Keywords
blood gas, altitude, Andean, blood pH, blood calcium, blood glucose, hematocrit, Huamachuco
blood gas, altitude, Andean, blood pH, blood calcium, blood glucose, hematocrit, Huamachuco
This revision is to incorporate changes suggested by 3 peer reviewers. Aside from minimal edits to improve readability, this version includes:
1. Confidence intervals for arterial blood gas parameters.
2. Correlation analysis between bicarbonate blood concentration and other measured blood gas parameters.
3. An improved discussion of the interplay between pH, bicarbonate, and dissolved carbon dioxide.
4. Expression of uncertainty regarding adaptation patterns among study participants.
5. Since one of the reviewers suggested we colorize the figure, we are uploading an updated figure that presents the same data in color.
See the authors' detailed response to the review by Latika Mohan
See the authors' detailed response to the review by Niroj K. Sethy
See the authors' detailed response to the review by Erica Heinrich
Approximately 25.2 million people live at an altitude greater than 3,000 meters above sea level, in an environment marked by decreased atmospheric pressure1 which causes a series of downstream physiological effects that must be compensated to maintain homeostasis. Arterial blood gas analysis (ABGA) offers a window into these mechanisms for residents and sojourners at different altitudes.
ABGA is also an important tool for monitoring and diagnosing cardiopulmonary disfunction, which requires comparison of reference values to a result.2 However, establishing reference intervals is a significant challenge, especially for ABGA at high altitude, for three reasons: (1) few studies have examined reference intervals among healthy people because the test is frequently ordered for unwell patients,2,3 (2) obtaining arterial blood is a more difficult procedure that causes more pain to the patient than routine venous phlebotomy,4 and (3) different ethnic groups have different compensating mechanisms for life at high altitude: for instance, South American natives adapt by increasing hemoglobin, while Tibetan natives improve blood circulation.5,6
To provide additional ABGA values at high altitude and to better understand altitude adaptation mechanisms between ethnic groups, we performed arterial blood analysis on young healthy volunteers resident in Huamachuco, Peru (elevation 3,164 m) and compared them with published results at a variety of elevations and locations. We hypothesized that residents in Huamachuco will exhibit similar adaptations seen in other Andean high-altitude natives, regardless of biological sex.5,6
Although the research took place at Instituto de Educación Superior Tecnológico Público de Huamachuco (IESTPH) due to its high-altitude location, none of the authors are affiliated with IESTPH, and IESTPH does not have, to the best of our knowledge, an ethics committee capable of approving the study. Because of this, The Training, Teaching and Research Committee (ethical approval committee) of Florencia de Mora – ESSALUD Hospital I approved this study, on 01 April 2019. We sought approval from the Florencia de Mora Hospital as the first author is a doctor based at this hospital. The administration of IESTPH was informed of the project with a formal letter. The National University of Trujillo Postgraduate School also ethically approved this study; VHBZ was affiliated with that institution at the time of the study.
Volunteers were informed of the risks of arterial blood sampling and provided their written informed consent for arterial blood sampling and for the publication of the results. Participation in the study was strictly voluntary with no benefits provided, except for personal analysis results. There was one participant that was under the age of 18 (17 years old). In this case, the participant and the participant’s father signed the consent form for use with minors.
This cross-sectional convenience-sample study was not preregistered.
IESTPH was chosen for the study site because it was high-altitude location that could accommodate the equipment necessary for arterial blood analysis. The GPS coordinates and altitude of the study location were measured using a Samsung Galaxy A3 with the “Precise Altimeter” application.
A letter from the director or IESTPH was sent to students of the same institution inviting them to participate in the study. Recruitment took place between May to August 2019. On the day of the study, August 26, 2019, the fasting (>8 h) volunteers were informed of the risks of arterial blood sampling. Written informed consent for arterial blood sampling and for the publication of the results was obtained from all participants. Although the study took place in 2019, we are publishing this work now due to delays caused by the COVID-19 pandemic.
Inclusion criteria for the entire study were age between 17 and 30 years and residence in Huamachuco district for at least 5 consecutive years prior to the study and no recent travel to low-altitude locations. Exclusion criteria included self-report of strenuous exercise more than 60 minutes per day, use of tobacco, antiplatelet agents, anticoagulants, diuretics, corticosteroids, beta-blockers, or some beta-stimulants, or recent travel to low elevations. Exclusion criteria also included self-report or clinical signs upon evaluation of cardiovascular, pulmonary, or hematologic disease. Participants with self-reported diabetes and/or diabetes determined by arterial blood test (defined here as arterial blood glucose greater than 7.2 mM to account for the arteriovenous glucose difference7,8), had BMI >30, or had abnormal axillary temperature (not between 35.5 and 37.0°C9) were allowed to continue their participation, but their ABGA results were not used in the aggregated data. However, data from participants with diabetes was combined with other participant data to estimate diabetes prevalence.
Volunteers were questioned by a nurse from IESTPH to determine whether the participant met inclusion/exclusion criteria. The nurse measured the height, body mass, pulse rate, blood pressure, and axillary temperature of each participant. Volunteers were also asked to self-report their biological sex. Body mass index (BMI) was calculated by dividing body mass by the square of the height. If the volunteers wished to continue, physicians (authors VHBZ and/or LJFR) obtained one sample of arterial blood (1 mL) from the right brachial artery of the volunteers using standard sterile technique and a heparinized needle (Westmed Pulset 3cc syringe). Blood samples were stored on ice in a cooler prior to analysis.
Personnel from a commercial laboratory (BermanLab, Trujillo, Perú) analyzed blood gas parameters of the samples at IESTPH less than 15 minutes after sampling to avoid contamination during storage and transport. The blood gas analyzer (Stat Profile Prime CCS, Nova Biomedical) passed operational qualification in Huamachuco and was used to measure pH, partial pressure of oxygen (pO2) and carbon dioxide (pCO2), plasma ionized calcium (iCa2+), glucose (Glc), lactate (Lac), and hematocrit (Htc, %). Bicarbonate (HCO3−) and oxygen saturation (sO2) were calculated from the measured parameters.
The most likely experimental source of bias in this experiment is air contamination of the sample before or during analysis. Air contamination of the sample would artificially elevate pO2 without causing large changes in other parameters. This bias was addressed by careful sampling technique, rapid analysis of the sample, and removal of suspiciously high pO2 measurements by statistical methods. A second source of possible bias is due to the inclusion of unhealthy individuals in the ABGA, such as those with diabetes, obesity, or abnormal axillary temperature. If ABGA results were from a patient with diabetes, obesity, or abnormal axillary temperature or if the ABGA results were suspected of air contamination, the results were eliminated from the aggregated arterial blood analyses. However, these results were included in our analysis of the prevalence of diabetes and impaired fasting glucose.
ABGA results with pO2 outlier values were eliminated from the dataset (Tukey’s fence, k = 1.3). Shapiro-Wilk normality tests, Mann Whitney U tests, and linear regression analyses were used to analyze the dataset, compare different groups, and compare these results with previously reported data at a significance level of p < .05 using a Bonferroni correction where appropriate. Quantitative variables were reported as means and standard deviations or by quartile depending on the distribution. If the Mann Whitney U test revealed a significant difference between the sexes, data was reported for males and females separately. Confidence intervals were calculated using the t-distribution for ABGA parameters, and the Wilson score interval was calculated for diabetes prevalence values. We used Microsoft Excel for Mac version 16.73 and R version 4.3.0 to analyze the data. Since all participants completed a single blood test on the same day, there was no missing data.
Huamachuco district has an area of 424 km2, and a 2017 population of 66902 including 16456 17 to 30 year old inhabitants.10 A topographic analysis of the district indicates that it varies from 2,200 to 4,600 m, but most of the population lives below 3,400 m. The largest city, also named Huamachuco, is located at about 3,200 m. IESTPH, where the samples were taken, is located at 7.815833 S, 78.03917 W and had a GPS altitude of 3,164 m.
The study size was based on available IESTPH students that volunteered and met acceptance criteria. A total of 56 participants (21 male and 35 female) volunteered for the study. Ten participants (4 male and 6 female) were excluded from ABGA after blood sampling because they had diabetes, unusual body temperature, obesity, or outlier arterial pO2. Some participants excluded at the interview, axillar temperature, or by excessive BMI freely decided to have their blood sampled and analyzed to know their results.
The 46 participants included in our ABGA analysis were between 17 and 28 years old, with a median age of 20 years. Ages were skewed toward lower values: the Shapiro-Wilk tests showed a significant departure from normality for age, W(46) = .885, p < .001. Body height was greater in males than females (Mann Whitney U test, p < 0.001): females had a median height of 1.51 m, and males had a median height of 1.60 m. Statistically significant differences between the sexes in other anthropomorphic parameters were not found. Body mass index had a normal distribution (Shapiro-Wilk, W(46) = .97, p = .31) and had an average of 23.4 and a standard deviation of 2.8. No participant had a BMI > 30, high blood pressure or diabetes. The median blood pressure was 100/60 mmHg, with a variation of less than 20 mmHg. The median pulse rate was 67 with a range of 55 to 89 min-1. Most of the participants had an axillar temperature of 36.6°C, but the range was 36.0 to 37.0°C.
The summary values of the resulting analyses are recorded in Table 1.
Values are expressed as minimum, first quartile, median, third quartile, maximum and 95% confidence interval (95% CI) as calculated using the t-distribution, as some results showed a statistically significant departure from normality. Males and females are separated where a statistically significant difference was found.
There were significant differences for Htc % (Mann Whitney U test, p < 0.001) and HCO3− (Mann Whitney U test, p = 0.0019). Other ABGA parameters failed to reach statistical significance between sexes with Bonferroni correction.
We compared 21 combinations between pH, pO2, pCO2, iCa2+, Glc, Lac, Htc, and HCO3− using regression analysis and Bonferroni correction. Two comparisons achieved statistical significance: (1) glucose (mM) predicted lactate (mM) (R2 = .24, F(1,44) = 13.96, p < .001. β = .42, p < .001, α = -0.34, p = .57) and (2) pCO2 (kPa) predicted HCO3− (mM) (R2 = .71, F(1,44) = 107.16, p < .001. β = .378, p < .001, α = 4.58, p = .007). pH and pCO2 and pH and glucose had inverse relationships that were close to reaching statistical significance.
Living in the reduced atmospheric pressure of a high-altitude environment causes a series of physiological adaptations to maintain adequate blood oxygenation. These adaptations have been shown to vary between ethnic groups5,6 making it necessary to sample different healthy populations to establish reference intervals. To our knowledge, this is the first systematic ABGA of healthy northern Peruvians living at high altitudes. Furthermore, this study includes analysis of arterial iCa2+, Glc, and Lac, which are also not frequently measured among healthy high-altitude residents.
A growing body of ABGAs of healthy residents of the Americas at different elevations makes a systematic comparison of pO2 and altitude possible. Figure 1A visualizes the published pO2 for healthy residents of the Americas living at different altitudes. The results collected over the past half century11–30 reveal that the inverse relationship between meters above sea level and pO2 (kPa) can be modeled with a linear equation up to about 4,500 m (R2 = 0.87, F(1,40) = 273.13, p < .001, β = -0.00133, p < .001, α = 12.5, p < .001). Inspection of Figure 1A reveals that there is a lack in published ABGA results between 100 to 1000 m. It is likely that data in this range will be useful in confirming or rejecting a linear model for pO2 and altitude. The median value of pO2 determined here is 1 kPa (7.50 mmHg) higher than the predicted value of the regression, although the data range overlaps the trendline and the point is within the spread of the data.
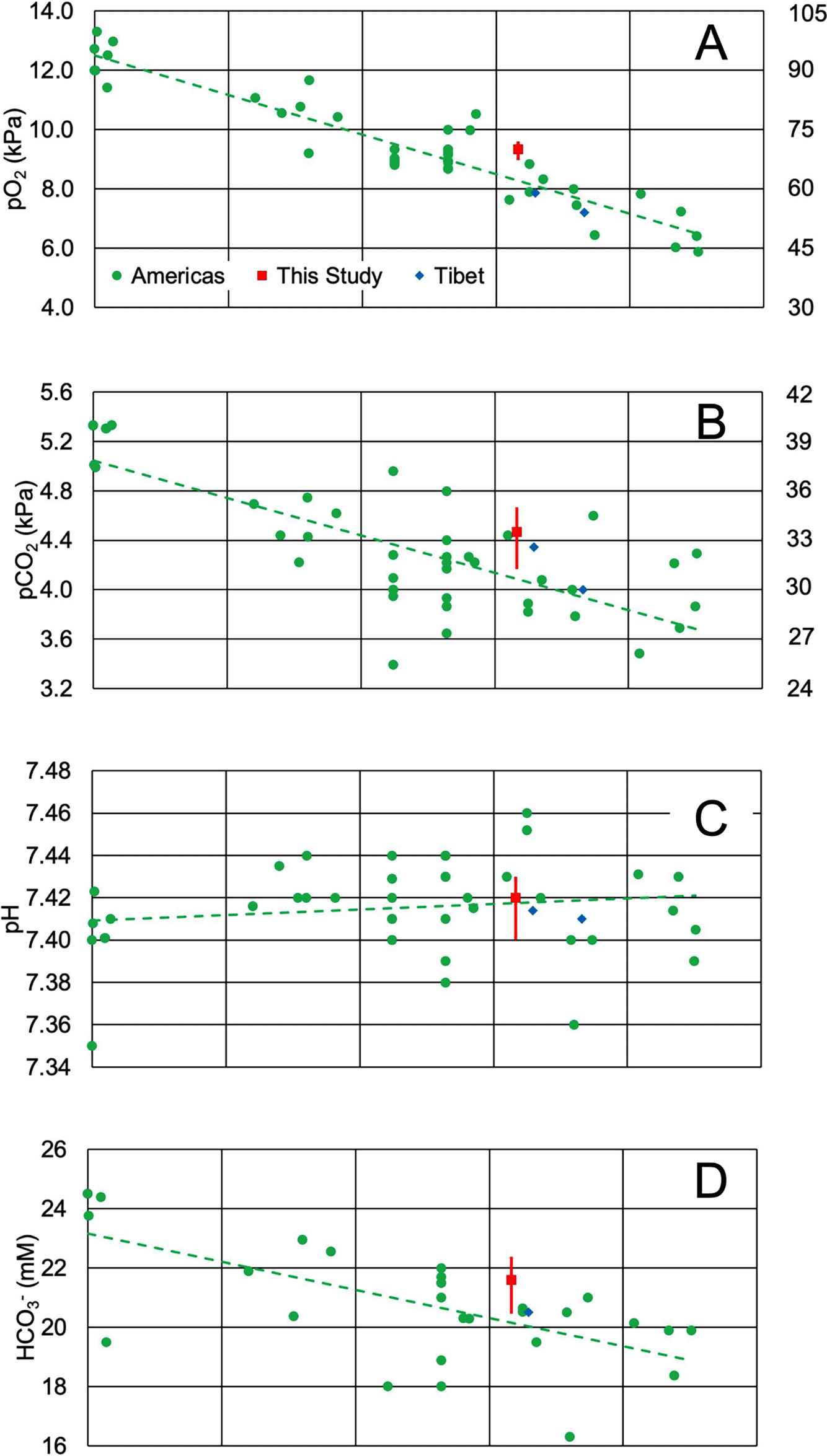
Each panel represents a different blood parameter and its relationship to altitude (meters above sea level): (A) pO2 (kPa), (B) pCO2 (kPa), (C) pH, and (D) HCO3- (mM). Green circle data points are averages or medians from previously published results in the Americas; where possible, age range was considered to coincide with the age range in this study.11–30 The red square data point is the median determined here, with whiskers representing the interquartile range. Data from native Tibetans are represented as blue diamonds.31,32 Previously published results from the Americas (green circle data points) were used to calculate the regression lines in the figure and regression statistics in the text.
One possible explanation is that arterial blood oxygen decreases with age and the ages of the participants in this study were skewed toward young adults.2,11 When possible, only subjects that resembled the ages of the participants were included in the regression, but selection criteria and age range reporting differed between studies; older participants in the published studies included in the regression would systematically lower the regression line (Figure 1A). A second possibility was hyperventilation during sample acquisition, but breathing rate among participants was not monitored. Changes in equipment use over the years may also have added systematic errors to the data.
Andeans have been shown to have elevated oxygen saturation compared to Tibetan highlanders.5,6 In fact, the results of this study confirm higher pO2 for Andean residents, as two studies that measured similar parameters in Tibet31,32 fell slightly below the regression line (Figure 1A, diamonds). Furthermore, the results from Tibet were approximately 2 kPa (15 mmHg) below our result. Therefore, these results suggest support for the hypothesis that Andeans have higher oxygen saturation than Tibetans at similar altitudes, although more experimental data from random population samples at different altitudes in Tibet and Peru more would help confirm this observation.
Blood pH is tightly regulated through several buffering systems, the most important of which is the bicarbonate-carbonic acid system, which is derived from dissolved carbon dioxide. Like pO2, pCO2 (kPa) has an inverse relationship with altitude in m (R2 = .57, F(1,40) = 53.27, p < .001. β = -.0003, p < .001, α = 5.05, p < .001, Figure 1B), although the slope of the correlation is less steep and altitude explains less of the variation in pCO2 than it does with pO2 (Compare Figure 1A and B). This decrease in pCO2 as altitude increases is usually explained by hyperventilation to compensate for decreased oxygen partial pressure at high altitude. We find that our results largely follow this trend. Unlike some other studies,2,12,33 a statistically significant difference between sexes for pCO2 was not observed.
Although altitude and pCO2 were moderately correlated, no such relationship is present for pH, which remains almost constant regardless of altitude in m (R2 = .025, F(1,40) = 1.01, p = .320, β = .00000264, p = .320, α = 7.41, p < .001, Figure 1C). The results determined here nearly exactly overlap the regression line. Given that dissolved CO2 has an inverse relationship with pH, the pH should also increase with altitude, but this is compensated by renal excretion of nonvolatile bases.34,35 These changes also require modification of the Siggard-Anderson acid-base chart to account for altitude. Our data support moving the normal target of a plot of pH vs pCO2 downward, but the variance of our results suggests a widening of the vertical size of the target.34
This increased bicarbonate excretion is also reflected in an inverse relationship between HCO3− (mM) and altitude in m (Figure 1D; R2 = 0.43, F (1,27) = 20.13, p < .001, β = -.00095, p < .001, α = 23.16, p < .001). Since HCO3− is derived from pH and pCO2, it follows that if pH is relatively stable, HCO3− would also decrease if pCO2 also decreases. We found no correlation between blood pH and actual HCO3− or pH and pCO2. This is likely because maintenance of pH within a narrow range result ultimately in a lower concentration of oxygen, carbon dioxide and bicarbonate, creating a new equilibrium at a lower buffer concentration but the same pH. A study at sea level did find weak correlations between HCO3− , pH and pCO2, with higher pH having lower pCO2.2 It is likely that this study was able to find these correlations because of its larger size, but the difference is over 0.1 pH unit. The HCO3− concentration determined here is higher than the regression line, but the spread of the data overlaps the trendline, and males had statistically significantly higher HCO3− than females.
Among healthy individuals, calcium is maintained within a narrow range. Departures from this range lead to several severe symptoms, including tetany, vomiting, coma, and neurological disturbances.36 The reference interval for iCa2+ is between 1.05 and 1.30 mM, which is slightly higher than the range (0.95 to 1.13 mM) found here.36 Similar studies in the Andes also reported average results below the lower limit of the reference range.13,14 Since iCa2+ is dependent on several factors, including albumin content, vitamin D, and blood pH, it is not yet possible to determine whether lower iCa2+ is a result of altitude or another confounding factor. Correcting iCa2+ for pH did not change values significantly: the largest adjustment was not greater than 0.02 mM.
Lac concentration is a measure of anaerobic respiration and tissue hypoxia and has a reference range of between 0.3-0.8 mM, but this has been called into question.36,37 The range of values determined here is both wider and has a higher median than the reference range. A study at higher altitude also reported higher Lac.14 These results may reflect a variety of activity levels among study participants, especially considering that the test site was in mountainous terrain. Alternatively, reduced oxygen at high altitude can promote anaerobic metabolism, but high-altitude residents have been shown to have Lac levels similar to those of lowlanders after exercise.38 We found a positive correlation with Lac and Glc concentration, which may be because of their relationship in the Cori cycle, but this hypothesis requires further testing.
Due to the selection criteria, only subjects with normoglycemia and reasonable ABGA results were included in the data analysis. However, it is unlikely that fasting arterial blood Glc would be impacted by outlier ABGA results, which provides an opportunity to measure diabetes and impaired fasting blood glucose (IFBG) prevalence in the entire study population of 56 people. Diabetes and IFBG are usually defined in terms of glucose concentration in venous blood plasma; arterial blood was measured here. For fasting individuals, the arteriovenous glucose difference is on the order of 0.2 mM, which revises the cutoff to ≥ 7.2 mM for diabetes and between 5.8 and 7.2 mM for IFBG.7,8 Given these criteria, the prevalence was 2/56 (3.6%, 95% CI [0.98%, 12%]) for diabetes, and 10/56 (17.9%, 95% CI [10%, 30%]) for IFBG. Aggregated diabetes prevalence in Peru is approximately 6% and IFBG prevalence is approximately 15-20%. However, highland prevalence is lower for both diabetes and IFBG, where it is estimated to be approximately 4.5% and 17.4%, respectively.39 These results are almost exactly what we observe as well, further supporting that living at high altitude correlates with lower diabetes prevalence.
Htc. Htc, a measure of the red blood cell content of blood, has been shown to vary between biological males and females, as well as between different high-altitude resident populations. Andeans and acclimatized Europeans tend to respond to high altitude by increasing hemoglobin and Htc% in a dose-response manner.5,6,40 The hematocrit determined here (Table 1) falls on the high end of the sea-level reference interval (42-52% and 37-47% for males and females respectively), suggesting similar adaptations seen in other Andean high-altitude natives.5,6,36
We observe that in resident Andean highlanders pO2 and pCO2 decrease, Htc% increases, and pH and iCa2+ do not change very much with altitude. Thus, Northern Peruvians seem to have similar adaptations seen in other Andean high-altitude natives. However, these results show higher than expected pO2 and pCO2 for the altitude of the analysis. This is likely due to the young age range of the participants and the small study sample.
This study used a convenience sample cross section of young and healthy Andeans, which means it is not likely applicable to older people or those with chronic conditions, those with different altitude adaption profiles, or sojourners to the high-altitude environment. Additional sample taking of a more demographically balanced cross section and controls preventing air exposure of the sample are necessary to confirm these results and make the generalizable to the population of Huamachuco. Furthermore, comparing the results as a function of barometric pressure rather than altitude can eliminate some variability.
We hope that these results can be helpful in further establishing altitude- and ethnicity-dependent data-supported values for ABGA as well as Glc, Lac, and iCa2+, which will aid in the diagnosis and treatment of cardiopulmonary disease at high altitude.
Figshare: Dataset for “Arterial blood gas analysis of healthy residents in Huamachuco, Peru (3,164 m)”. https://doi.org/10.6084/m9.figshare.22720630. 41
This project contains the following underlying data:
• Huamachuco-calculations-110623.xlsx (Dataset for the article “Arterial blood gas analysis of healthy residents in Huamachuco, Peru (3,164 m)”. All raw data collected, most calculations, and results reported in the figure and table are included in this spreadsheet.)
Figshare: STROBE Checklist for “Arterial blood gas analysis of healthy residents in Huamachuco, Peru (3,164 m)”. https://doi.org/10.6084/m9.figshare.22720723. 42
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The authors wish to thank the direction of Instituto de Educación Superior Tecnológico Público de Huamachuco. La Universidad Privada Antenor Orrego is providing publication funds.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: High-altitude medicine, pulmonary physiology, control of breathing
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Suresh K, Chandrashekara S: Sample size estimation and power analysis for clinical research studies.J Hum Reprod Sci. 2012; 5 (1): 7-13 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Hypoxia, High altitude biology, Human response to hypoxia
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: High-altitude medicine, pulmonary physiology, control of breathing
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: High altitude physiology, Neurophysiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 26 Sep 23 |
read | ||
|
Version 1 26 Jul 23 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)