Keywords
Chrysanthemum cinerariifolium (Trev.), Cyclin D, doxorubicin, IC50, OSCC, PI3K
This article is included in the Oncology gateway.
Chrysanthemum cinerariifolium (Trev.), Cyclin D, doxorubicin, IC50, OSCC, PI3K
Cancer is a disease characterized by uncontrolled growth and abnormal cell spread, often resulting in death (American Cancer Society, 2020). According to Global Cancer Statistics, there were approximately 18.1 million new cases of cancer and 9.6 million deaths worldwide from cancer in 2018 (Ferlay et al., 2019). According to the World Cancer Research Fund (2018), lips and oral cavities cancer are the most common type of cancer. More than 90% of cancers in the oral cavity are oral squamous cell carcinoma (OSCC) (World Cancer Research Fund, 2018).
The genetic and epigenetic mutations in oncogenes or tumor suppressor genes lead to cell cycle dysregulation, inhibition of growth suppressor factors, and resistance to apoptosis (Lopez and Lopez, 2020). Mutations in the PI3K/Akt pathway contribute to the development of OSCC (Aali et al., 2020). The PI3K pathway involves cellular cell functions, including growth, angiogenesis and proliferation (Lakshminarayana et al., 2018). Alteration of this pathway leads to cell cycle dysregulation and contributes to the development of OSCC (Li et al., 2018). The activated PI3K pathway promotes cell proliferation and inhibits apoptosis. PI3K regulates the Akt protein, which then phosphorylates p21, causing the complex interaction of cyclins and cyclin-dependent kinases (CDK’s) and resulting in cell proliferation (Kidacki et al., 2015).
Cell proliferation also involves genes that play a role in cell cycle control (Saawarn et al., 2012). The cell cycle is a process of regulation of cell proliferation with several stages, including S, G2, M and G1 phases. This process requires cyclin/CDK interactions (Jain, 2019). Cyclin D1 is a protein that is overexpressed to more than 50% in the incidence of cancer (Qie and Diehl, 2016). Overexpression of cyclin D1 causes a shortening of the G1 phase, which results in abnormal cell proliferation and additional genetic lesions (Abid and Merza, 2014). Poor prognosis of OSSC is characterised by the low cell differentiation associated with overexpression of cyclin D (Ramos-García et al., 2019).
The rapid proliferation of OSCC causes most OSCC to be diagnosed at an advanced stage. Various treatments have been used to treat OSCC, but long-term survival is less than 50% (Kumar et al., 2015). Various treatments of OSCC that are frequently used are surgery, radiotherapy or a combination of radiotherapy and surgery, and chemotherapy. One chemotherapy drug commonly used for OSCC is doxorubicin (DX). DX works by inhibiting topoisomerase II, causing the termination of the cell cycle’s G2/M phase, which can subsequently induce apoptosis. However, chemotherapy drugs sometimes cause side effects, such as drug resistance (Mansoori et al., 2017). Due to these side effects, the treatment of OSCC requires combination therapy (Dasari and Tchounwou, 2014).
Reducing the dose of DX is required to minimize the side effect of doxorubicin (Fan et al., 2017). Combination chemotherapy can be applied in OSCC treatment to increase the therapeutic effect and reduce the side effects of chemotherapy drugs such as DX. The combined use of chemotherapy drugs and herbal plant compounds such as polyphenols have been shown to have low toxicity, which is particularly advantageous as it can reduce the dose of chemotherapy drugs (Mostafa et al., 2020). C. cinerariifolium (Trev.) (CC) extract can inhibit the growth of the T47D breast cancer cell line by inhibiting the cell cycle at G0-G1 and S phases (Mutiah et al., 2020). A previous study by Listiyana et al. (2019) revealed that the best cytotoxic activity against T47D cells was observed in CC stems. Therefore, the present study aimed to investigate the effect of the combination of ethanol extract of CC stems and DX) in inhibiting PI3K and cyclin D, which are proteins that play a role in increasing cell proliferation in OSCC.
CC was obtained from Punten Village, Batu City, East Java and identified at UPT Materia Medica Batu, East Java, Indonesia (no: 074/153/102.20-A/2-22). CC were harvested and the stems cut. The stems were cleaned, dried in the sun, and sorted. The dried stem samples were ground to a powder and then added with 96% ethanol in a ratio of 1:20. The mixture was extracted using UAE (Ultrasonication Assisted Extraction) for 2 min with three replications. Next, the filtrate was evaporated using a rotary evaporator at 50°C to produce a crude extract and concentrated using an oven at 40°C.
The in vitro study was conducted at the Biomedical Central Laboratory, Universitas Brawijaya. Human oral squamous carcinoma cell lines SCC-9 were purchased from American Type Culture Collection/ATCC, Virginia (catalog number: CRL-1629). Cells were cultured in a complete medium that consisting of 1:1 mixture of Dulbecco’s modified Eagle’s medium and Ham’s F12 medium (1.2 g/L sodium bicarbonate, 2.5 mM L-glutamine, 15 mM HEPES and 0.5 mM sodium pyruvate), supplemented with 90% of 400 ng/mL hydrocortisone and 10% fetal bovine serum.
This study used five concentration series of CC stem extract (700, 350, 175, 87.5, 43.5 and 21.875 μg/mL) and DX (800, 400, 200, 100, 50 and 25 nM). SCC-9 cell line was maintained with a complete medium in 96-well plates and then incubated for 24 h. After 24 h of incubation, the medium was removed and washed using Phosphate Buffered Saline (PBS). Then, each concentration of CC and DX was added into each well with three replications and incubated for 24 h. After 24 h, the medium was removed and washed using PBS, then added with Cell Counting Kit-8 (CCK-8) Reagent (Dojindo Laboratories, Japan). The absorbance of each sample was determined at 450 nm using a microplate reader. The IC50 value of CC and DX was determined using GraphPad Prism 8 (Graphpad Software, La Jolla, Canada, USA).
A combination dose test was carried out based on the IC50 value of CC and DX with seven combinations including 7/8 IC50 CC + 1/8 IC50 DX, 6/8 IC50 CC + 2/8 IC50 DX, 5/8 IC50 CC + 3/8 IC50 DX, 4/8 IC50 CC + 4/8 IC50 DX, 3/8 IC50 CC + 5/8 IC50 DX, 2/8 IC50 CC + 6/8 IC50 DX, 1/8 IC50 CC + 7/8 IC50 DX.
SCC-9 cells were grown in 96-well plates and then incubated for 24 h. After 24 h, the medium was removed and washed using PBS. Each combination of CC and DX was added into each well with three replications and incubated for 24 h. The medium was then removed and washed using PBS. Cell Counting Kit-8 (CCK-8) Reagent (Dojindo Laboratories, Japan) was added to each well. The absorbance of each sample was determined at 450 nm using a microplate reader. CompuSyn software was used to evaluate the synergistic combination of CC and DX. The results from this software were combination index (CI) values. The interpretation of the CI value is <0.1 = Strong synergist, 0.1–0.3 = Powerful synergist, 0.3–0.7 = Synergist, 0.7–0.9 = Light synergist, 0.9–1.1 = Additives, 1.1–1.45 = Light antagonist, 1.45–3.3 = Antagonist, >3.3 = Powerful antagonist.
The combination dose for this test was based on the synergism evaluation of the CC and DX combination, which showed synergistic results. This study used six treatment groups, including: control cells without treatment, IC50 value of CC, IC50 value of DX and three combinations of CC and DX [7/8 IC50 CC + 1/8 IC50 DX (dose 1), 6/8 IC50 CC + 2/8 IC50 DX (dose 2), and 4/8 IC50 CC + 4/8 IC50 DX (dose 3)].
PI3K levels were measured using the enzyme-linked immunosorbent assay (ELISA). SCC-9 cells were grown in 24-well plates and then incubated for 24 h. After 24 h, the medium was removed and washed using PBS. Then, each treatment was treated to the cells with three replications and incubated for 24h. The medium was removed and washed using PBS. RIPA Lysis Buffer (RIPA Lysis Buffer-MB-030-0050, Rockland) was added and incubated at 2–8°C for 5 min. Cells were scraped rapidly and transferred to tubes on ice. Cells were centrifuged at 8,000 × g for 10 min at 4°C. The supernatant was then analyzed using the Human Phosphoinositide-3-kinase-interacting Protein 1, PIK3IP1 ELISA Kit (BT Lab, Cat No. E5870Hu, Shanghai Korain Biotech Co., Ltd, China) to measure PI3K levels in ng/mL.
The cyclin D expression was observed by the immunofluorescent assay. The medium was aspirated, incubated with 4% formaldehyde in PBS for 15 min at room temperature, and then rinsed three times with PBS. The first step for immunostaining was blocking the buffer for 60 min. During this step, cyclin D primary antibody (Cat No. bs-0623R, Bioss Antibodies Inc., USA) was prepared by diluting it with antibody dilution buffer, then aspirating the buffer solution. Cyclin D primary antibody was added and incubated for 24 h at 4°C. Then, the samples were rinsed three times with PBS for 5 min. Then, samples were added with fluorochrome-conjugated secondary antibody diluted in antibody dilution buffer and incubated for 1-2 h at room temperature in the dark. Samples were rinsed with PBS and then coated with Prolong Gold Antifade Reagent (#9071) or Prolong Gold Antifade Reagent with DAPI (#8961). Cyclin D expression was visualized using Olympus IX71 Fluorescent Microscope with 40x magnification, then photographed with Olympus Cell Sens software version 3.2. The pixel intensity in the cell nucleus reflecting the expression level was quantified using Image J software (Fiji) and presented as fluorescence (signal) intensity or integrated density (IntDen) value.
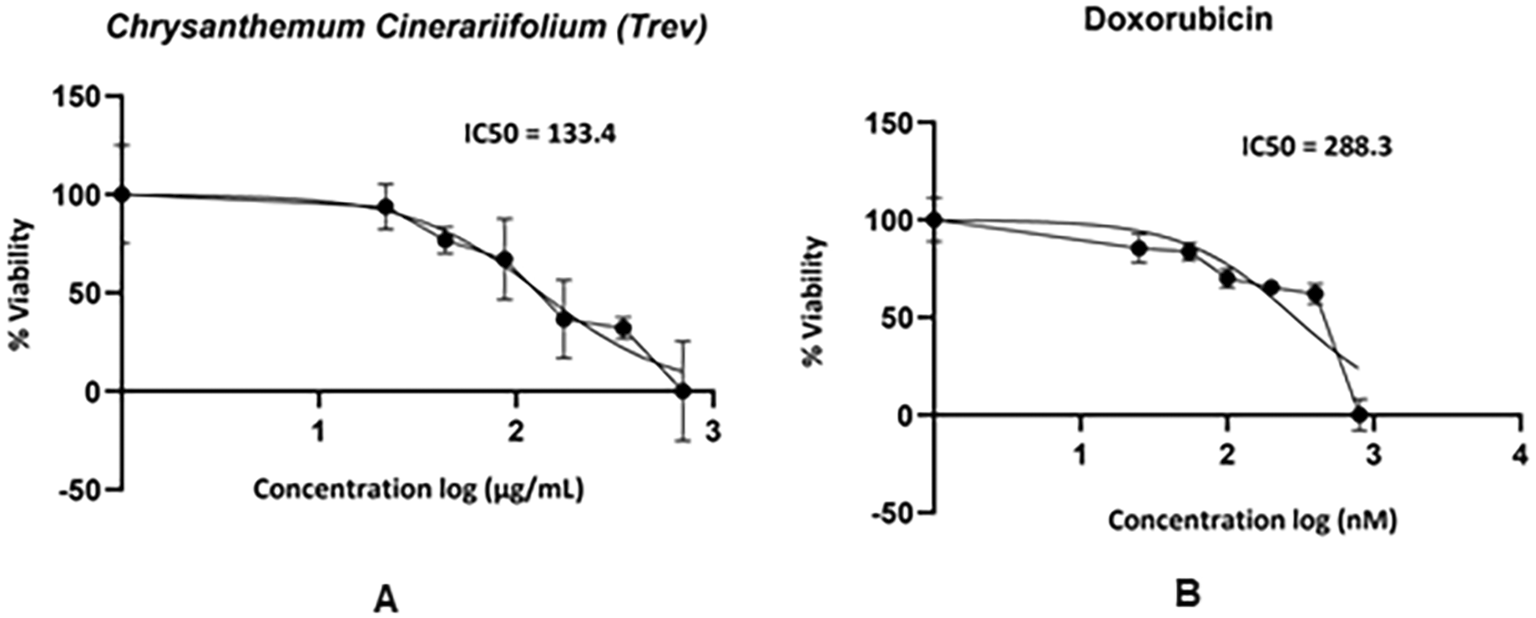
The absorbance value of the SCC-9 cell line after being treated with CC and DX can be seen in Table 1. The higher concentration exhibited a lower absorbance value. It was indicated that the higher concentration of CC and DX decreased the cell viability of SCC-9 cell lines. The results also showed that IC50 values of CC and DX were 133.4 μg/mL and 288.3 nM, respectively (Figure 1).
Synergism evaluation of the CC and DX combination, obtained from CompuSyn software, is presented in Table 2. From the seven combinations of doses analyzed, only three combinations showed synergistic effects, including 7/8 IC50 CC + 1/8 IC50 DX, 6/8 IC50 CC + 2/8 IC50 DX, and 4/8 IC50 CC + 4/8 IC50 DX with CI values respectively 0.694, 0.634 and 0.698. CompuSyn analysis also showed that the combination of CC and DX had a dose reduction index (DRI) > 1, indicating a mutual strengthening effect.
The results revealed that the SCC-9 cell line without treatment (control group) had the highest levels of PI3K (4.483 ± 0.59 ng/mL) (Figure 2). PI3K levels decreased significantly (p < 0.05) in all treatment groups. Interestingly, dose 2 showed the lowest levels of PI3K compared to all treatment groups, with PI3K levels of 0.715 ± 0.22 ng/mL. PI3K levels in the CC and DX combination group significantly decreased compared to the single CC and DX treatment group. Doses 1, 2 and 3 all were significantly different to the IC50 DX group (p = 0.001, 0.000 and 0.002, respectively), while when compared to the IC50 CC group, only doses 1 and 2 were significantly different (p = 0.001 and 0.000, respectively). From these results, CC and DX could reduce PI3k levels in single and combined treatments.
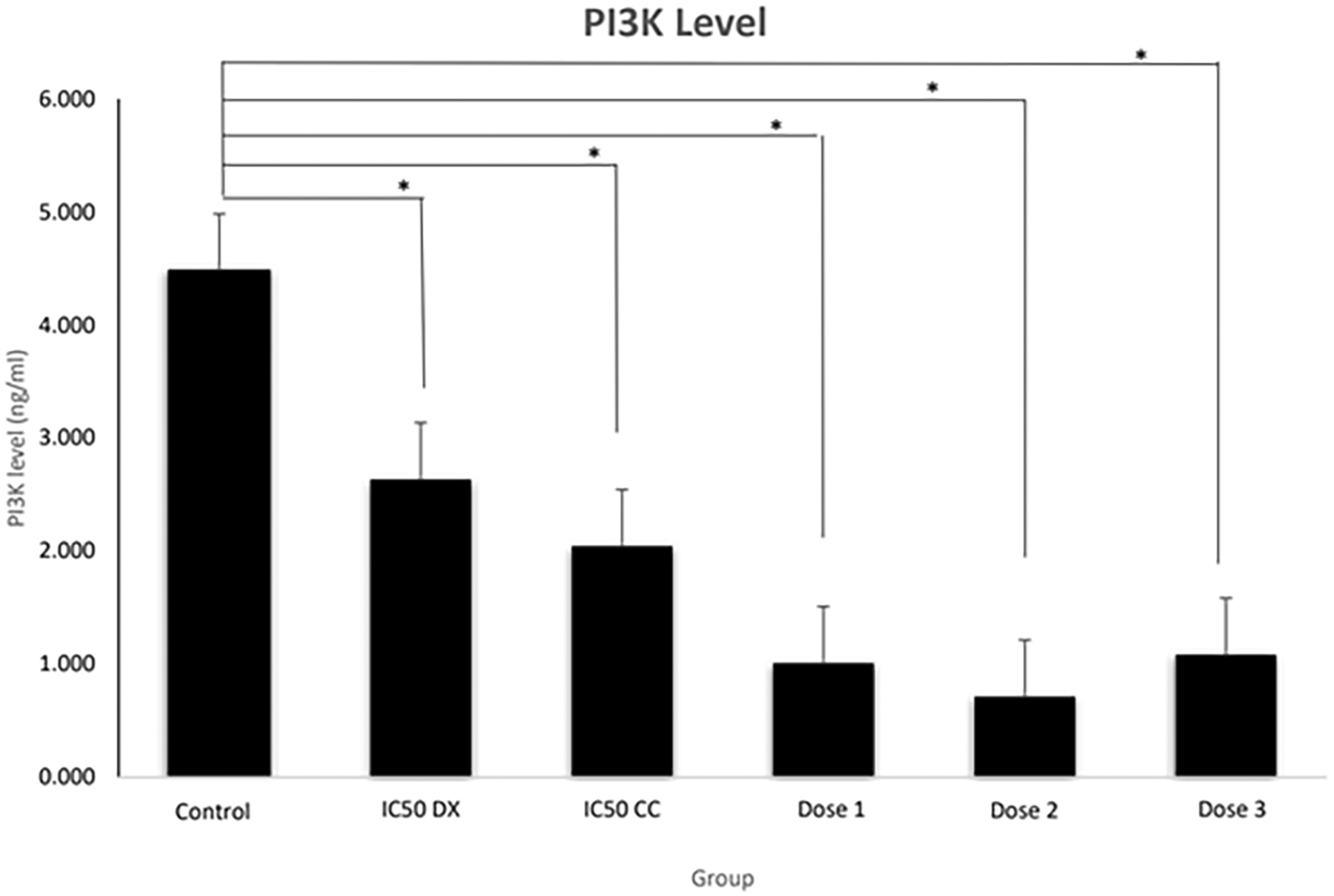
PI3K levels (in ng/ml) were measured by ELISA. Control = medium + SCC-9 (without treatment), IC50 DX = medium + SCC-9 + IC50DX. IC50 CC = medium + SCC-9 + IC50 CC, Dose 1 = medium + SCC-9 + (7/8 IC50 CC + 1/8 IC50 DX), Dose 3 = medium + SCC-9 + (6/8 IC50 CC + 2/8 IC50 DX), Dose 3 = medium + SCC-9 + (4/8 IC50 CC + 4/8 IC50 DX). Data are expressed as mean ± SD, *p < 0.05.
Cyclin D expression was observed from pixel intensity in the cell nuclei of the SCC-9 cells and presented as fluorescence (signal) intensity or integrated density (IntDen) value (Figure 3A). The results showed that CC and DX significantly decreased cyclin D expression in both single and combination treatments. The control group has the highest IntDen value, indicating that the control group expressed the highest Cyclin D. Cyclin D expression significantly declined (p < 0.05) in all treatment groups except the IC50 of DX. The lowest cyclin D expression was observed in dose 2 (Figure 3B).
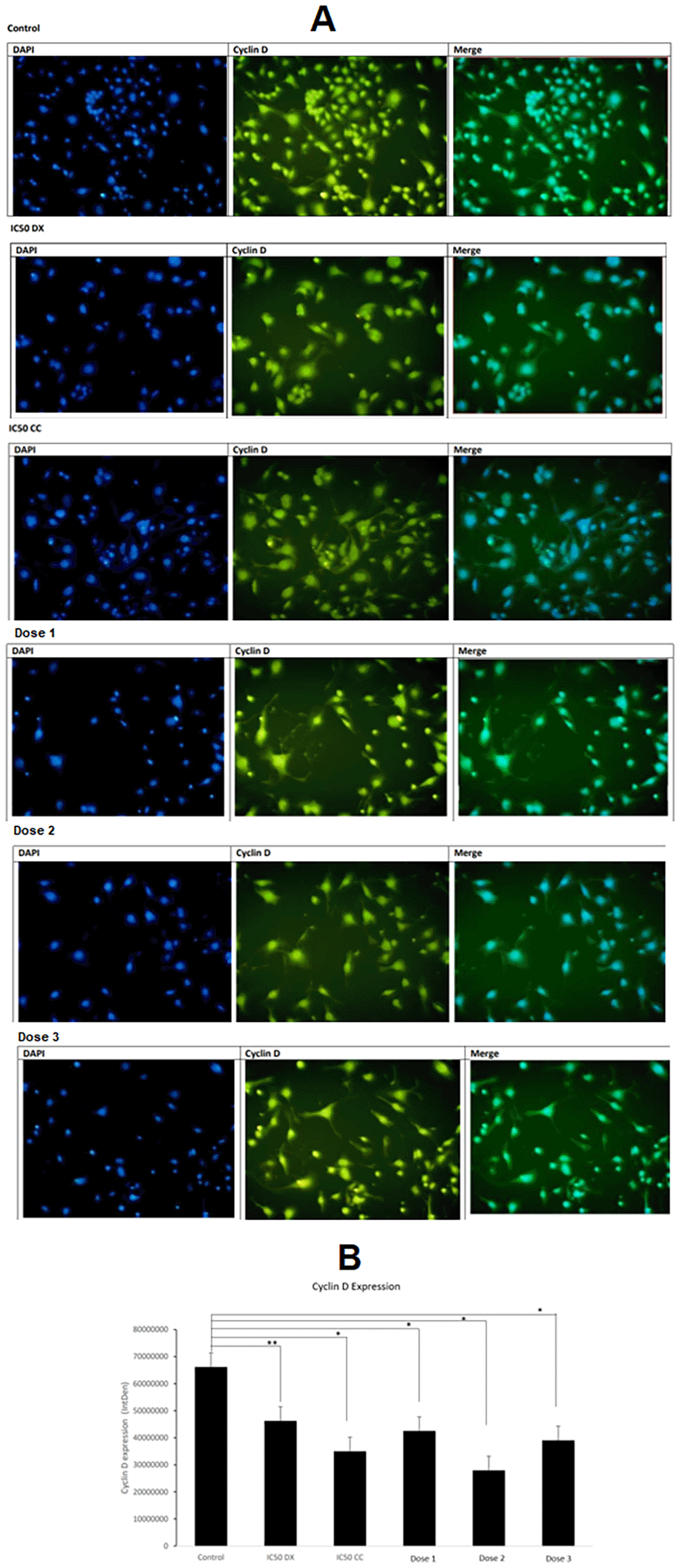
A) The fluorescence image was obtained from the fluorescent microscope. Cyclin D was expressed in the nucleus. Blue indicates cell nuclei with DAPI staining, and yellow indicates Cyclin D expression with FITC. The expression of Cyclin D is indicated by pixel intensity in the cell nucleus and fluorescence (signal) intensity or integrated density (IntDen) value. B) The graph shows the average Cyclin D expression. Control = medium + SCC-9 (without treatment), IC50 DX = medium + SCC-9 + IC50DX. IC50 CC = medium + SCC-9 + IC50 CC, Dose 1 = medium + SCC-9 + (7/8 IC50 CC + 1/8 IC50 DX), Dose 3 = medium + SCC-9 + (6/8 IC50 CC + 2/8 IC50 DX), Dose 3 = medium + SCC-9 + (4/8 IC50 CC + 4/8 IC50 DX). Data are expressed as mean±SD, *p < 0.05.
DX is a chemotherapeutic agent that is widely used in cancer treatment, but this drug produces toxicity and drug resistance. In this study, DX, in combination with herbal plants, minimized the side effects and increased the therapeutic effect. Based on Table 1, the higher concentration of DX and CC caused a decrease in the cell viability of SCC-9 cells. El-Hamid et al. (2019) showed that DX could increase caspase-3 levels, thereby increasing cell apoptosis in the OSCC cell line. Several research studies also proved that CC could be used as an anticancer agent. C. cinerariifolium extract contained flavonoids and terpenoids (Mutiah et al., 2020). Flavonoids could suppress the proliferation of OSCC by stopping the cell cycle and inducing apoptosis (Listiyana et al., 2023a). Terpenoid compounds have anticancer activity in breast cancer (Bishayee et al., 2011). Listiyana et al. (2019) revealed that CC has a cytotoxic activity on T47D breast cancer cells.
The present study showed that the IC50 value of CC stem extract on the SCC-9 cell line was 133.4 μg/mL. Costa et al. (2017) stated that the strong anticancer activity of the extract is indicated by an IC50 value <500 μg/mL and weak anticancer activity is indicated by an IC50 value >500 μg/mL. The stem extract of CC and DX has strong anticancer activity on the SCC-9 cell line due to IC50 value <500 μg/mL. The IC50 value of DX is close to Abdolmohammadi et al. (2008) research, which stated that the IC50 value of doxorubicin on T47D cells was 250 nM.
An increase in PI3K and cyclin D can cause excessive cell proliferation of OSCC. Figure 2 showed that untreated SCC-9 cell lines had the highest PI3K levels. Excessive cell proliferation in OSCC may also be due to gene mutations that encode various components of signalling pathways in proliferation, such as PI3K and cyclin D. In OSCC, mutations and amplification of the PI3K gene occur, especially at advanced stages (Kozaki et al., 2006). Ferreira et al. (2017) found that PI3K expression was seen in >90% of OSCC patients, and there was an increase in the gingival tissue, hard palate, and alveolar ridge by immunohistochemistry methods. The untreated SCC-9 cell line expresses the highest cyclin D. Excessive expression of cyclin D1 causes a shortening of the G1 phase, resulting in abnormal cell proliferation (Saawarn et al., 2012). The poor prognosis in OSSC is markedly associated with low cell differentiation and overexpression of cyclin D (Ramos-García et al., 2019).
PI3K levels decreased significantly in all treatment groups, indicating that the ethanol extract of CC stem and DX could inhibit PI3K expression. PI3K levels at the combined dose significantly decreased compared to the CC and DX single treatment group. CC significantly reduced cyclin D expression in a single treatment and combined with DX. Interestingly, the lowest PI3K and cyclin D expression levels were observed in dose 2. Furthermore, the combination in dose 2 had the best synergistic value (CI = 0.634) compared to doses 1 and 3, based on CompuSyn analysis (Table 2). Therefore, it can be concluded that dose 2 is the best combination dose for inhibiting the proliferation of the SCC-9 cells.
CC and DX have the same mechanisms of action in inhibiting cell proliferation, especially in cell cycle inhibition. CC inhibits the G0-G1 and S phases of T47D cells (Mutiah et al., 2020), whereas DX cause G2/M phase termination in T47D cells (Abdolmohammadi et al., 2008). Dose 2 was a combination dose with a lower IC50 percentage of doxorubicin than CC. This study revealed that the combination of CC and DX could inhibit OSCC proliferation through the inhibition of PI3K and cyclin D. The use of the combination of CC and DX which have a synergistic mechanism of action, is expected to reduce the dose DX needed in OSCC therapy.
C stem ethanol extract and DX inhibited SCC-9 cells with IC50 values of 133.4 μg/mL and 288.3 nM, respectively. The combination of CC and DX for dose 2 (6/8 IC50 CC + 2/8 IC50 DX) exhibited a high decrease in PI3K and cyclin D expression in SCC-9 cells. Therefore, the combination of CC and DX synergistically declined OSCC proliferation by inhibiting PI3K and cyclin D expression.
Figshare: Synergistic effect of the combination of Chrysanthemum cinerariifolium (Trev.) and doxorubicin in inhibiting PI3K and Cyclin D in oral squamous cell carcinoma in vitro study, https://doi.org/10.6084/m9.figshare.22584580.v1 (Listiyana et al., 2023b).
This project contains the following underlying data:
- Synergistic effect of the combination of Chrysanthemum cinerariifolium (Trev.) and doxorubicin in inhibiting PI3K and Cyclin D in oral squamous cell carcinoma in vitro study.xlsx
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Thank you to the Doctoral Study Program of Medical Science, Faculty of Medicine, Universitas Brawijaya, for their support. The author would like to thank the BOPTN Litabdimas LP2M UIN Maulana Malik Ibrahim Malang grant from the Ministry of Religion of the Republic of Indonesia.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: -
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Oral cancer cell biology research, chemotherapeutic therapy, targeted therapy, immunotherapy, surgical therapy, personalized precision therapy.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 25 Jul 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)