Keywords
vestibular incision subperiosteal tunnel access, VISTA, platelet rich fibrin matrix, PRFM, platelet rich fibrin, PRF, gingival recession, randomized controlled trial
This article is included in the All trials matter collection.
This article is included in the Datta Meghe Institute of Higher Education and Research collection.
vestibular incision subperiosteal tunnel access, VISTA, platelet rich fibrin matrix, PRFM, platelet rich fibrin, PRF, gingival recession, randomized controlled trial
Gingival recession (GR) is a common patient related complaint, which occurs when the position of the marginal gingiva shifts unfavorably. Recession presentation has been observed to follow two broad patterns: localized and multiple types. Multiple gingival recession (MGR) is more common than its localized variation with higher aesthetic demand because of the more common traumatic origin. The large areas of the avascular root surface that needs to be covered further complicates the MGR management.1 Management of single or MGR with various surgical techniques relies on a variety of parameters, including the extent of the defect, whether or not keratinized tissue surrounds the defect, the thickness of the gingiva surrounding the defect, and the patient compliance.2
The fundamental goal of treating buccal or labial recession defects is to reconstruct the gingival architecture, and whether efforts are made to enhance tissue properties. Traditionally, pedicle grafts, free soft-tissue grafts, and guided tissue regeneration (GTR) have all been used to address recession defects. The decision to choose one modality over another from the variety depends on the number, type, and patient-related aspects of the defects.1 The critical patient element to be considered before choosing a particular procedure is high aesthetic demand with a requirement for low postoperative distress. Both bilaminar and coronally advanced flap (CAF) methods have been used to correct MGR. According to a systematic review, the clinical parameters can be improved by CAF with or without a connective tissue graft (CTG).2 Amongst them, CAF with CTG is regarded as the gold standard for periodontal root coverage (RC) and soft tissue augmentation. It has some drawbacks, including the need to harvest from a donor tissue, the scarcity of available tissue, and the increased risk of post-harvest morbidity.3
The tunneling technique introduced by Allen in 1994 is yet another significant addition in CAF.4 Since its introduction in 1994, numerous procedural changes have been suggested. Vestibular incision subperiosteal tunnel access (VISTA), a minimally invasive subperiosteal tunnel approach, was introduced by Zadeh in 2011. It allows access to multiple teeth through a single vertical incision.3 It also simplifies the tunneling method and enhances the profile of wound healing.5,6 Additionally, the VISTA in combination with different graft materials produced intriguing results.3,7–9 The successful treatment of multiple recession defects (MRD) can be achieved with the minimally invasive VISTA approach which has several benefits. The possibility of lacerating the gingiva of the teeth being treated is decreased by the vertical incision made medial to the defect. Additionally, subperiosteal dissection minimizes gingival margin (GM) tension during coronal advancement and keeps the interdental papillae blood supply intact. GR coverage is improved with the VISTA procedure when the GM is moved coronally to the cemento-enamel junction (CEJ) with an augmented graft or membrane and fixed in a secure position to avoid recurrence at an early stage of recovery.10
Clinicians are always on a quest for an appropriate bioactive surgical adjuvant that can control inflammation while simultaneously promoting healing. The events that follow the postoperative period determine complex wound remodeling and tissue survival. Recombinant growth factors, for example, have been demonstrated to promote periodontal wound healing when used as an adjuvant.11 A second-generation platelet concentrate called platelet rich fibrin (PRF) was introduced in 2005 by Choukroun et al.12 One such bio-healing substance that is important for both effective hard- and soft-tissue repair is PRF. It delivers vital healing dynamics that lead to reduced postoperative pain and advanced tissue recovery.12 Multiple cell lines with immunological activity have shown a good response to PRF, and viable platelets in PRF release the six required growth factors.13 The advantages of PRF in different surgical procedures have been assessed and found to be adequate.13,14 Additionally, its beneficial effects on many cell lines, particularly the fibroblastic cell line, have been ascertained.15 Recent histological research examined CTG with or without PRF in treating recession and said that the PRF group had early vascularity and tissue maturation.16
Autologous platelet concentrates are a promising new approach to periodontal regenerative therapy. Numerous studies have examined the efficiency of platelet rich plasma (PRP) and PRF in a variety of therapeutic conditions that demand rapid healing and have discovered favorable clinical and radiographic findings.17–19 Due to these encouraging results, it is necessary to compare and contrast PRF with the recently created platelet rich fibrin matrix (PRFM), which involves a streamlined technique without artificial biomodification. The creation of autologous PRFM as a growth factor delivery method is an advancement in the dental field. It consists solely of centrifuged blood without any additions or biochemical processing. PRFM is a therapeutic biomaterial with great capability for bone and soft tissue regeneration, which combines the qualities of fibrin, platelets, leukocytes, growth factors, and cytokines.20 Compared to a typical human blood clot, the autologous biomaterial PRFM contains a dense concentration of platelets. Growth factors found in its alpha granules impact every cell and the development of every tissue involved in wound healing. It releases growth factors (GF) abundantly and these are essential elements in the course of wound healing through signaling transduction mechanisms. Soft tissue and bone regeneration have demonstrated an immense potential role in regenerative therapy.21 In vitro research by Carroll et al.22 from 2005 revealed that viable platelets in PRFM released six GFs throughout their seven-day study, primarily platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and fibroblast growth factor (FGF).
The present study was undertaken to compare the efficacy of the PRFM membrane with that of the PRF membrane using the VISTA approach for obtaining RC in patients with Miller’s class I/II MRDs. Since the minimally invasive VISTA approach permits better access, gingival margin stabilization and coronal positioning, PRFM and PRF overcome the drawbacks of CTG.
The current randomized, controlled, parallel designed clinical study was conducted in 20 subjects as determined by a sample size calculator (OpenEpi, version 3, open-source calculator – SSMean) by comparing two means at 95% confidence interval and a computer-generated random number was generated (10 male and 10 female). We adhered to the CONSORT checklist for reporting clinical trial protocols.23 Patients with an age range of 25 to 50 years (mean age of 33.7 ± 7.11) with MGR defects on the buccal and/or labial surfaces of the teeth were enrolled in the outpatient department of Periodontics, Sharad Pawar Dental College and Hospital, Wardha, Maharashtra. Approval for the research protocol was given by the Institutional Ethics Committee (DMIMS) Wardha, Maharashtra, (IEC No. DMIMS (DU)/IEC/2020-21/9415, date of approval: 24/12/2020). Clinical trial registration: CTRI/2021/07/035240, registered 29 July 2021.
The purpose and essence of the research were explained to each subject before starting the technique, and the written informed consent of each subject was obtained.
Participants were recruited from the outpatient department of Periodontics, Sharad Pawar Dental College and Hospital, Wardha, Maharashtra.
Healthy subjects without any systemic illness with MGR (>1) on their labial/buccal surfaces, GR depth ≥ 2 mm, and presence of adequate width of keratinized gingiva (WKG) were included. To match all the characteristics of included subjects at baseline the age group of the subjects was in the range of 25 to 50 years.
Subjects with poor oral hygiene following “etiotropic/phase I periodontal therapy” and exhibiting plaque scores greater than or equal to 1, mobile teeth, any systemic diseases, suspected or known allergies to drugs or study materials, use of tobacco in any form, immune-compromised subjects, alcoholics, lactating or pregnant women, and subjects with compromised immune systems were excluded.
After initial therapy and before surgery for this study, all subjects with at least two or more adjacent GR defects were chosen. A computer-generated random number (Research Randomizer software (Version 4.0) (an open-access alternative is Random Allocation Software (Version 2.0))) was generated following a simple randomization technique, with an equal number of subjects allocated in the test and control groups. Once the allocation sequence was generated, a single examiner (CS) allocated the patients into two groups. A second examiner (SH) was blinded to the allocation sequence and the examiner who gave the intervention was blinded. Participants and the examiner were both blinded during the allocation system. The test group was managed by VISTA and PRFM membrane (n = 10) while the control group was treated with VISTA and PRF membrane (n = 12). A re-evaluation was performed at three and six months of therapy for the estimation of outcomes.
Custom made occlusal acrylic stents were made to standardize the probe position and angulations. Alginate impression was prepared to make a cast model of both jaws. An acrylic stent was prepared on the cast model at baseline and six months. The occlusal stent was covering the occlusal surfaces of the teeth and occlusal surface of at least one tooth distal and mesial to it. At the deepest site of the involved tooth a reference point was made for positioning of the UNC 15 periodontal probe (University of North Carolina, Hu-Friedy, Chicago, USA). The apical margin of the stent was considered as a fixed reference point. (To prevent measurement variability, a fixed reference point was established at baseline to duplicate the same location during the subsequent visits.) Assessment of the primary outcomes was carried out with the help of the following clinical measurements. The probing pocket depth (PPD), relative gingival margin level (RGML), and relative attachment level (RAL) was assessed with a UNC-15 calibrated periodontal probe (University of North Carolina, Hufriedy) and rounded up to the nearest millimetre marking – in case of doubt, lower values were considered. All probing measurements were recorded in the individual tooth at peak recession depth (mid-facially). These clinical recordings were noted at baseline and at the six months recall visit.
The UNC-15 calibrated periodontal probe was positioned in the slot given on the custom-made stent and a measurement was taken from the stent’s bottom edge to the gingival margin, which corresponds to the RGML. The distance to the lower border of the prefabricated stent was then noted as the RAL while the probe was still held at the base of the pocket. By subtracting the RGML from the RAL value, PPD was recorded. WKG was recorded from the most apical portion of mucogingival junction (MGJ) to the margin of gingiva on the mid labial aspect of the experimental teeth with the help of William’s graduated probe (GDC Single End Probes #3 (Pcpunc15). REC was calculated from the CEJ to the gingival margin with the help of a UNC-15. Gingival thickness (GT) was calculated 3 mm below the gingival margin, under topical anesthesia and using an endodontic reamer with a rubber stopper.
For a detailed description please refer to the research protocol in the underlying data.24,25
For the production of PRFM, 10 mL of blood was obtained from the antecubital vein through venipuncture for one minute from the patients. Within 30 seconds, the samples were transferred to the Meresis PRFM kit (*R-4C, REMI Laboratory Instruments, Mumbai, India) and positioned in the centrifuge. A single-spin centrifuge (Remi R-8C 16×15 ml Laboratory Centrifuge with Angle Rotor Head) was used to centrifuge the sample for 10 minutes at 3000 rpm. The upper layer of PRFM clot was obtained and placed in a PRFM box after centrifugation and pressed to produce PRFM membranes.22
A sample of 10 mL of blood was collected from the antecubital vein of each patient, placed in sterile glass test tube and centrifugated at 3000 rpm for 13 minutes in a centrifuge machine (Remi R-8C 16×15 ml Laboratory Centrifuge with Angle Rotor Head). After centrifugation, a fibrin clot from the upper layer was obtained, and the remaining red blood cells were removed. To obtain the PRF membrane, the clot was transferred to a PRF box and compressed (Choukroun et al. 2006).26
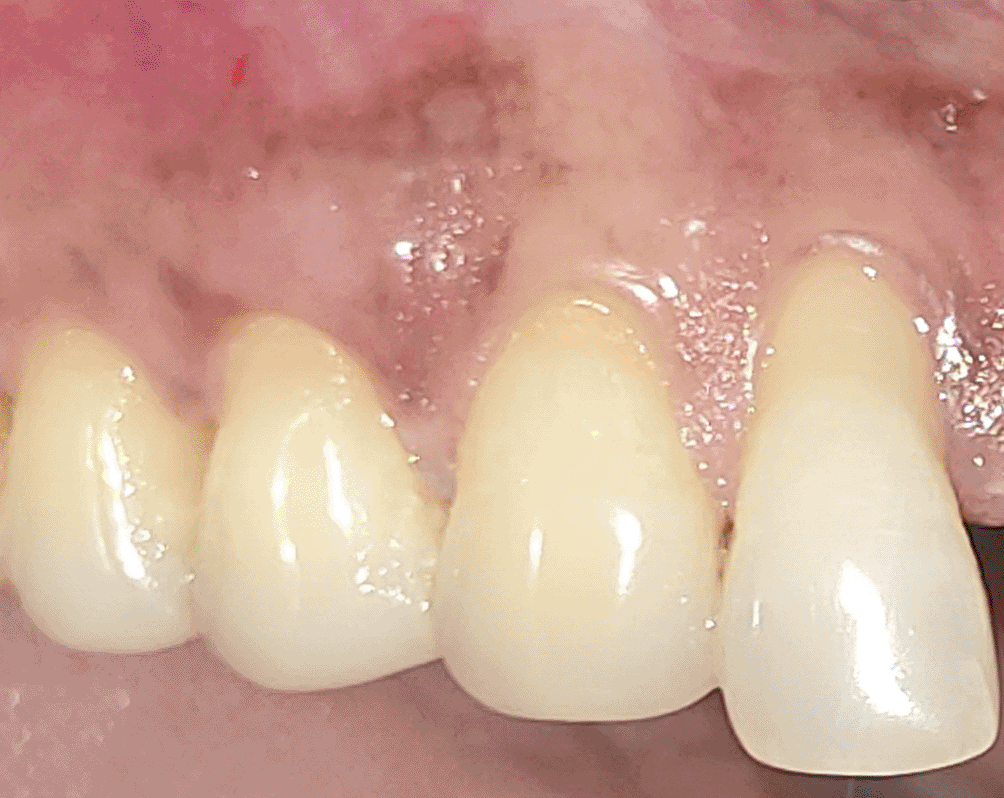
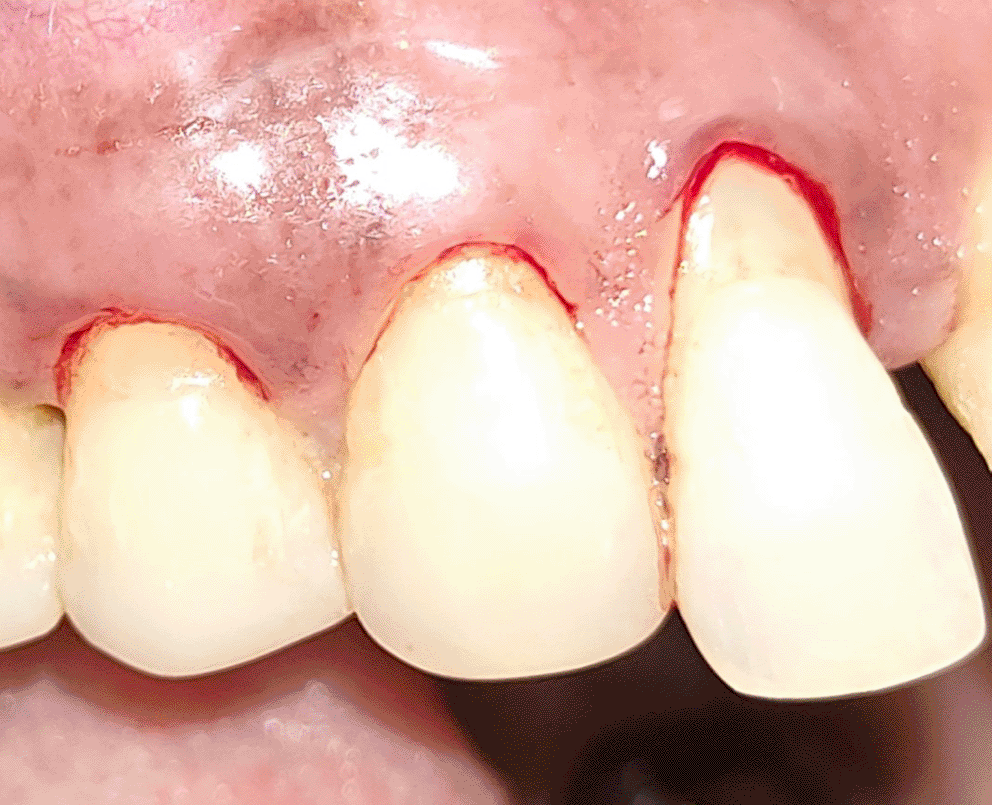
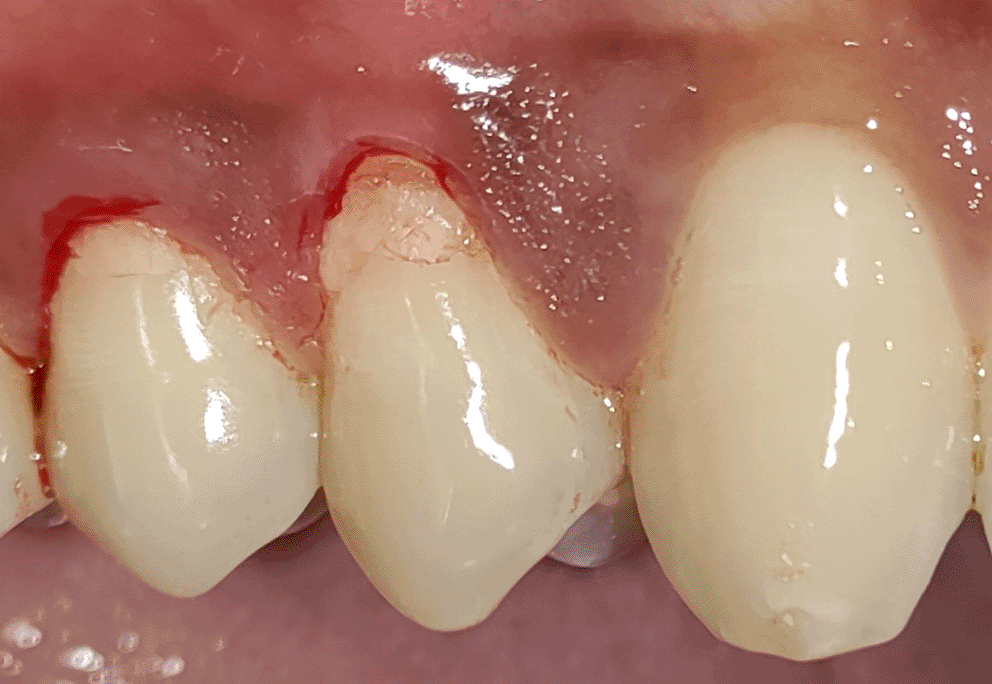
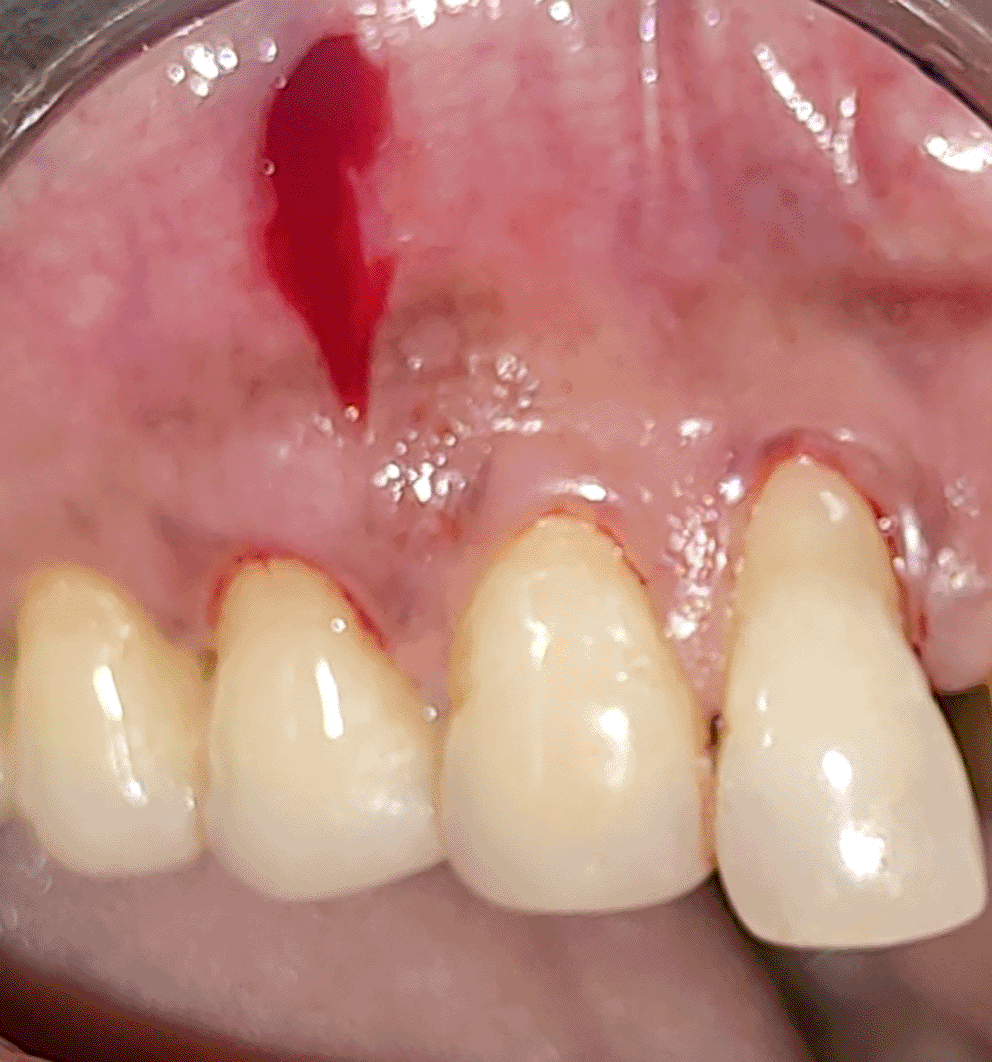
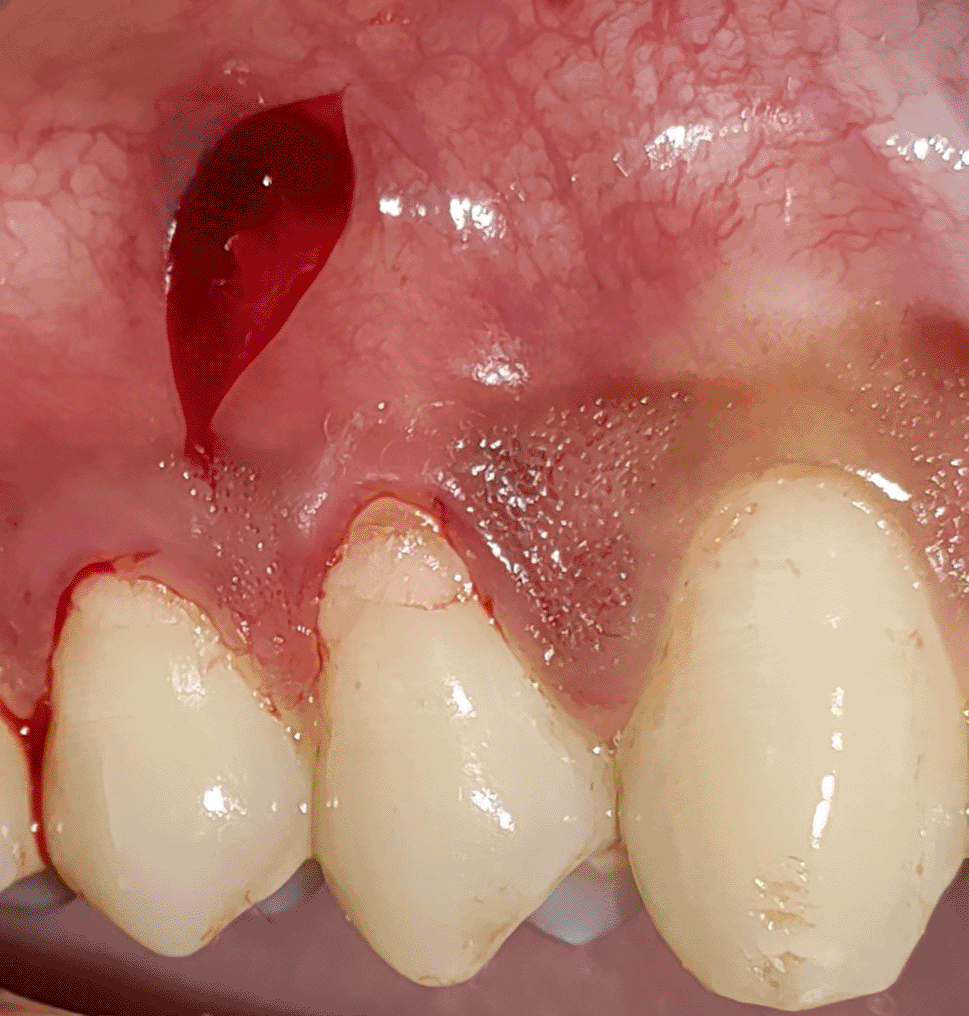
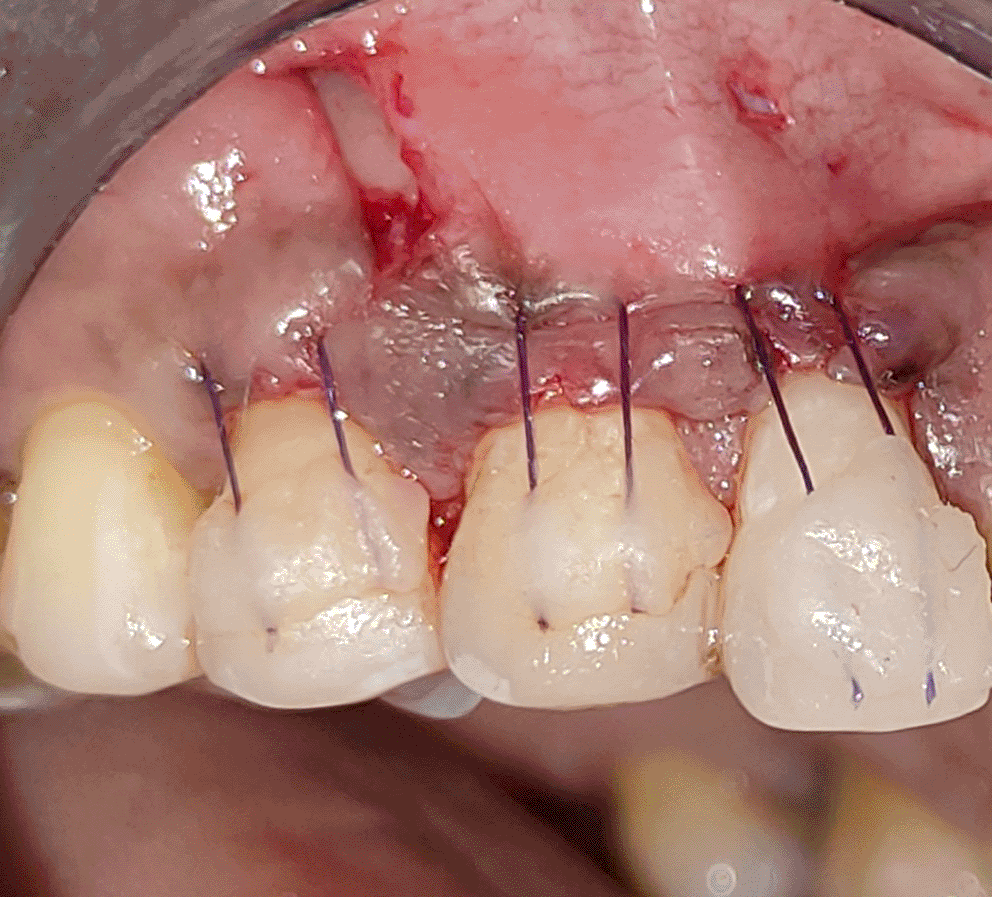
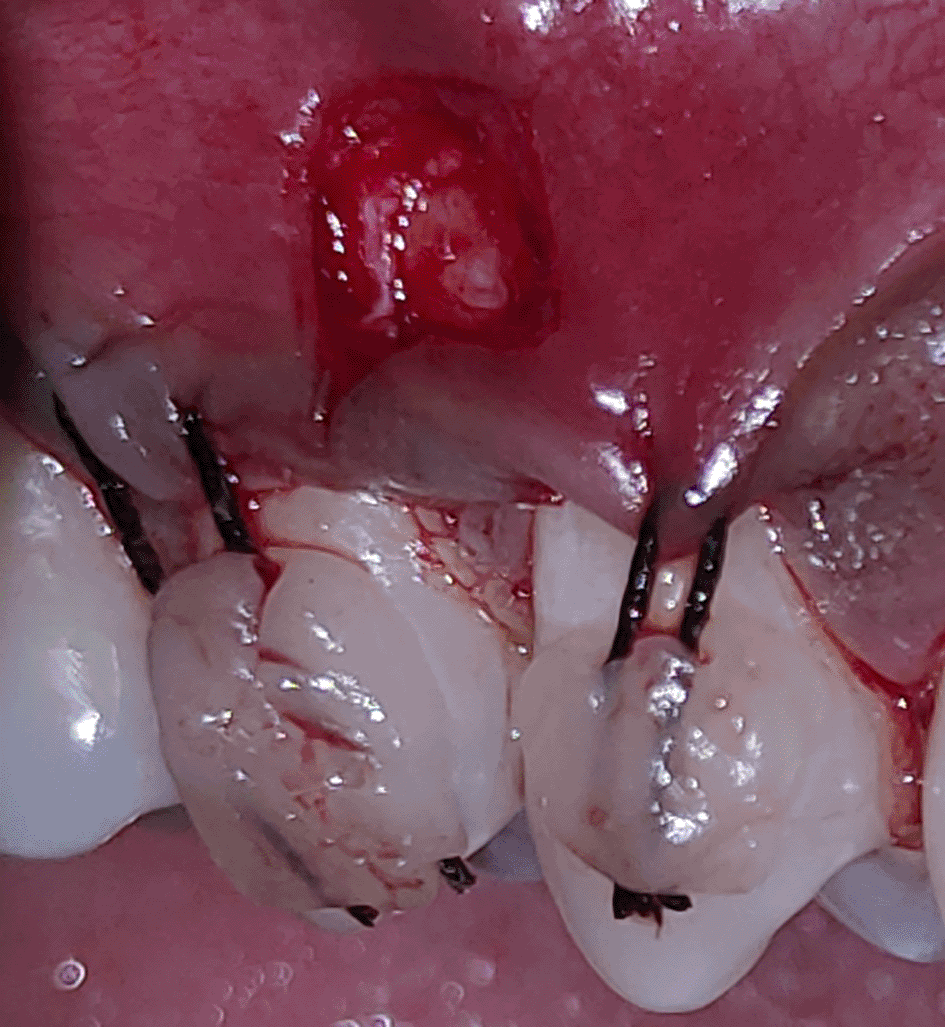
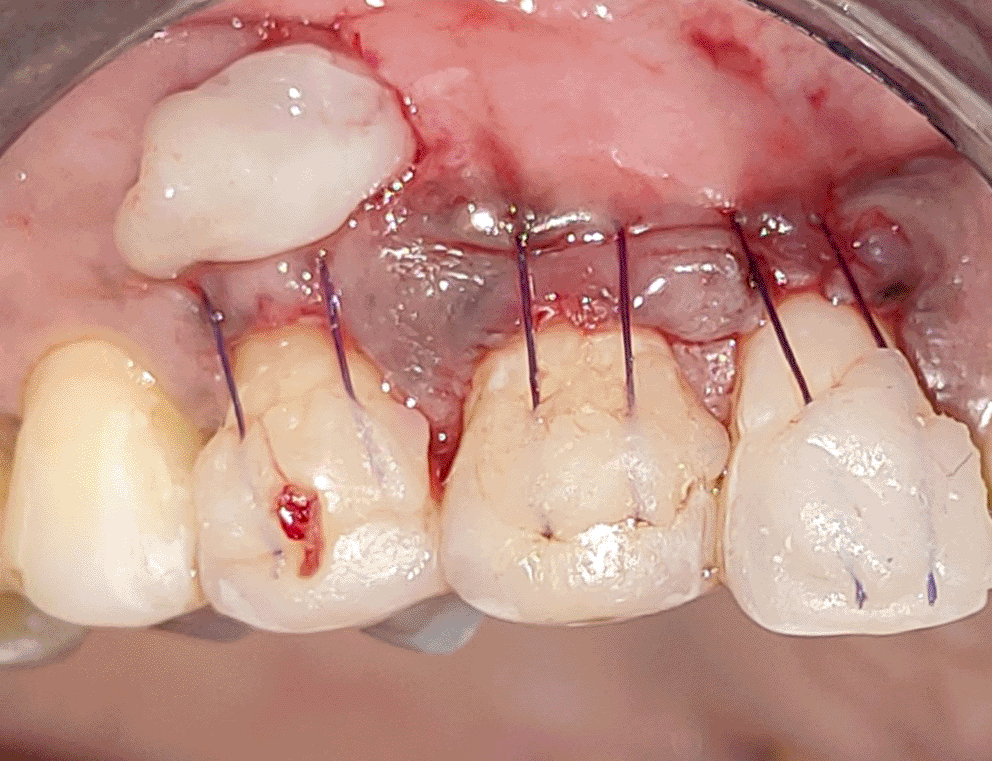
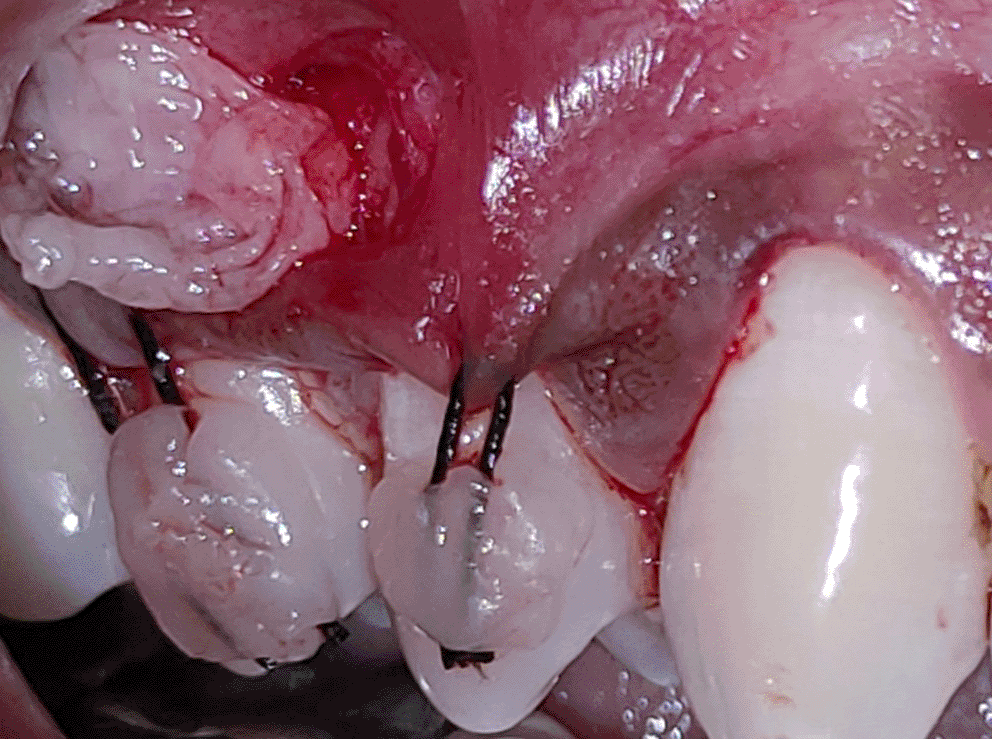
All the patients were educated to use oral rinse 0.2% chlorhexidine gluconate (CHX) (Hexidine Mouthwash ICPA health products LTD) for 30 seconds before the treatment. Infection control and full asepsis were maintained during the surgical process. After a 2% Xylocaine containing 1:80,000 concentration of epinephrine (Ligno-Ad local anesthetic, Proxim Remedies, India) anesthetic was administered, the denuded root surfaces were thoroughly cleaned and planed using curettes and an ultrasonic device (Woodpecker HW-3H). Gingival recession seen with three teeth numbered as 23, 24, 25 as shown in Figure 1 and gingival recession seen with two teeth numbered as 14, 15 as shown in Figure 2. Crevicular incision given as shown in Figure 3 and Figure 4. In the vestibule mesial/distal to the surgical site, a full-thickness vertical incision 8–10 mm in length was made as shown in Figures 5 and 6. The vertical incision was not extended all the way to the gingival edge but functioned as a portal for the expansion of the subperiosteal tunnel. The osseous plate was exposed, and a blunt Orban’s knife was used to expand the tunnel up to one or two teeth beyond the surgically corrected recession location. Furthermore, without penetrating the papillary tip, the sub-periosteal tunnel was expanded into the inter-papillary area. The slow apical motion of the knife via the papillary area connects to the vestibular tunnel, allowing the mucogingival covering the MRD to migrate coronally. This subperiosteal tunnel was moved coronally and passively placed over CEJ, which will cover the MRD. Coronal anchoring sutures were engaged 2–3 mm apically to the gingival border of each tooth. To avoid apical displacement of the marginal gingiva, the suture was joined with a resin composite button at the mid-coronal position of each tooth’s buccal aspect as shown in Figures 7 and 8. After coronal stabilization, using a small periosteal elevator, a freshly made PRFM membrane was inserted into the tunnel in the test group (as shown in Figure 9) and a PRF was inserted in the control group (as shown in Figure 10) and they were uniformly distributed throughout all defects. The vertically placed incision was sutured for the primary closure when the membrane had fully adapted as shown in Figures 11 and 12. A Coe pack was used to cover the whole surgical site. A pre- and post-operative view of the test group at six months is shown in Figures 13 and 14. Figures 15 and 16 show a pre- and post-operative view of the control group at six months.
After the procedure, a non-steroidal anti-inflammatory, consisting of a blend of Ibuprofen 400 mg and Paracetamol 325 mg and antibiotic Amoxicillin 500 mg TID were prescribed for five days. For three weeks following surgery, participants were instructed not to brush their teeth at the treated areas. For 14 days, all individuals were asked to oral rinse with 0.2% CHX two times daily. Patients were informed to avoid any damage to the pack. Removal of the periodontal pack was done after seven days followed by suture removal after 14 days, healing was observed. Proper care was taken while saline irrigation and polishing with rubber-cup & polishing paste, to avoid damage to the involved sites. Patients were asked to clean the treated area with 0.2% CHX soaked cotton, for additional seven days before brushing using Charter’s approach. The recall periods were set at one, three and six month intervals after the surgical procedure.
The mean and standard deviation (Mean ± SD) scores for all clinical measures were documented. The statistical significance of the mean data was assessed using conventional statistical techniques. Student-paired t-tests were applied for data comparison within each group from baseline to six months, whereas student unpaired t-tests were utilized for data comparison across groups. If the probability value (p) was ≤0.05 it was significant and ≥0.05 it was non-significant. All data were assessed using SPSS 11.0 (SPSS Inc, 2003) (RRID:SCR_019096) software.
Twenty-eight patients were screened of which eight patients did not fulfill the inclusion criteria. Randomization of 20 patients were done and 10 patients were allocated to each group. No patients were lost to follow-up and 20 patients were analyzed at three and six months as shown in Figure 17.
Twenty systemically healthy patients with a mean age of 33.7 ± 7.11 in the test group and 35.1 ± 8.23 in control group (Age range: 25 to 50 years) presenting with 48 labial/buccal multiple (≥2 teeth) GR defects (>2mm) were treated in the present study.
The healing process was uneventful throughout the research. Until the first postoperative visit, the periodontal dressing remained in place. No patient had any post-operative complications. None of the participants withdrew before the completion of study and all were pleased with the outcome.
Table 1 displays the mean full mouth plaque index (FMPI) and full mouth papillary bleeding index (FMPBI) score at baseline and six months for both groups. We found a statistically significant decrease in FMPI and FMPBI score at six months (p < 0.05) in both groups. The mean FMPI scores during the six-month study period remained low (<1), in both groups which could be because of reinforcement of oral hygiene instructions.
(Mean ± SD in mm).
All the investigated parameters in both groups at baseline were observed to be statistically non-significant (p >0.05), indicating the same starting point for both procedures. Clinical parameters RGML, REC, RAL, PPD, WKG and GT revealed a significant reduction (p < 0.05) after six months compared to baseline in both groups (Tables 2 and 3).
(Mean ± SD in mm).
(Mean ± SD in mm).
The comparison between mean RGML reduction in the test (2.58 ± 0.50 mm) and control groups (2.37 ± 0.49 mm) at six months indicated no significant difference (p = 0.23) in both groups by 0.20 ± 0.83 mm. Comparison of mean CAL gain among groups at six months indicated no statistically significant difference (0.20 ± 1.06 mm). The mean reduction of PPD for the test group was 0.5 ± 0.51 mm when compared to the control group 0.5 ± 0.51 mm at six months, and no significance difference was found (p > 0.50). At six months, the comparison of the mean increase in WKG in the test group (2.04 ± 1.36 mm) with the mean gain in WKG in the control group (1.5 ± 1.35 mm) showed no significant difference (p-0.18). The difference between the mean GT rise in the test group (0.77 ± 0.15 mm) and the mean GT rise in the control group (0.82 ± 0.17 mm) was statistically non-significant. At six months, when comparing mean REC between both the groups, a reduction in the test group was observed but it was non-significant (Tables 4 and 5).
(Mean ± SD in mm).
(Mean ± SD in mm).
In the test group, mean percentage of defect coverage was 89.23 ± 15.04% and the predictability for complete CRC was 61.6 ± 43.11% i.e., 14 of 25 defects demonstrated 100% CRC. In the control group, mean percentage of defect coverage was 85.06 ± 17.71% and the predictability for CRC was 55 ± 19.45% i.e., 8 of 24 defects treated showed 100% CRC (Table 6).
(Mean ± SD in mm).
Clinicians face significant therapeutic challenges in managing GR defects including restoring the protective anatomy of the MGJ complex, reestablishing the aesthetic harmony between soft tissues and neighboring tooth structures, and ideally regenerating alveolar bone, periodontal ligament, and cementum, which is lost. Such therapeutic challenges become even greater when treating MRD, where challenges include inadequate tissue availability and increased post-harvesting morbidity. In the treatment of gingival recession defects, platelet concentrates have emerged as an alternative, which contain cytokines, platelets, stem cells giving a more predictable and reproducible outcome to restore the amount of keratinized tissue, RC and aesthetic outcome.27 In recent years, the usage of autologous blood derivatives (PRP/PRF/PRFM) for periodontal defects has also aimed to provide a novel and highly efficient technique for regeneration in root coverage procedures with less patient morbidity and fewer post-operative complications. Since the PRP/PRF era derivatives have been used for various soft tissue augmentation procedures. In the current study, 24 recession defects were treated with PRFM membrane with the VISTA approach in the test category whereas 24 recession defects were managed with PRF membrane using the VISTA technique in control group.
Zadeh (2011)3 observed complete root coverage for all GF mediated, minimally invasive VISTA treated teeth, as well as 1 to 2 mm gains in keratinized gingiva after a 12-month follow-up period. At the 20-month observation period these improvements were sustained. According to the findings of our study, both groups had significantly improved clinical parameters after six months. At six months, both treatment groups showed a significant GR reduction (2.58 ± 0.50 mm in the test group and 2.37 ± 0.49 mm in the control group).
Jankovic et al. (2012)28 carried out an RCT and reported that utilizing PRF membrane accelerated wound healing and this occurs by the virtue of growth factors trapped in the PRF mesh, which are gradually released, accelerating the regenerative capability. The fibrin network structure is crucial to the enhanced PRF healing process20 and reduces subjective discomfort of patients and provides acceptable clinical results in GR treatment compared to CTG. The use of CTG versus PRF in the management of Miller Class I and II GR using conventional RC techniques was compared in several systematic reviews.29–31 Both the types of above-mentioned treatment modalities have shown improvement in the condition of GR defects, with regard to CAL gain, RD reduction and increase in keratinized tissue.
To the best of our knowledge, the use of PRFM membrane in RC is rare, with just a few case studies documenting its usage especially in socket preservation, GR, and depigmentation. The outcome of a single case report utilizing the CAF method using peripheral blood mesenchymal stem cells and PRFM corresponds to RC of 60.0% and CAL gain of 3 mm at three months. (Belludi, et al., 2020).32 The present study yielded better results. Additionally, there aren’t enough clinical studies that use PRFM for RC. The findings of the above-mentioned study are in congruence with the outcomes obtained in the present investigation.
Therefore, in order to achieve RC, the current investigation was conducted to evaluate the effectiveness of the VISTA+PRFM membrane to manage MRD. A statistically significant reduction in mean recession defects was seen in the test group six months after surgery in comparison to the baseline. At six months, the mean GR in the test group reduced from 3 ± 0.83 mm to 0.41 ± 0.58 mm, reflecting a mean RC of 89.23% and a CRC of 14 out of 24 recession defects treated (61.6%).
Six months after surgery, the mean RD in the control group decreased from 2.91 mm to 0.54 mm, correlating to a mean of 85.06% recession defect coverage. The CRC was present in 13 out of 24 (55%) corrected recession defects. A study by Garg et al. (2017)33 compared the effects of VISTA with or without PRF membrane for the treatment of Class I and III MRD and it can be used to compare the outcomes of this research for the usage of PRF membrane utilizing the VISTA approach to treat multiple GR. The authors concluded that for Class III recession defects, VISTA along with PRF-membrane provides better results in terms of reduction in recession depth that ranged from 50% to 80%.
The mean CAL gain after six months in the current study was 2.95 ± 0.69 mm in the test group and 2.75 ± 0.89 mm in the control group,24 which was statistically not significant. Since no histological findings are obtainable due to ethical considerations, it is only possible to hypothesize the healing type achieved in the test group (VISTA+PRFM membrane). Debnath and Chatterjee (2018),34 on the third day after depigmentation, found that all the patients presented with good healing in PRF and PRFM, in comparison to seven patients with a poor healing score of 63.6% and four patients with a good healing score. Both groups in the present research had mean PPD reductions of 0.5 mm and 0.5 mm at six months, which were statistically not significant. Other authors have discussed related findings in the literature, for example, Geeti et al.7
The mean WKG in both experiment (VISTA+PRFM membrane group: 2.04 ± 1.36mm) and control group (VISTA+PRF membrane group: 1.5 ± 1.35mm) at six months was not significant in the current study. Mohamed et al. (2020),35 in their study evaluated VISTA + PRF for root coverage and concluded similar results.
The significance of GT for describing or achieving RC and clinical outcome stability has been underlined by recent studies. GT increased statistically in the current research in both the test (0.77 ± 0.15 mm) and control (0.82 ± 0.17 mm) groups. This increase may be the result of the membrane’s spacing impact or the effect of GF on the proliferation of PDL and gingival fibroblasts. Thamaraiselvan et al. (2015)36 assessed the clinical outcome of PRF membrane in GR and observed a significant increase in GT from 0.95 ± 0.14 to 1.25 ± 0.23 mm.
The test and control site outcomes for RC, PPD, CAL, REC, WKG, and GT were similar, according to the study’s overall assessment, while PRFM showed slightly better results for RC, WKG, CAL gain, and REC reduction. This can be ascribed to the VISTA approach, a minimally invasive procedure that lowers trauma to the surgical site while simultaneously preserving the major blood arteries of the flap and the blood supply to the area, improving the nourishment of the placed membrane. This may be partially attributed to the positive group results, the small sample size, and defects (Miller’s Class I and II), which are known to exhibit predictable RC.
Treatment with VISTA+PFRM membrane is effective for multiple gingival recession defects in terms of significant coverage of gingival recession, which corresponds to 89.23%. However, VISTA+PRF corresponds to 85.06% of root coverage.
Written informed consent for publication of the patients’ details and their images was obtained from the patients.
Figshare: Underlying data for ‘Comparative evaluation of platelet rich fibrin matrix (PRFM) membrane and platelet rich fibrin (PRF) membrane using the vestibular incision subperiosteal tunnel access (VISTA) approach technique for the treatment of multiple gingival recession in humans: A double-blind, parallel-group, randomized controlled clinical trial’, https://www.doi.org/10.6084/m9.figshare.22354444. 24
Figshare: CONSORT checklist for ‘Comparative evaluation of platelet rich fibrin matrix (PRFM) membrane and platelet rich fibrin (PRF) membrane using the vestibular incision subperiosteal tunnel access (VISTA) approach technique for the treatment of multiple gingival recession in humans: A double-blind, parallel-group, randomized controlled clinical trial’, https://www.doi.org/10.6084/m9.figshare.22354435. 23
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0)
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Platelet concentrate, Implant, Peridodontal medicine, regenerative periodntal surgery, Stem cells in dental practice,
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Periodontolgy, Implantology, Mucogingival surgery, Platelet Concentrates
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Periodontics, Biomaterials, Implants, Clinical Trial, Systematic review, Rehabilitation.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 1 24 Jul 23 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)