Keywords
Apoptotic, antioxidant, cisplatin, HepG2, sitagliptin, HCC, control group, level
This article is included in the Oncology gateway.
Apoptotic, antioxidant, cisplatin, HepG2, sitagliptin, HCC, control group, level
Hepatocellular carcinoma (HCC) is the most common tumor type of the liver and is highly aggressive. Approximately 90% of people with a cirrhosis diagnosis also have HCC. Causes of HCC have been identified, including viral infections, alcohol consumption, usage of aflatoxins, and genetics (Ioannou et al., 2007).
About 75% of all cases of liver cancer occur due to HCC, making it the seventh most prevalent cancer and the second largest cause of mortality (Bray et al., 2018). There were predicted to be 713 new cases and 686 fatalities in Iraq in 2020 (IARC, 2020). According to GLOBOCAN 2020, liver cancer rates have recently increased sharply in many nations. Included in this set of nations are the countries of Iraq, Iran, Nepal, Afghanistan, Azerbaijan, and Qatar (Zhang et al., 2022). The world’s highest rates are found in Asia and Africa (McGlynn et al., 2021).
Treatment for HCC typically consists of one or more of the following: surgical procedures, ablation, trans-arterial chemoembolization, and chemotherapies like cisplatin.
Cisplatin is activated upon entry into a cell. Cisplatin’s cytoplasmic chloride atoms are exchanged for water molecules. This hydrolyzed molecule is a highly powerful electrophile that may react with a wide variety of nucleophiles found in nucleic acids. Cisplatin inhibits tumor cell proliferation and induces apoptotic cell death by binding to the N7 on purine residues, thereby degrading DNA (Fraval et al., 1978).
In biology systems, anticancer drugs cause oxidative stress, which causes lipid peroxidation and the production of many electrophilic aldehydes. These oxidative stress byproducts may impede the effectiveness of anticancer treatments by slowing the progression of the cancer cells’ cell cycle (Conklin, 2004). Therefore, combining cisplatin with other drugs with antioxidant and apoptotic characteristics reduces concentration to avoid drug resistance and toxicity.
Recently, significant anticancer effects of dipeptidyl peptidase-4 (DPP-4) inhibitors in cancer cells have been observed. In particular, the anti-diabetic medication sitagliptin was approved by the USA Federal Drug Agency (FDA) in 2006 as an inhibitor of DPP-4 (Aschner et al., 2006). Glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are incretin hormones deactivated by the enzyme DPP-4. The hormone incretin increases insulin secretion from beta cells while decreasing glucagon secretion from beta cells (Scott, 2017). Since sitagliptin blocks DPP-4 activity, it also extends the effects of incretin hormones. Consequently, significant postprandial augmentation of insulin secretion and reduction of glucagon secretion is observed (Amritha et al., 2015).
Sitagliptin has been proven to improve the prognosis of several different types of cancer, including breast (Tseng, 2017), kidney (Kabel et al., 2018), and colon (Amritha et al., 2015), and modify the oxidative stress equilibrium during chemotherapy (Salama et al., 2022). Based on the above, our study attempted to assess the antioxidant and apoptotic activity of the sitagliptin in HepG2 liver cancer cell-line.
The cells were cultured into a 96-well plate with Roswell Park-Memorial Institute-1640 (RPMI-1640) liquid medium that supplied by Gibco/UK and then incubated for 24 hours to promote the formation of a cellular monolayer (80% growth phase). Later, the old medium was discarded, and 200 μL of medium containing the test medicines was added (Meerloo et al., 2011). Five primary groups were utilized including the control group, which were: Control group (untreated HepG2 cells), cisplatin-treated HepG2 group, sitagliptin-treated HepG2 group, cisplatin plus sitagliptin-treated group which received a combination of different concentrations of cisplatin plus fixed concentration of sitagliptin (250 μg/mL), and the last group treated with a combination of different concentrations of sitagliptin plus fixed concentration of cisplatin (25 μg/mL). The plates were then kept in an incubator for 48 hours. The supernatant from each well was collected at the end of the exposure time and placed in a 1.5 mL sterile Eppendorf tube, and subsequently frozen at -20°C until measured by specific MDA and BCL-2 ELISA assay kits.
BCL-2 concentration measurement
Human BCL-2 ELISA kit purchased from (BT LAB, Shanghai, China) was evaluated. The assay procedure was performed according to BT LAB protocol. Briefly, the standard stock solution was diluted by 1:2 to 1:16. A 50 μL of the standard was added to the standard well, 40 μL of the sample to the sample wells, and 10 μL of anti-Bcl2 antibody and 50 μL of streptavidin-HRP to the sample and standard wells. A sealant was used to close off the plate, allowing it to sit at 37°C for an hour. Repeatedly, the plate was washed in the wash buffer. Each wash should last between 30 seconds and 1 minute, and use 300 μm of wash buffer. 50 μL of substrate solution A, then repeated with substrate solution B was added to each well. After adding the substrate solutions, a color appears directly proportional to the concentration of Human BCl-2. Absorbance was taken at 450 nm when the process was stopped with an acidic stop solution. Then, a microplate reader that had a wavelength of 450 nm was used to measure each well’s absorbance value.
MDA concentration measurement
Human MDA ELISA kit purchased from (BT LAB, Shanghai, China) was evaluated. The assay procedure was performed according to (BT LAB protocol). Briefly, the standard stock solution was diluted by 1:2 to 1:16. A 50 μL of the standard was added to the standard well, 40 μL of the sample to the sample wells, and 10 μL of anti-Bcl2 antibody and 50 μL of streptavidin-HRP to the sample and standard wells. A sealant was used to close off the plate, allowing it to sit at 37°C for an hour. Repeatedly, the plate was washed in the wash buffer. Each wash should last between 30 seconds and 1 minute, and use 300 μm of wash buffer. 50 μL of substrate solution A, then repeated with substrate solution B was added to each well. After adding the substrate solution, a color appears directly proportional to the concentration of human MDA. Absorbance was taken at 450 nm when the process was stopped with an acidic stop solution. Then, a microplate reader with a wavelength of 450 nm was used to measure each well’s absorbance value.
The effect of cisplatin on BCL-2 level
After incubating the HepG2 cells with cisplatin alone, a significant reduction in BCL-2 level (p≤0.001) was seen after exposure to all utilized concentrations of cisplatin in comparison with the control group, as shown in Figure 1.
The effect of sitagliptin on BCL-2 level
After incubating HepG2 cells with sitagliptin, a significant decrement was noticed in BCL-2 level at all sitagliptin concentrations applied in comparison with the control group, as illustrated in Figure 2. Statistical calculations revealed that at a sitagliptin concentration of 31.25 μg/mL, BCL-2 level was decreased significantly at p≤0.01, while at higher concentrations, a reduction in BCL-2 level was highly significant (p≤0.001).
Comparison between the effect of cisplatin alone versus combination of cisplatin plus sitagliptin on BCL-2 level
Comparing the apoptotic effect of cisplatin alone versus combination of cisplatin plus sitagliptin showed significantly lower BCL-2 level (p≤0.001) after exposure to combination than that obtained with cisplatin alone, as displayed in Figure 3.
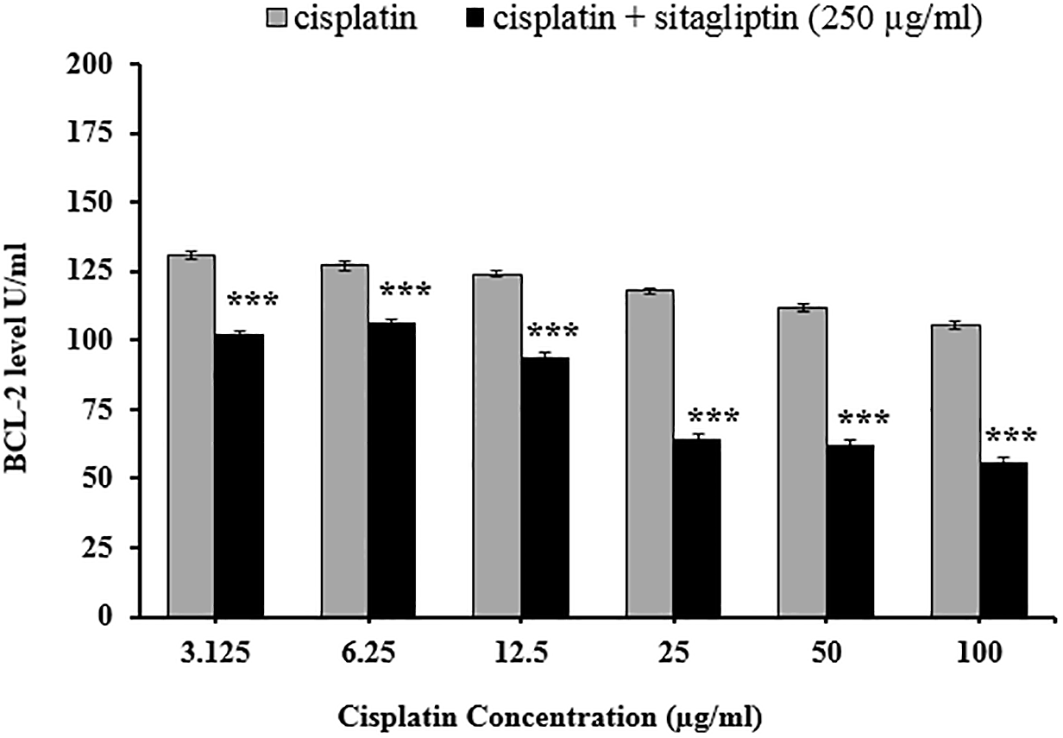
*** p≤0.001.
Comparison between the effect of sitagliptin alone versus combination of sitagliptin plus cisplatin on BCL-2 level
As described in Figure 4, evaluating the apoptotic effect of sitagliptin alone versus combination of sitagliptin plus cisplatin demonstrated a highly significant decrement in BCL-2 level (p≤0.001) after exposure to combination versus sitagliptin alone.
The effect of cisplatin on MDA level
The results showed a significant increase in MDA level at p≤0.001 after exposure to all used concentrations of cisplatin in comparison with the control group, as shown in Figure 5.
The effect of sitagliptin on MDA level
The results showed a significant decrease in MDA level at p≤0.05 and p≤0.01 for the concentrations 500 μg/mL and 1000 μg/mL, respectively. However, the other concentrations of sitagliptin (31.25 μg/mL, 62.5 μg/mL, 125 μg/mL and 250 μg/mL) did not show significant changes in MDA level in comparison with the control group, as shown in Figure 6.
Comparison between the effect of cisplatin alone versus combination of cisplatin plus sitagliptin on MDA level
Comparing the MDA level after exposing the HepG2 cells for cisplatin alone versus combination of cisplatin plus sitagliptin showed significantly lower MDA level after exposure to combination than that obtained with cisplatin alone, as demonstrated in Figure 7.
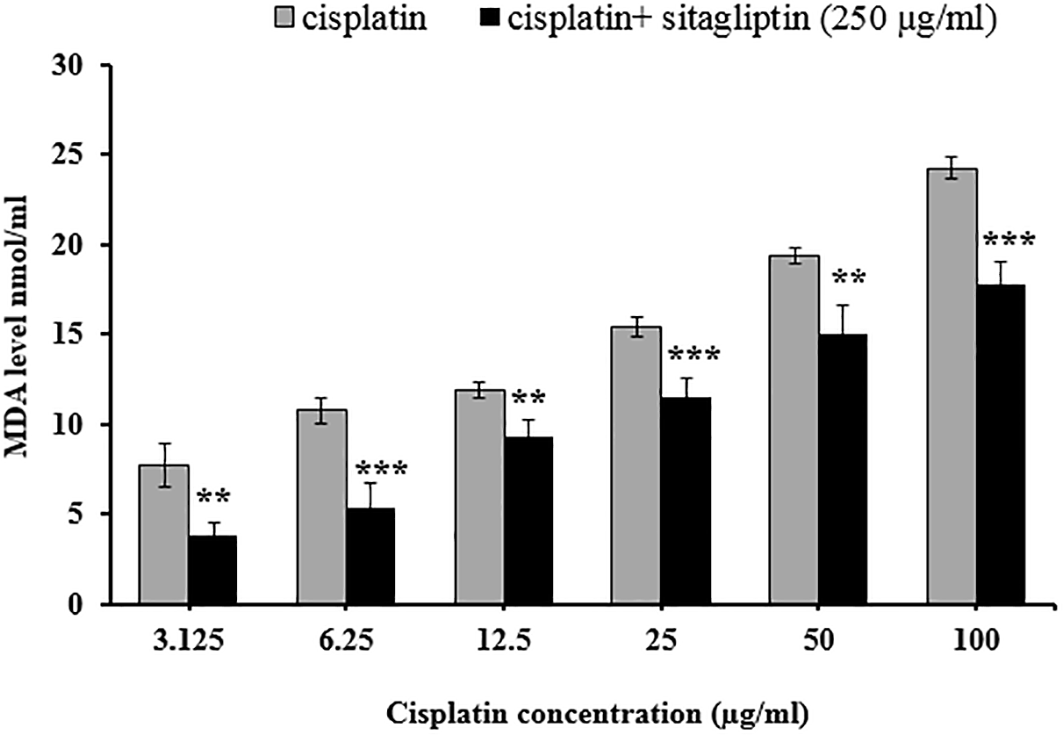
** p≤0.01, *** p≤0.001.
Comparison between the effect of sitagliptin alone versus combination of sitagliptin plus cisplatin on MDA level
Finally, exposing HepG2 cells to sitagliptin alone or combination of sitagliptin plus cisplatin further confirmed the antioxidant capability of sitagliptin. Indeed, as the concentration of sitagliptin increase, lower MDA levels were detected in both groups. In addition, significantly higher MDA levels (p≤0.001) were seen in cell treated with combination of sitagliptin plus cisplatin as expected, as shown in Figure 8.
It is believed that inducing apoptosis is an effective method for treating cancer, given that the number of cancer cells depends on their development and death rate. On the other hand, cancer cells have developed various methods to resist death caused by apoptosis (Nachmias et al., 2004). One of these methods is the excessive production of anti-apoptotic proteins belonging to the BCl-2 family, leading to death resistance and reducing therapeutic efficacy (Letai, 2005). However, a potent cytotoxic response can be achieved by downregulating these proteins. The apoptotic pathway that begins inside mitochondria is strongly controlled by proteins of the BCl-2 family (Gross et al., 1999; Cheng et al., 2001).
Our study indicated that the apoptotic effect of cisplatin on the HepG2 cell-line is explained by decreased BCl-2 level. Consistently with these findings, Xu et al. (2007) found that cisplatin and/or phenol decreased BCL-2 expression and increased Bax expression in human hepatoma cell lines, especially in the combination group. Moreover, compared to the control group, our finding showed a significant decrease in BCL-2 level in the HepG2-treated sitagliptin group, suggesting the apoptotic effect of sitagliptin on these corresponding cancer cells. Similarly, other study showed that sitagliptin suppressed cell growth and caused apoptosis in immortalized and primary glioblastoma cells (You et al., 2023). Based on the findings of Salama et al. (2022) in the Ehrlich solid carcinoma experimental model, sitagliptin showed anti-tumor activity through multiple mechanisms involving inhibition of cell proliferation and induction of tumor apoptosis. Interestingly, sitagliptin reduced apoptosis in normal cells as previously reported (Marques et al., 2014; Abo-Haded et al., 2017). Furthermore, the combination of sitagliptin and cisplatin demonstrated a synergistic apoptotic effect on cancer cells by significantly lowering BCL-2 levels. Yet, no previous data were reported concerning the effect of sitagliptin plus cisplatin combination on BCL-2 level.
Overproduction of reactive oxygen species (ROS) leads to oxidative stress, which triggers mitochondrial malfunction and death (Liu et al., 2022). Tumor survival, growth, spread, and angiogenesis have all been linked to oxidative stress (Reuter et al., 2010). Multiple studies have indicated an increase in mitochondrial ROS as the mechanism of cisplatin toxicity (Martins et al., 2008). MDA, a marker of lipid peroxidation, was evaluated to determine the level of oxidative stress. The results of our study showed a significant increase in the MDA level of HepG2 cells when treated with cisplatin alone, which highlights the oxidative stress inducing capability of cisplatin. Studies on cisplatin cytotoxicity have indicated lowered hepatic GSH and elevated hepatic MDA, supporting the hypothesis that cisplatin cytotoxicity results from elevated oxidative stress in hepatic tissue (Aboraya et al., 2022). Moreover, liver tissue from cisplatin-injected animals showed increased oxidative stress (increased MDA and decreased GSH) (Taghizadeh et al., 2021). A recent study used A549 and DU145 cell lines found that exposure to cisplatin significantly increased intracellular ROS levels, and it was suggested that mitochondria are the origin of the ROS response induced by cisplatin in cancer cells (Marullo et al., 2013). On the other hand, the MDA level in the normal renal cells of mice treated with cisplatin elevated by an average of 1.5-fold, which triggered renal cell death due to ROS-dependent kidney damage (Soni et al., 2018).
Combining cisplatin with other compounds containing antioxidant characteristics has been considered to reduce its toxicity. For example, the ability of sitagliptin to increase the activity of nuclear factor erythroid 2-related factor 2 (Nrf2), an inducer of various antioxidant enzymes, may be responsible for the antioxidant action (Li et al., 2019). Our result indicated that only higher concentrations (1000 μg/mL and 500 μg/mL) of sitagliptin showed a significant decrease in the MDA level of the HepG2 cell line in comparison to the control group. These findings are supported by several other studies which demonstrated the possible antioxidant impact of sitagliptin in various tissues and conditions, such as diabetic nephropathy (Marques et al., 2019) and Alzheimer’s disease (Li et al., 2019). In addition, the effect of a combination of sitagliptin plus cisplatin on MDA levels further demonstrated the oxidative stress-reducing ability of sitagliptin. This antioxidant effect clearly improved as the concentration of sitagliptin increased because the concentration of cisplatin was constant.
The antioxidant protective effect of sitagliptin may reduce cisplatin cytotoxicity in addition to potentiating its apoptotic activity. Importantly, the antioxidant beneficial effect of sitagliptin in combination with chemotherapeutic agents was also mentioned by Salama et al. (2022), who demonstrated that when sitagliptin combined with doxorubicin, the level of MDA was significantly decreased compared to both the control and doxorubicin-treated groups, suggesting partial protection from the oxidative stress against normal cells.
Sitagliptin could have antioxidant effect in higher concentrations. Moreover, sitagliptin revealed apoptotic activity against HepG2 cell-line based on BCL-2 measurement. The apoptotic effect of sitagliptin clearly enhanced the apoptotic activity of cisplatin, which may help in reducing the dose and subsequently the side effects of cisplatin in cancer treatment protocols.
Figshare: ELISA 2.xlsx, https://doi.org/10.6084/m9.figshare.23616819.v1 (Alameen et al., 2023).
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Su K, Wang F, Li X, Chi H, et al.: Effect of external beam radiation therapy versus transcatheter arterial chemoembolization for non-diffuse hepatocellular carcinoma (≥ 5 cm): a multicenter experience over a ten-year period.Front Immunol. 2023; 14: 1265959 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical and basic research on hepatocellular carcinoma
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 10 Aug 23 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)