and its haploid genome previously sequenced by Wolfe et al. (Wolfe, Kuo et al., 2012; more fully described in Hess & Pringle, 2014). We re-sequenced the same single spore cultivar to take advantage of improved sequencing technologies. AmanitaBASE 10175 was collected in Córdoba, Argentina in 2014 and it was originally identified as A. thiersii by G. Robledo (Robledo voucher: G201); from this point forward, we refer to 10175 as an Amanita sp. We also refer to the genomes generated from each mushroom specimen by their AmanitaBASE numbers. DNA extraction for genome sequencing and library preparation followed protocols described by Wang (Wang et al., 2023). Genomes were sequenced on the Illumina HiSeq 2500 short reads platform with 251 bp paired-end reads (Wang et al., 2023).
To assemble the genomes of A. thiersii 10802, A. foetens 10801, and Amanita sp. 10175, the raw reads were first trimmed using bbduk from the BBMap suite ver. 38.32 (kmer length 23; Bushnell, 2016). The genomes were then assembled using SPAdes ver. 3.5.0 with default parameters using two libraries (Prjibelski et al., 2020).
Saprotrophic Amanita species are closely related to each other and basal to ectomycorrhizal Amanita (Wolfe, Tulloss et al., 2012). To clarify the phylogenetic relationship among specimens collected as either A. thiersii or A. foetens, we obtained DNA sequences of the nuclear regions ITS, NucLSU (28S), and NucSSU (18S), and of the mitochondrial regions MitLSU, and MitSSU loci, from all saprotrophic or asymbiotic Amanita available from NCBI as of March 11, 2022. We included all sequences meeting the following criteria: 1) the sequence was from a specimen (Collector’s ID) associated with at least two of the five loci of interest, and 2) the mushroom corresponding to the sequence was not identical to any represented by our own genomes. We included NCBI data from two A. thiersii specimens; one of them (Collector’s ID SKay4041_het) is directly related to our sequenced single spore cultivar. It is the dikaryotic parent of our genome A. thiersii 10802, in other words, A. thiersii 10802 is the monokaryotic offspring of SKay4041_het. Because the diploid SKay4041_het data captures all of the genetic information of the original specimen, we omitted the haploid genome of A. thiersii 10802 from the 5-locus analysis. In total, we included data from eight asymbiotic Amanita species, each species represented by between one and three specimens, and from two specimens of an outgroup species (Pluteus cervinus) which also met our criteria (Table 1). We also identified and extracted the five loci from our remaining genomes (10801 and 10175) by querying the genomes with known sequences of closely related species using blastn from the BLAST+ suite (Altschul et al., 1990). Sequences corresponding to the best BLAST hit were obtained using seqinr in R (Charif & Lobry, 2007; Team, 2016).
Table includes species name, the identifier given by the mushroom collector “Collector’s ID”, and all the NCBI accession numbers associated with that mushroom used in this analysis for the five loci.
We aligned each sequence set using MAFFT ver. 7.490 (code and tags can be found on GitHub; Katoh et al., 2002). Resulting alignments were used to construct maximum-likelihood phylogenies with IQtree ver. 1.6.12. Our pipeline first used the ModelFinder tool to find the best nuclear or mitochondrial substitution model for each alignment (Kalyaanamoorthy et al., 2017), and then ran 1000 bootstraps using the ultrafast bootstrap approximation method (Nguyen et al., 2015). To construct a single phylogeny using the data of all five single-locus phylogenies, we concatenated alignments. The concatenated sequence was used to reconstruct a maximum-likelihood phylogeny to create the best tree to fit the data, with informative branch lengths corresponding to genetic distances. The five-locus phylogeny was created using IQtree run with partition models to distinguish the loci based on the ModelFinder tool, and bootstrapped 1000 times using the ultrafast bootstrap approximation (tags found on GitHub; Dunkirk, 2023). We verified the results of this method by also creating a consensus tree using ASTRAL (Zhang et al., 2018). The trees were rooted with P. cervinus as outgroup.
As a final analysis and to contextualize the close relationship between A. thiersii and A. foetens, we downloaded all 2,237 Agaricales ITS sequences available from NCBI on October 1, 2022, and we included these with the ITS sequences we used to generate the five-locus phylogeny. Sequences were aligned with MAFFT using the ‘-auto’ parameter and trimmed with trimAL using the ‘-automated1’ parameter (Capella-Gutiérrez et al., 2009). The resulting trimmed alignment of 279 bp was used in IQtree to construct a maximum likelihood phylogeny using the ‘test’ parameter to find the best model as constrained within ‘raxml’ options. All identical sequences were removed by default. All pairwise distances in the resulting tree were obtained from the ‘mldist’ file and filtered to only include comparisons within the same genus. Only the lowest distance comparison within and between species was kept for a given sequence. We visualized the data as a histogram to compare pairwise distances between intraspecific and interspecific species, as named in the database.
To contextualize the genomes of A. thiersii 10802, A. foetens 10801 and Amanita sp. 10175 within the genus Amanita, we downloaded all publicly available Amanita genomes, both asymbiotic and mycorrhizal, in addition to those of Volvariella volvacea and Pluteus cervinus, both used as outgroups (genomes downloaded from NCBI between March 29 and 31, 2022; Table 2). We identified a set of fungal Benchmarking Universal Single-Copy Orthologs (BUSCOs) from each of 15 genomes using the program BUSCO (ver 3.0.2 run with Laccaria bicolor as reference; Simão et al., 2015). We subset this dataset to include only those BUSCOs present as single copies in all 15 taxa (n = 55 BUSCOs). We aligned the sequences of each BUSCO with MAFFT and constructed single-BUSCO maximum-likelihood phylogenies for each resulting alignment with IQtree.
Table includes data downloaded from NCBI detailing the species name, genome assembly accession number, and genome ID associated with the genome.
| Species | NCBI GenBank Genome Assembly Accession | NCBI GenBank genome ID |
|---|---|---|
| 10175 Amanita sp. | SRR23983940 | PRJNA947219 |
| 10801 Amanita foetens | SRR23983939 | PRJNA947219 |
| 10802 Amanita thiersii | SRR23983941 | PRJNA947219 |
| Amanita bisporigera | GCA_001983365.1 | ASM198336v1 |
| Amanita brunnescens | GCA_001691785.2 | ASM169178v2 |
| Amanita inopinata | GCA_001691775.3 | ASM169177v3 |
| Amanita jacksonii | GCA_000497225.1 | AmaJack1.0 |
| Amanita muscaria | GCA_001691765.1 | ASM169176v1 |
| Amanita phalloides | GCA_001983385.1 | ASM198338v1 |
| Amanita polypyramis | GCA_001691755.2 | ASM169175v2 |
| Amanita pseudoporphyria | GCA_003316615.1 | ASM331661v1 |
| Amanita rubescens | GCA_015039365.1 | Amarub1 |
| Amanita subjunquillea | GCA_020011035.1 | ASM2001103v1 |
| Pluteus cervinus | GCA_004369065.1 | Plucer1 |
| Volvariella volvacea | GCA_001691835.3 | ASM169183v3 |
We took two approaches to generate subsequent multi-gene species-trees: first, we concatenated the alignments and made a single tree, and second, we used a consensus tree method to generate a consensus tree. First, concatenated sequence data were used to generate a maximum-likelihood phylogeny using IQtree run with a partition model, using methods parallel to the methods used to generate the five-locus tree (described above). Second, ASTRAL ver. 5.7.8 was used to reconstruct a consensus tree (Zhang et al., 2018) based on the phylogenies constructed for each individual BUSCO. Essentially, using ASTRAL, the BUSCO species tree was created by using single-gene-BUSCO trees as inference and by considering discordance among the single-gene trees. As an extra check to verify the topology of the BUSCO species trees, we used additional methods. To determine the number of informative gene-trees which showed topology in concordance with the consensus phylogeny, we re-ran ASTRAL and measured quartet support (Zhang et al., 2020), and we calculated gene concordance with IQtree ver. 2.1.2 (Minh et al., 2020) using the single-gene BUSCO trees and the maximum-likelihood phylogeny previously generated using IQtree. The quartet support option in ASTRAL indicates, at each branch, how much conflict there was between gene-trees in the resulting consensus tree (Zhang et al., 2018). The concordance factor option in IQ-tree indicates the percentage of locus-trees which support that branch (Minh et al., 2020). The tree was rooted with P. cervinus and V. volvacea as outgroup taxa.
U.S.A. populations of A. thiersii are characterized by a lack of genetic diversity, suggesting sexual reproduction is absent or involves genetically similar pairs (Wolfe, Kuo et al., 2012). In fungi, successful sexual reproduction typically requires the interaction of compatible mating type genes, named as Homeodomains 1 and 2 (HD1 and HD2). To determine if our three genomes include the mating type loci required for sexual reproduction, we used the methods described above to extract the genes HD1 and HD2. To identify HD2, we queried genomes 10175, 10801, and 10802 using the tblastn function of BLAST with the amino acid (AA) sequences of the HD2 gene identified from an earlier annotated genome of A. thiersii (Hess et al., 2014), from the genome of another closely related asymbiotic Amanita, A. inopinata (Hess et al., 2014), and from Coprinopsis cinerea (Stajich et al., 2010). To identify HD1, we queried the same genomes using tblastn with the AA sequences of HD1 from A. inopinata, and C. cinerea.
The published, annotated genome of A. thiersii (Hess et al., 2014) does not appear to include the HD1 gene region and so we could not use it as a query. To explore this dynamic further, we searched for HD1 in a publicly available transcriptome (NCBI ID: SRX037158; Wolfe, Kuo et al., 2012) sequenced from a culture of the dikaryotic parent mushroom of the single spore used to generate genome 10802. We first used the HD1 nucleotide sequence from Amanita sp. 10175 as query in an SRA BLAST (Sequence Read Archive Basic Local Alignment Search Tool) of the transcriptome. Next, we downloaded all output sequences and used the program EGassembler to merge sequence fragments into a single consensus sequence (Masoudi-Nejad et al., 2006). We used the resulting consensus sequence as the next query to SRA BLAST, once again searching in the transcriptome, and repeated our searches until no new transcriptomic reads could be incorporated into the consensus.
Because the HD2 gene includes introns, we used the annotated genome of A. thiersii to locate and remove them (Hess et al., 2014). After removing introns, we used the tool Expasy to translate the DNA sequences of both HD1 and HD2 into AA sequences (Duvaud et al., 2021). Homeodomain proteins are typically identifiable by three helices (Hull et al., 2002). To confirm the presence of the three helix motif, we used a position-specific iterative predictor, PSIPRED, to predict and confirm secondary structural motifs (McGuffin et al., 2000). We checked the PSIPRED predictions using blastp (Altschul et al., 1990). Next we searched for HD1 and HD2 in other Amanita and V. volvacea (Hess et al., 2018). To compare HD1 and HD2 among species, we aligned only the conserved three-helix homeodomain AA sequences using MAFFT (default settings; Katoh et al., 2002).
To confirm the absence of mating-type genes in assemblies as the result of true deletions and not error related to genome assembly, we aligned raw reads from 10802 to the genome of 10801 that contained both mating type genes using BWA mem (Li, 2013). Resulting alignments were visualized in Integrative Genomics Viewer (Thorvaldsdóttir et al., 2012) to confirm the absence of reads aligning at mating type loci.
Amanita thiersii and A. foetens are morphologically very similar, but key differences are apparent in the original species’ descriptions. Features which appear identical include gill characteristics, ring location, and basidiospore shape (Figure 1). Features which appear similar include the general appearance of mushrooms, more specifically their color, height, cap size and wart characteristics; the integrity of the ring on developing mushrooms; and basidiospore size. Conflicting or ambiguous descriptions relate to the structure of the volva, details of stipe and ring morphology, and, notably, mushroom scent.
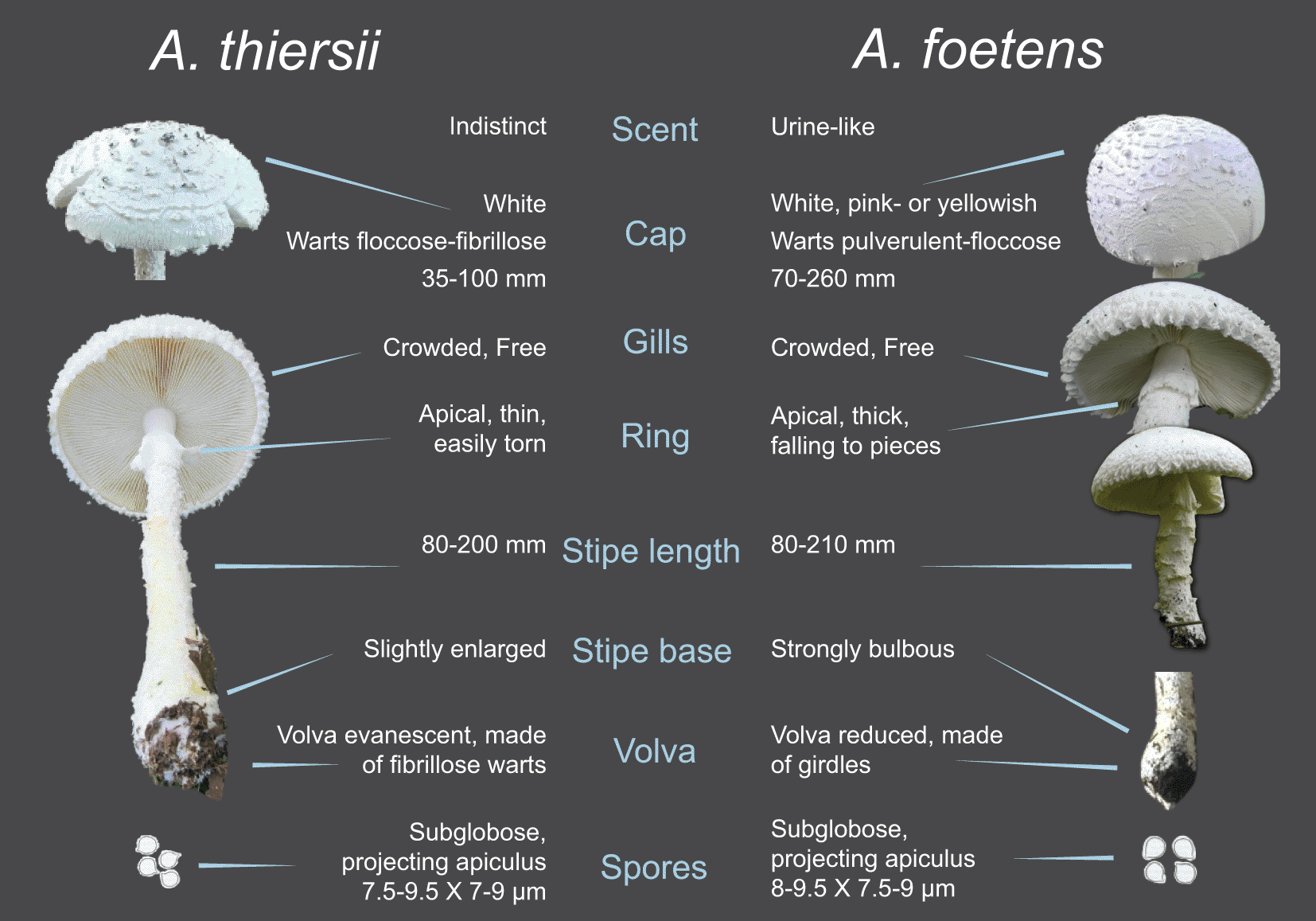
Morphology summarized from the original descriptions by Singer (1953) and Thiers (1957) and secondary descriptions by Bas (1969) for notable characteristics. Photos under creative commons license (Cc-by-sa-3.0) or reproduced with permission from Bas (1969).
Both species are described as entirely white in color (A. foetens may also be pink or yellowish), with medium to large mushrooms and convex caps (Bas, 1969). Caps possess abundant floccose or fleshy warts with crowded and freely attached gills. The caps of A. thiersii appear to be slightly smaller than caps of A. foetens but ranges are not disjunct. The mushrooms are described as either “rather thick-fleshed” (A. thiersii; Bas, 1969) or with a “rather sturdy fruit body” (A. foetens; Bas, 1969). Stipes are reported as textured as opposed to smooth (Bas, 1969). The stipe of A. thiersii is described as being equally wide across its height, but with a slight bulb at its base (Thiers, 1957). The stipe of A. foetens is described as “white, firm, broad” with a strongly bulbous base (Singer, 1953). Stipe height for both species is reported similarly at between 80-200 (A. thiersii) or 80-210 (A. foetens) mm (Bas, 1969; Thiers, 1957). Basidiospores appear to have the same shape: globose to subglobose, and mushrooms drop a white spore print (Singer, 1953; Thiers, 1957). Basidiospores are amyloid and less than 10 × 10 μm (Bas, 1969). Basidia lack clamps, a feature typical for stirps Thiersii, the subset of the genus Amanita housing both A. thiersii and A. foetens (Bas, 1969).
Other characters in the two species’ descriptions are either difficult to compare or are ambiguous. Within the genus Amanita, the volva is a key distinguishing feature. If A. thiersii and A. foetens are the same species, we would expect to find similar or identical volval descriptions. The volva of A. thiersii is described as usually evanescent “or present as a series of irregular rows of easily detached, fibrillose warts along the base of the stipe” (Thiers, 1957). The volva of A. foetens is described as strongly reduced or absent “or represented by some girdles” (Singer, 1953). Although these descriptions use different words, fibrillose warts versus girdles, they both allude to a volva made up of evanescent pieces of mushroom tissue around the stipe; a contrast to the obvious, persistent, and cup-shaped volva of many Amanita. Both descriptions point to the lack of a volva, or a volva present as remnants only, but because it is difficult to interpret the original descriptions further, how the volva might compare to each other remains ambiguous.
Less ambiguous are other differences, including differences in the internal morphology of the stipe; the stipe of A. thiersii is described as “stuffed to hollow” (Thiers, 1957) while the stipe of A. foetens is described as “solid, firm” (Bas, 1969; Singer, 1953). After revisiting Bas’s (1969) species descriptions one of us (Tulloss) a taxonomic expert and specialist of the genus, concluded fresh specimens of the species can be distinguished based on stature. The stature of A. thiersii is significantly more “gracile” than the stature of A. foetens (Bas, 1969: Fig. 85 vs. Fig. 88). The word gracile is used to mean slender, and a comparison of the ratios of stipe length to stipe width can stand in for the qualitative term “gracile.” In A. thiersii the ratio ranges from 6.9 to 7.3 (based on data of Bas, 1969) while in A. foetens the ratio ranges from 10 to 20 (also using data from Bas, 1969).
Descriptions of the ring also emerge as distinct. While both species’ rings are described as white, membranous, and located apically on the stipe (Bas, 1969), A. thiersii’s ring is described as thin and “easily torn, sometimes disappearing” (Bas, 1969) while A. foetens’ ring is described as “rather thick”, “frequently fragmentary” (Singer, 1953), and as “falling to pieces” (Bas, 1969). While both rings appear fragile, the distinction of thin versus thick cannot be ignored.
Finally, the scent of A. thiersii is reported as indistinct (and we ourselves have never found a strong-smelling A. thiersii), whereas the smell of A. foetens is “resolutely stinking” and in mature specimens, like urine (Singer, 1953). In the aggregate, the morphological differences recorded for A. thiersii and A. foetens suggest they are different species. Later we discuss the usefulness of these diagnostic characters to the field biologist.
The two species were not equally represented across iNat and MO. There were a total of 24 observations of A. foetens in iNat, of which we used 15 (we removed nine observations with low quality data grades), and no observations in MO. We manually added the locations of the isotype of A. foetens collected from Tucumán, Argentina (AmanitaBASE 10801/MICH4948) and the mushroom collected as Amanita sp. from Córdoba, Argentina (AmanitaBASE 10175) to our dataset (total observations included in subsequent analyses: 17). There were a total of 286 observations of A. thiersii in iNat, of which we used 155 (we removed 131 observations with aberrant morphologies or low-quality data grades), and 15 observations in MO. We manually added the location of our genome-sequenced sample from Kansas, U.S.A. (AmanitaBASE 10802) to our dataset (total observations included in subsequent analysis: 171). Because the nomenclature within iNat and between iNat and MO is inconsistent (iNat uses the pseudonym Saproamanita thiersii, while A. foetens remains as Amanita foetens (Hawksworth, 2016; Redhead et al., 2016; Tulloss et al., 2016; at the date of download, MO did not use the generic name Saproamanita), we use the names Amanita thiersii and Amanita foetens to describe records from both iNat and MO (except on Figure 2, where for clarity we use the names used in the databases themselves).

A. Map of U.S.A. and Mexico plotted with observations of A. thiersii (as blue squares from MO, and blue circles named as Saproamanita thiersii from iNat). Mushroom A. thiersii 10802, the source of a sequenced genome, plotted as a black X. Geographic distribution of A. thiersii as published in Wolfe, Kuo et al. (2012) shown as small red dots. B. Map of Argentina and Uruguay plotted with observations of A. foetens (iNat) as orange circles, A. thiersii (MO) as blue squares, and Saproamanita thiersii (iNat) as blue circles. Mushrooms Amanita sp. 10175 and A. foetens 10801, sources of sequenced genomes, plotted as black X and +, respectively. Axes of A. and B. reference latitude and longitude.
Both species are observed within North and South America and not on other continents. While two observations were made from Taiwan and South Africa (iNat observations 118324696 and 117233579, respectively), neither observation matched either species’ morphology. Amanita foetens was observed predominantly in Argentina (n=15), as far south and east as Buenos Aires and as far north and west as Tucumán. It was also found in Uruguay (n=2). The fungus was most frequently observed in the Argentinian province of Buenos Aires (n=13). Amanita thiersii was found overwhelmingly in the U.S.A. (n=160), but its range appears to be expanding. Wolfe et al. (2012) reported it as far north as Illinois and east to Kentucky (red dots on Figure 2A), but the fungus now appears as far north as Wisconsin, and as far east as Pennsylvania and Maryland (Figure 2A). It has also been reported from Florida. Amanita thiersii appears to be newly common in Illinois, with 16 observations recorded from the state since 2012. But in the U.S.A., A. thiersii was most frequently observed in Kansas (n=38), Maryland (n=31), and Texas (n=28). Although MO records observations of A. thiersii in Argentina (n=1) and Uruguay (n=1), the records likely reflect a bias towards recording this well-known species. Moreover, within MO, all Argentinian A. thiersii observations have comments urging observers to name the observations as A. thiersii or “Amanita stirps Thiersii,” and not A. foetens. MO users do not appear to use the name A. foetens. The iNat records of A. thiersii from Mexico (n=4) are among the first observations of the fungus in that country (see below). One of us (Tulloss) keyed out one of the Mexican mushrooms and confirms it is A. thiersii (Tulloss, 2020). Mexican A. thiersii appear to be collected from lawns; images of the observations show mushrooms growing with lawn grasses and in one case, mulch (see iNat observation 53329070). Lawns are also where U.S.A. A. thiersii are found. There are also four iNat records of “A. thiersii” in Argentina, one from Mar del Plata, Argentina, and the others from Buenos Aires. The record from Mar del Plata is the southernmost record in South America. We are skeptical the Argentinian A. thiersii records are real, and later we discuss the issue.
Most mushrooms in both databases were observed around urban centers. By contrast, the two Argentinian specimens used for genome sequencing were collected far from any city; the isotype of A. foetens (AmanitaBASE 10801/MICH4948) was collected in a grass pasture in the Tucumán region (+ on map, Figure 2B) and the specimen originally described as A. thiersii (AmanitaBASE 10175), which helped spark our study, was collected from a grassy paddock outside of Córdoba, between Tucumán and Buenos Aires, Argentina (X on map, Figure 2B). The A. foetens observations in Argentina and Uruguay are typically pictured in grass lawns, although some are featured in a mulch or heavily wooded environment (see iNat observation 12341687).
We used sequences of the ITS, NucLSU, NucSSU, MitLSU, and MitSSU loci to clarify the phylogenetic relationships of our specimens and other saprotrophic, asymbiotic Amanita. We downloaded between two and five gene sequences from a total of 17 mushrooms representing ten different species from NCBI (Table 1), and also used our data from AmanitaBASE specimens 10801 and 10175. (Because publicly available sequences from the dikaryon A. thiersii Skay4041_het represent the parent genome of our haploid (monokaryotic) genome A. thiersii 10802, we omitted our genome 10802 from this analysis.)
The topology of the concatenated five-locus species tree (Figure 3) is congruent with the topologies of each of the single-locus trees and the consensus tree (not shown). Specimen Amanita sp. 10175 is nearly identical to A. foetens 10801, evidence it is A. foetens and is not A. thiersii. Notably, mushrooms of A. thiersii and both A. foetens 10801 and Amanita sp. 10175 are more closely related to each other than they are to any other saprotrophic Amanita, a clustering strongly supported by bootstrapping. However, A. thiersii specimens from North America form a separate monophyletic clade from A. foetens 10801 and Amanita sp. 10175, and bootstrap support is moderately strong. While the samples from North and South America appear to be in two distinct monophyletic groups, the genetic distance between A. thiersii 10802 and A. foetens 10801 in our distance matrix is only 0.000001: the two species appear very closely related to each other (Figure 4). However, in this analysis of the ITS locus, interspecific measures of genetic distance are frequently very low (Figure 4). The overlap between intraspecific and interspecific genetic distances is striking.
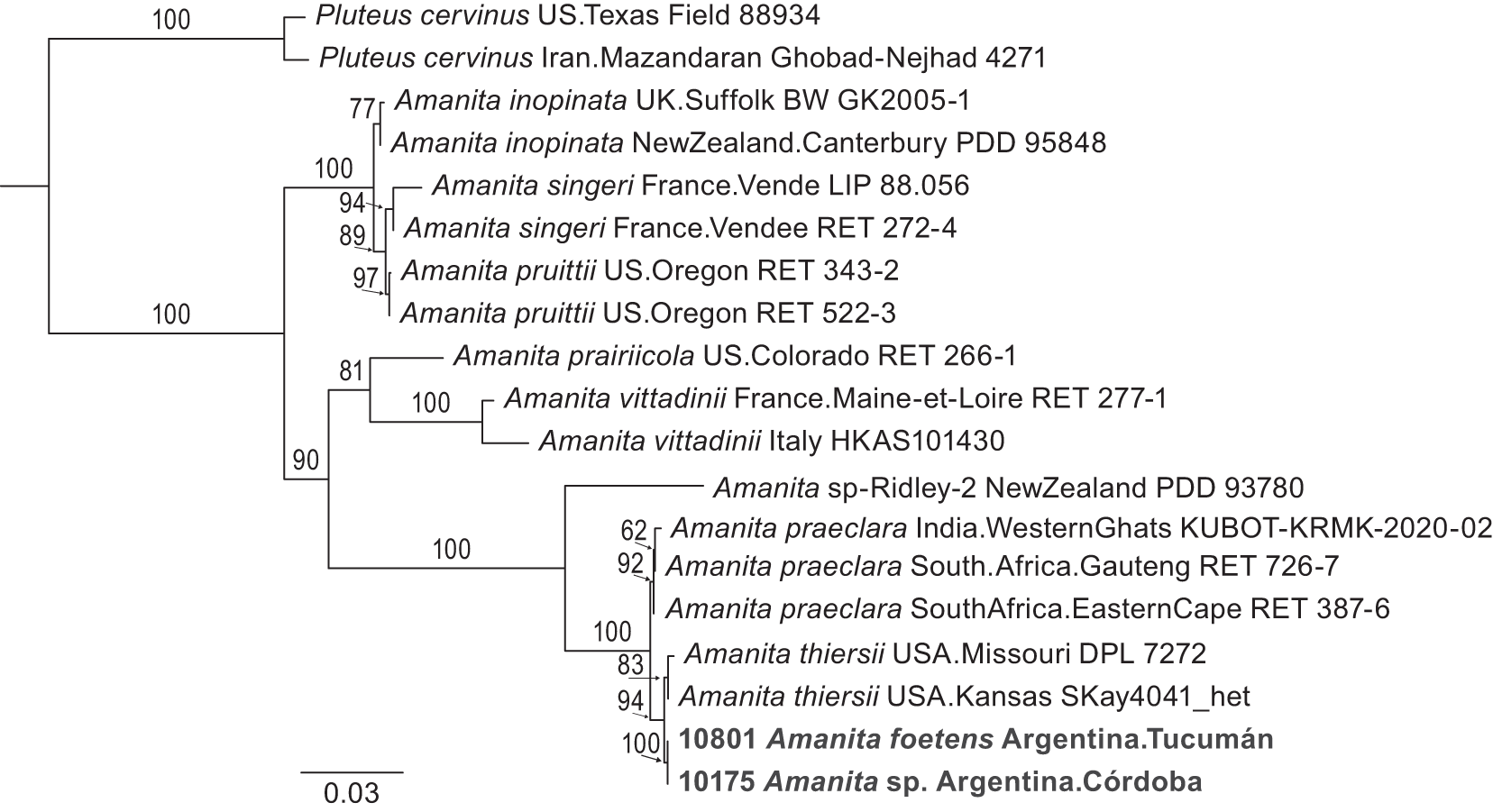
Single phylogeny generated from five concatenated loci of saprotrophic Amanita, with Pluteus cervinus used as outgroup. Branch lengths correspond to genetic distances.
The BUSCO analysis resulted in a different number of single-copy BUSCOs from each of the genomes, ranging from 197 in A. pseudoporphyria to 279 from A. inopinata. The BUSCO completeness values for our own genomes were good for genomes 10175 (94.8%) and 10802 (95.1%) and moderate for genome 10801 (81.7%). We used the sequences of 55 BUSCO genes which were found as single-copy genes from each of 15 Amanita genomes (including our genomes of AmanitaBASE specimens 10801, 10175 and 10802) to elucidate phylogenetic relationships between the three Amanita spp. specimens and other symbiotic and asymbiotic Amanita from across the genus (Table 2). Both methods (concatenation and consensus) used to construct a BUSCO species tree resulted in identical topologies for A. thiersii and A. foetens (Figure 5; concatenated sequence tree not shown).
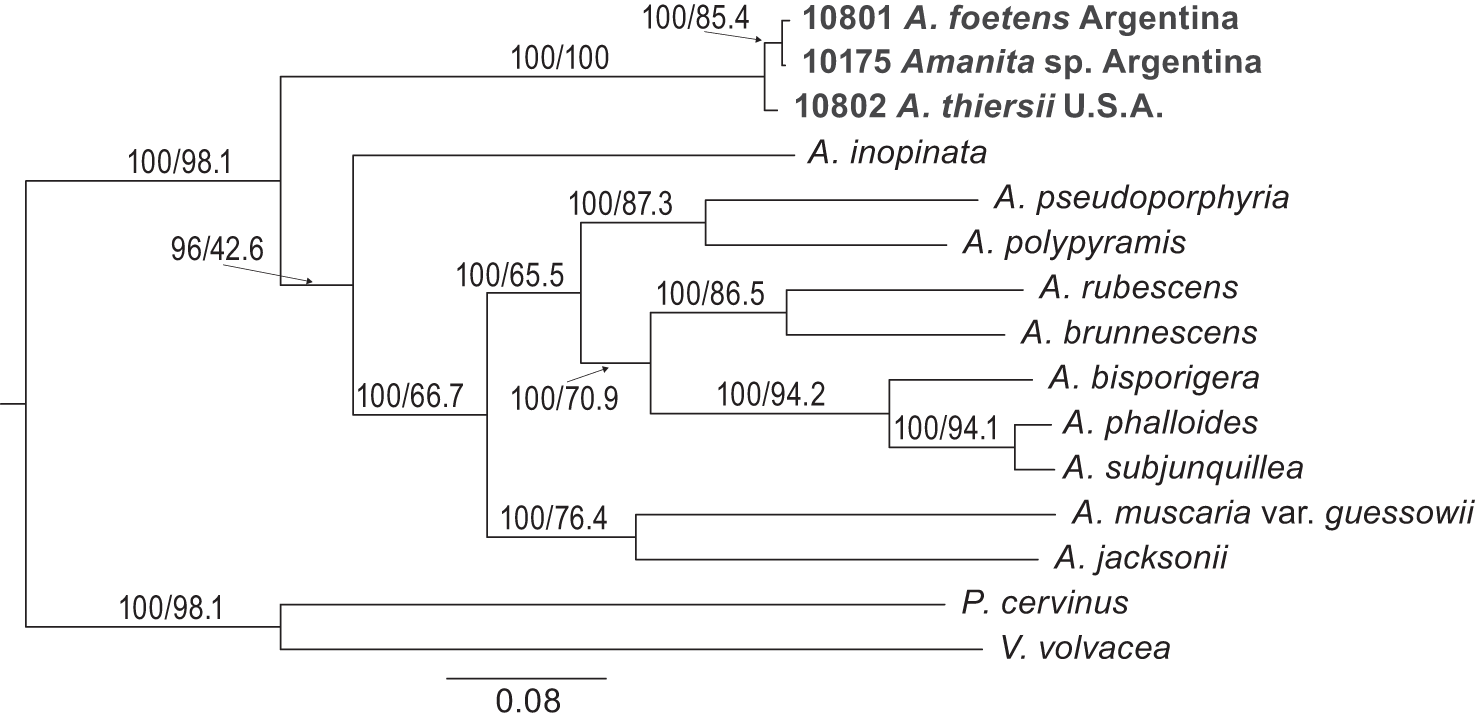
A 55-BUSCO-gene phylogeny of Amanita based on all available Amanita genomes from NCBI with P. cervinus and Volvariella volvacea used as outgroups. The phylogeny was generated with IQtree (with concatenated sequences), and a phylogeny reconstructed with ASTRAL has the same topology. Branch supports indicate ASTRAL’s quartet support test and IQtree’s concordance factor (shown before and after slashes, respectively).
In the BUSCO-species tree, our three specimens form a monophyletic group distinct from all other Amanita, and the clustering is supported by high levels of ASTRAL quartet support and IQtree concordance factor support (Figure 5). Consistent with the first analysis (Figure 3), the topology of the BUSCO phylogeny shows the Argentinian samples 10801 and 10175 as clustering together to form a monophyletic clade, additional evidence Amanita sp. 10175 is the same species as A. foetens 10801. These two are consistently separate from A. thiersii 10802, additional evidence A. foetens and A. thiersii are distinct species.
The presence of the mating type genes HD1 and HD2 in a genome would signal the potential for sexual reproduction by an individual. Using AA sequences as queries, we were unable to find the HD1 gene in the A. thiersii 10802 genome assembly (a finding we confirmed using genome alignment methods), but we could isolate partial sequences in both Amanita sp. 10175 (AA length=222) and A. foetens 10801 (AA length=103). We also isolated a sequence of HD1 from the transcriptome of A. thiersii SKay4041_het (AA length=533), a transcriptome sequenced from a dikaryotic mushroom, the parent of the germinated basidiospore used to generate the genome sequence A. thiersii 10802. In other words, while the transcriptome of the dikaryotic parent does have HD1, the genome of one of its nuclei does not: the second nucleus of the parent must be the source of the transcriptome’s HD1. The HD1 sequence found in the genome of Amanita sp. 10175 possesses a single AA substitution distinguishing it from the HD1 sequence found in the genome A. foetens 10801 and in the transcriptome of A. thiersii SKay4041_het across the conserved three-helix homeodomain region (AA length=60; Figure 6A, HD1; AA substitutions shown with asterisks). Using AA sequences as queries we were able to identify the complete sequence of HD2 in each of our three genomes and the transcriptome of A. thiersii SKay4041_het (AA lengths=289), and each sequence possessed the typical three-helix structure of the conserved homeodomain motif (AA length=60; Figure 6B, HD2). The HD2 sequences possess only one AA substitution, between Amanita sp. 10175 and A. foetens 10801. This is the first report of mating type loci for these Amanita spp.
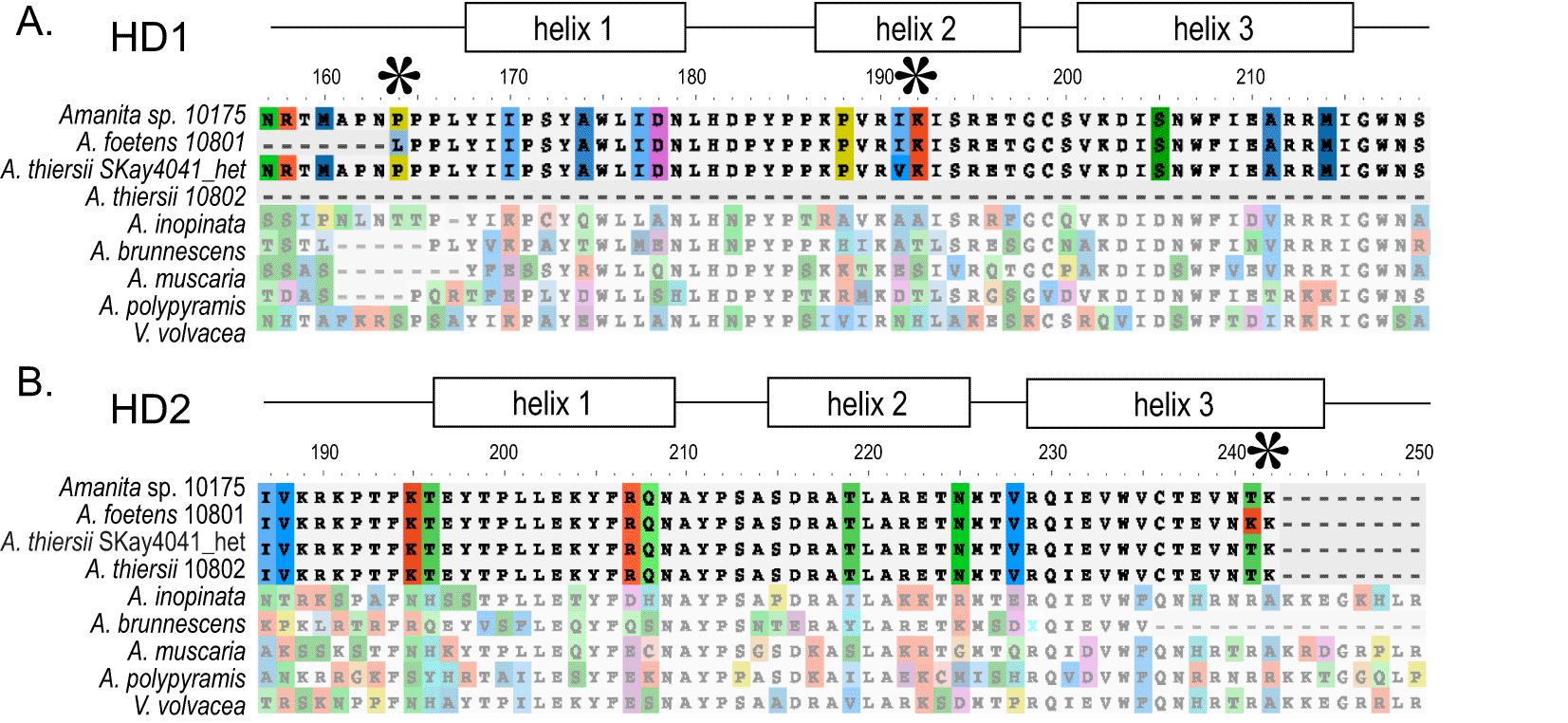
Amino acid sequences of mating type loci HD1 (A) and HD2 (B). A. thiersii 10802 is missing the HD1 gene, but a transcriptome generated from the dikaryotic parent of 10802 (A. thiersii Skay4041_het) houses a transcript of HD1, suggesting one of the nuclei of the dikaryotic parent houses HD1 while the other does not. Sequence similarity among A. thiersii and A. foetens is very high, with only one or fewer amino acid differences in the conserved homeodomain region of both mating type genes. Asterisks are near key amino acid differences.
Species’ descriptions, ranges, and phylogenies contextualized by other taxa in the genus Amanita each suggest A. thiersii and A. foetens as two different species. The morphological differences distinguishing them may enable identification in the field. However, identifying fungi based on morphology alone is often difficult (Houbraken et al., 2020; Looney et al., 2020), and if a specimen is old or weathered, key characters may be absent (a full description of the characters used to identify Amanita species is provided by Tulloss (Tulloss, 2023). While it may be possible to identify fresh material as A. thiersii or A. foetens using features of the stipe and smell; by measuring stipe length and width, and deciding if a specimen smells and, if so, what it smells like, in practice many will find these to be difficult field characters (or not know to measure or record them). For example, the smell of A. thiersii mushrooms is described as indistinct, while mushrooms of A. foetens are supposed to smell like urine (Figure 1). But collectors posting to MO report A. thiersii as having a range of scents, from indistinct and “scentless” to “smells like urine”, “has a fishy, bad odor”, all the way to “the odor was unpleasant, a bit like a sweaty locker room”. Collectors posting to iNat do not include details of A. foetens smell. While morphology emerges as formally useful, it may not serve as a practical guide for choosing whether a particular mushroom is A. thiersii or A. foetens. It is also possible the original species descriptions (especially of A. thiersii) are missing descriptions of intraspecific variability in scent, or variability between e.g. young and old mushrooms.
The geographic origin of any material is likely to be a more useful diagnostic for most collectors. Both iNat and MO document A. thiersii as growing throughout much of North America. The fungus continues to expand its range and is now found throughout the U.S.A. east of the Rocky Mountains, from Texas and Florida up to Wisconsin, Pennsylvania and Maryland (Figure 2, compare to red dots denoting range of A. thiersii in Wolfe, Kuo et al., 2012). The continuing spread of A. thiersii is remarkable. In the U.S.A., A. thiersii is most often recorded from typical lawn environments, e.g., iNat observations 94653088 from Kansas and 60276048 from Indiana. Notably, some of what are now the eastern-most collections, for example collections in Florida, show the mushroom growing from dead leaves, for example iNat observation 74461419, described at “woodroad’s edge” and pictured without any grass in sight. The fungus is hypothesized to be moving north and east in response to climate change (Hobbie et al., 2017).
With this hypothesis in mind, the northward and eastward movements of the fungus are perhaps less surprising than the discovery of A. thiersii in Mexico. In Mexico the fungus is found directly north of Mexico City and to the south in Oaxaca. However, we do not know if the Mexican observations reflect the ongoing range expansion or the discovery of the native range. We hypothesize the discovery of A. thiersii in Mexico represents a new range for the fungus, and not the discovery of a native range, basing our hypothesis on the habitats of the Mexican mushrooms. As is true for A. thiersii in the U.S.A., in Mexico A. thiersii appear to grow in lawns, for example iNat observations 86939921 and 86236720 from México, Mexico. Lawns of grasses are habitats grown by humans and the current scarcity of collections from natural habitats suggests a link between human activity and the fungus.
Amanita foetens mushrooms have not been reported in North America. Instead, the observations of A. foetens from iNat suggest it is a South American mushroom of urban and anthropogenic habitats, including lawns, pastures, and gardens. It grows in both Argentina and Uruguay. The preponderance of urban observations may reflect a simple bias; perhaps people in cities are more frequently using the database, as compared to people outside of cities. The fifteen observations are clustered in Buenos Aires Province, Argentina. By contrast, the two Argentinian mushrooms we used in genome sequencing were found far from urban habitats. The isotype and other specimens used in the original species’ description were discovered in a pasture near Tucumán (although the exact coordinates are unknown and the location is given simply as “Pie del Periquillo, Tucumán Province, Argentina”). The specimen which began our debate about A. thiersii and A. foetens, Amanita sp. 10175, was collected similarly in a paddock in the hills near Córdoba, well outside of the city center.
Observations of A. thiersii or “Saproamanita thiersii” (depending on the database) are also reported from Argentina and Uruguay. Because the name A. foetens is not used by MO collectors, it is difficult to know if records of South American “A. thiersii” in MO are actually records of A. foetens; in fact, because of the general confusion, iNat “Saproamanita thiersii” records may also represent misidentifications. The difficulty highlights the complications of using data in public databases. In iNat, Argentinian “Saproamanita thiersii” were sometimes observed from locales very close to iNat observations of A. foetens. In MO, the question of whether A. thiersii and A. foetens are the same species has been debated for nearly a decade, and the community has actively encouraged the naming of Argentinian samples as “A. thiersii” or “Amanita stirps Thiersii” (the latter convention encompasses both A. thiersii and A. foetens). Either A. thiersii is also growing in Argentina alongside A. foetens, or the Argentinian MO and iNat records are misidentifications (the records are A. foetens mislabeled as A. thiersii or S. thiersii). We favor the second hypothesis. Comments within MO offer support for our hypothesis, for example, MO observation 200425 is annotated with “… Of note to me is that this amanita [sic] was not found in its typical lawn habitat, but with trees and shrubs.”.
Phylogenies consistently place mushrooms of A. thiersii and A. foetens apart from each other, although the two species appear to be very closely related. A discussion of intraspecific diversity of the ITS locus is beyond the scope of our current study, but we were struck by the overlap between intraspecific and interspecific comparisons of genetic distances (Figure 4). We considered an hypothesis of the two species as distinct populations of the same species, but using the morphological and geographic data as context, we consider the phylogenies as better supporting the hypothesis of two distinct species. Although they were collected 570 kms away from each other, specimen Amanita sp. 10175 consistently groups with the isotype of A. foetens and is clearly an A. foetens, additional evidence A. thiersii is absent from Argentina.
The species most closely related to A. thiersii and A. foetens is A. praeclara (A. Pearson) Bas, a species originally described from the Cape Province of South Africa as an “aberrant” Lepiota (Figure 3; Pearson, 1950). It is another “white to whitish” (Reid & Eicker, 1991) decomposer Amanita collected from lawns; the original collections were made in the “grassy ground of paddock” and from “football” [soccer] fields (Pearson, 1950). The fungus was recently reported from India (Kantharaja & Krishnappa, 2022), and the Indian record suggests another potential introduction involving this different asymbiotic Amanita. However, the habitat for the Indian A. praeclara is described as “on soil under in [sic] dry deciduous forest region”. Moreover, while South African A. praeclara are described as white staining yellow (sulfur- or lemon-yellow), the Indian A. praeclara appears to be somewhat different; the description states mushrooms are “white, covered with pale yellow to orange yellow lanose-floccose covering when young … staining pale yellow afterwards” (Kantharaja & Krishnappa, 2022). One of us (Tulloss) does not believe the Indian A. praeclara is the same as the South African A. praeclara. Once again, the question of whether or not a newly discovered species is native or introduced is unanswered. Regardless, Bas (Bas, 1969) grouped A. praeclara in the same stirps as A. thiersii and A. foetens, and his grouping is now confirmed by our DNA sequence data (Figure 3).
Invasive species often reproduce asexually, and clonal propagation can facilitate spread (Gao et al., 2018). In North America, A. thiersii lacks genetic diversity (Wolfe, Kuo et al., 2012). While we identified the mating type locus HD1 in the transcriptome of a dikaryotic strain of A. thiersii, the locus is missing from its monokaryotic offspring (which was cultured from a single basidiospore of the mushroom used to generate the dikaryotic strain, see also Elmore, 2020). The HD1 locus appears to be present in some nuclei and absent from others. Mushrooms are sexual structures and A. thiersii clearly grows mushrooms. But the lack of genetic diversity and existence of a nucleus missing HD1 suggests unusual mating dynamics, an analog to the biology of invasive Death Caps in California (Wang et al., 2023), and to other unusual basidiomycete mating systems (Coelho et al., 2017). We found no evidence for missing mating type loci in either of the A. foetens genomes, however, neither genome involved a monokaryotic culture.
For the moment, we recommend all mushrooms keyed to A. thiersii and A. foetens observed in North America be named as A. thiersii and all those found in South America be named as A. foetens. Using geography to choose names will simplify identification of morphologically ambiguous mushrooms. However, basing identification on geography will also obscure future introductions. If or when either species is introduced to the other continent, the introduction will be difficult to recognize. There is a great need for simple molecular tools enabling straightforward identification of specimens to species, and tools would be strengthened by greater efforts to sequence types and apparent novelties.
In the aggregate, morphological descriptions, data on occurrence and habitat, and phylogenies based on both a few loci and many specimens, as well as many loci of a few specimens, support A. thiersii and A. foetens as closely related but distinct species. The species appear morphologically and genetically distinct, and geographically isolated. We reject the hypothesis of A. thiersii as an A. foetens introduced to Texas from Argentina by humans. While our data do not establish A. thiersii as an introduced and now invasive species, they also do not establish it as native; the native range of A. thiersii remains unknown. It may have been introduced to North America from a country other than Argentina. For the first time, we report A. thiersii in Mexico. The question of why A. thiersii is spreading rapidly throughout North America remains open. In this instance, baseline data on fungal biodiversity have failed us; there are no baseline data for A. thiersii. Thus, we started with an enigma, and end with one as well.
Dryad: Mushroom Observations of Amanita thiersii and A. foetens, https://doi.org/10.5061/dryad.7h44j1008 (Dunkirk et al., 2023a).
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
NCBI: Are Amanita thiersii and Amanita foetens the same species? Accession numbers SRR23983939, SRR23983940, and SRR23983941, https://identifiers.org/NCBI/bioproject:PRJNA947219 (Dunkirk et al., 2023b).
This project contains the following underlying data:
Source code available from: https://github.com/noramushrooms/Amanita_thiersii.
Archived source code at time of publication: https://doi.org/10.5281/zenodo.7996518 (Dunkirk, 2023).
License: Open.
We thank Dr. Timothy James and the University of Michigan herbarium for sending us the voucher specimen of A. foetens.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Fungal systematics, fungal genomics
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Mycology, taxonomy, fungal ecology, ectomycorrhiza, tropical ecology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: mycology, phylogenetics, population genetics, social evolution, kin selection, symbiosis
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 20 Jul 23 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
We read your paper as part of our Genomes Journal Club at the Forestry and Agricultural Biotechnology Institute (FABI) at the University of Pretoria. We’d like to ... Continue reading Dear authors
We read your paper as part of our Genomes Journal Club at the Forestry and Agricultural Biotechnology Institute (FABI) at the University of Pretoria. We’d like to share a few of our thoughts and comments with you.
Overall, we thought the article was really well written. The use of citizen science data was particularly interesting and we feel this is something that biologists should strive to do more often. We also felt that the way transcriptome data was used to search for the HD1 gene was an elegant and thorough identification method.
The only "negative" comment was related to the flow of the results. We felt it would have made more sense to put the phylogenies after the species description section, and only after that to bring in the location data results.
Another comment that came up was over the necessity of the recommendation to name all the observations in North America as A. thiersii and those in South America as A. foetens. Most of us felt that we would have drawn no conclusions about the naming, but it should be noted that none of us are experts in taxonomy. However, this could be considered a fair recommendation, especially since (1) you mention the pros and cons of this choice, (2) you say it is only for the time being and (3) you emphasise the need for molecular identification tools. The only question that remains for us is if this conclusion is necessary, i.e. is there a need to standardise the naming by location.
Otherwise, it was a very interesting article which we all enjoyed reading!
We read your paper as part of our Genomes Journal Club at the Forestry and Agricultural Biotechnology Institute (FABI) at the University of Pretoria. We’d like to share a few of our thoughts and comments with you.
Overall, we thought the article was really well written. The use of citizen science data was particularly interesting and we feel this is something that biologists should strive to do more often. We also felt that the way transcriptome data was used to search for the HD1 gene was an elegant and thorough identification method.
The only "negative" comment was related to the flow of the results. We felt it would have made more sense to put the phylogenies after the species description section, and only after that to bring in the location data results.
Another comment that came up was over the necessity of the recommendation to name all the observations in North America as A. thiersii and those in South America as A. foetens. Most of us felt that we would have drawn no conclusions about the naming, but it should be noted that none of us are experts in taxonomy. However, this could be considered a fair recommendation, especially since (1) you mention the pros and cons of this choice, (2) you say it is only for the time being and (3) you emphasise the need for molecular identification tools. The only question that remains for us is if this conclusion is necessary, i.e. is there a need to standardise the naming by location.
Otherwise, it was a very interesting article which we all enjoyed reading!