Keywords
Monkeypox; Oral lesions; Outbreak; Systematic review; Zoonotic virus
This article is included in the Emerging Diseases and Outbreaks gateway.
This article is included in the Trends and Advances in Counteracting Mpox: A Global Public Health Emergency collection.
A zoonotic, double-stranded DNA virus belonging to the genus Orthopoxvirus, the mpox virus (MPXV) is most common in tropical regions of Central and West Africa. The frequency of monkeypox (mpox) cases, however, has sharply climbed globally since May 2022.
To establish the threat of mpox in terms of the oral lesions caused in sufferers.
After a thorough study of the literature identified in the PubMed, Web of Science, and Cochrane library databases using the PRISMA framework, 103 papers were found. Using inclusion and exclusion criteria, we chose research that was relevant for our review before shortlisting 14 papers that conformed to the review's guidelines.
In the 14 selected studies, it was found that oral lesions were among the first clinical signs of a mpox affliction, with ulcers on the dorsal surface of tongue lips being the most common areas affected.
The rarely observed oral lesions of mpox infection may help in the diagnosis and management of this condition. It is critical to keep in mind that recognising and detecting oral lesions in mpox patients opens the door to more research and efficient patient management.
Monkeypox; Oral lesions; Outbreak; Systematic review; Zoonotic virus
The introduction has been revised to align with updated nomenclature, now referring to the disease as "mpox" and the virus as "mpox virus (MPXV)". Additionally, Table 1 has been updated to reflect current data, with the inclusion of 157 reported deaths from non-endemic regions. A reference to the study published in The Lancet Microbe, which described viable virus in saliva (doi: 10.1016/S2666-5247(22)00291-9), has been incorporated to support the relevant assertion. Furthermore, the manuscript now includes the study by Hernaez et al. (Monitoring monkeypox virus in saliva and air samples in Spain: a cross-sectional study), with appropriate citation, which involved 44 confirmed mpox patients from the 2022 outbreak in Madrid and detailed clinical symptoms, including oral lesions, available in supplementary materials (Hernaez et al., 2023).
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
The mpox virus (MPXV) is not an uncommon zoonotic disease. The mpox virus (MPXV) is the name for the virus causing the disease monkeypox (mpox). Both the smallpox and mpox viruses (MPXV) are members of the Orthopoxvirus genus in the Poxviridae family. In the Democratic Republic of the Congo, the first case of mpox on a human was documented in 1970.1 Since that time, mpox has spread over western and central Africa. Up until recently, 47 non-endemic nations in Europe, North and South America, Asia, North Africa, and Australia had reported cases of the disease. More than 4,100 confirmed cases had been reported as of 2022, the count increasing by the minute. Notably, 87% of occurrences have happened in nations in Europe.2 It is thought that the termination of smallpox vaccination, which offered some cross-protection against mpox but was not the primary cause of the new outbreak, led to an increase in human-to-human transmission. Generally speaking, human-to-human transmission happens when contaminated objects including linens, bedding, electronics, and clothing come into direct physical contact with an infected person's ulcerated skin or mucosa, respiratory droplets, or both. Through the placenta, pregnant women can spread the virus to unborn children.3 mpox virus (MPXV) airborne transmission is still a contentious topic. Even if it does, it could not be the primary method of transmission. The incubation period for mpox infection lasts for 7–14 days or 5–21 days and is not contagious. Patients do not have any symptoms during this time.4
Table 1 represents the clinical characteristics of mpox as generally observed in sufferers. Notably, lesions in patients with mpox in non-endemic locations are more confined and have a different distribution of rashes, whereas symptoms in patients with mpox in endemic regions are more severe and result in a certain proportion of fatalities. Since at this moment 157 deaths have been reported.5
The beginning phase of mpox includes a fever, chills, headache, backache, myalgia, asthenia, and lymphadenopathy. Patients that are infected may be contagious during the prodromal stage. It's interesting to note that the fundamental distinction between the symptoms of mpox and smallpox is that smallpox does not result in lymphadenopathy but mpox does. Submandibular, cervical, axillary, and inguinal lymph nodes may be bilaterally or unilaterally affected in mpox.6 Lesions may form in the mouth, oropharynx, or throat after the prodrome before spreading to the skin. The face and extremities, such as the palms of the hands and soles of the feet, are typically where the skin rash is more intense. The lesions develop in stages, going from macule to papule to vesicle to pustule to crust. Until all lesions have crusted over, patients are deemed contagious. The sores are frequently characterised as hurting initially, then becoming irritating. After scabs have peeled off, scars with hyper/hypopigmentation may develop. Usually, the disease lasts 2-4 weeks. Patients in the latest outbreak's non-endemic areas present with unusual symptoms that are very dissimilar from those in western and central Africa. Genital, perianal, and perioral/oral rash, fever, lymphadenopathy, and swallowing pain are a few of these. Lesions on the oral mucosa and vaginal region may first emerge before or without spreading to other body areas, pointing to sexual contact as the likely mode of transmission.7 Many individuals first had pustules before becoming feverish. Patients with a few small, isolated skin lesions sometimes show no signs of pain. It's interesting to note that the same people can have lesions in various stages. Pain, haemorrhage, proctitis, and tenesmus can all result from anus and rectum lesions.
Patients in the endemic area have never before reported having these symptoms. Patients in the current outbreak locations generally experience milder symptoms than those in the endemic zone. There haven't been many documented hospitalizations, and the two most common causes were pain management and subsequent infection care.8 Prior to skin rashes, mpox might present with oral and oropharyngeal lesions. It is stated that mouth sores, along with fever and enlarged lymph nodes, are common symptoms in mpox patients. Notably, the CDC stated that lesions in the mouth and on the tongue were seen in 70% of people. These findings imply that the virus can be carried in the saliva and spread via oral-skin and/or oral-anogenital contact. Therefore, it's crucial for dentists and other dental professionals to be aware of and able to identify mpox oral lesions. Starting with macule, papule, vesicle, and pustule, the progression of oral lesions should resemble that of skin lesions.9
Ulceration with pseudomembrane occurs after the roofs of the vesicle or pustule break off. A male patient who was a returning tourist from Nigeria experienced right cervical lymphadenopathy, multiple 2-4-mm pustules, and central umbilication of the skin, particularly on the face, neck, and hands. The oral lesions were described as a single intact pustule on the lower labial mucosa and a few round 2-3 mm erosions on the mucosa, indicating that the original vesicles or pustules have already broken off.9
Hence, by the means of this systematic review and subsequent meta-analysis, we aimed to establish the threat of mpox in terms of the severity of oral lesions that are caused due to it.
This systematic review was performed as per the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) strategy and rules from the Cochrane group and the book Orderly reviews in Health care: Meta examination.10 We also utilised the PICO strategy to identify and evaluate the relationship between mpox and oral lesions. The population of interest included individuals with confirmed cases of mpox. The intervention of interest was the presence of mpox, while the comparison group consisted of individuals without mpox. The outcome of interest was the occurrence of oral lesions associated with mpox.
This systematic review aimed to analyse, by the means of selecting studies, to review the correlation between the incidence of mpox and the oral lesions occurring in patients because of it at various stages of the disease.
There were a total of 103 documents discovered after extensive search on the online journals and 54 of the papers were selected initially. Following that, 22 similar/duplicate articles were eliminated, which resultantly made 32 separate papers available at first. The abstracts and titles of submissions were then reviewed, and a further 18 papers were eliminated. Finally, 14 documents that met the inclusion and exclusion criteria were chosen, which included study articles and randomised/non-randomised control trials (Figure 1).
For this systematic review, we employed the PICOS (Participants, Interventions, Comparison, Outcome, Study design) framework for assessment of studies fit for our investigation.
The following were excluded from the scope of our systematic review: incomplete data, individuals in whom antimicrobial treatment had begun only recently, seminar presentations, scholarly articles, placebo controlled studies, and opinion articles.
Since the literature available on this topic is quite scant in volume, we did not limit our search in terms of the time period when the studies were published i.e. we took into account all the papers that were published with context to our topic (where the number of papers itself was found to be quite sparse in number).
Placebo-controlled studies were not included in the analysis. Also excluded were literature reviews and cases published in languages other than English.
Using relevant keywords, reference searches, and citation searches, the databases PubMed-MEDLINE, Web of Science, Cochrane, and Scopus were all searched. “Monkeypox,” “Oral lesions,” “Saliva,” “Zoonotic viruses” and “Oral manifestations” were the search terms used to access the database.
Two independent reviewers located the relevant papers by using the right keywords in various databases and online search tools. The chosen articles were compared, and a third reviewer was brought in if there was a dispute.
After choosing the articles, the same two reviewers independently extracted the following data: author, year of publication, country, kind of publication, study topic, population demographics (n, age), outcome measure(s), relevant result(s), and conclusion (s). The data was compared and any differences were discussed with the third reviewer. The evaluation of risk analysis was also performed, the detail of which have been furnished in Figure 2.
The AMSTAR-2 protocol11 was employed for the assessment of the risk of bias in our selected studies. As a critical evaluation tool for systematic reviews, AMSTAR 2 joins a number of other instruments that have been published for this purpose. It consists of a 16-point checklist, as shown in Table 2 below. The development of the original AMSTAR tool was based on two instruments that have received a lot of attention. Two instruments that have been released are exact copies of the original AMSTAR. The domains listed in the Cochrane risk of bias instruments for systematic reviews are identified by the AMSTAR 2 risk of bias items. These represent an agreement that was reached after input from more than 30 methodology specialists in each case.
| Studies selected | Question and inclusion | Protocol | Study design | Comprehensive search | Study selection | Data extraction | Excluded studies justification | Included study details | Risk of bias | Funding sources | Statistical methods | Risk of bias in meta-analysis | Risk of bias in individual studies | Explanation of heterogeneity | Publication bias | Conflict of interest |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Benslama et al (2022)12 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | N/A | Yes | Yes | Yes | Yes | Yes | Yes |
| Girometti et al (2022)13 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | N/A | Yes | Yes | Yes | Yes | Yes | Yes |
| Iamaroon et al (2022)14 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | N/A | Yes | N/A | Yes | Yes | Yes | Yes |
| Martins-Filho et al (2022)15 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | N/A | Yes | Yes | Yes | Yes | Yes | Yes |
| Noe et al (2022)16 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Patel et al (2022)17 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Peters et al (2022)18 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | N/A | Yes | Yes | Yes | Yes | Yes | Yes |
| Piero-Mestres et al (2022)19 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | N/A | Yes | Yes | Yes | Yes | Yes | Yes |
| Samaranayake et al (2022)20 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | N/A | Yes | N/A | Yes | Yes | Yes | Yes |
| Sukhdeo et al (2022)21 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | N/A | Yes | Yes | Yes | Yes | Yes | |
| Tarín-Vicent et al (2022)22 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | N/A | Yes | Yes | Yes | Yes | Yes | |
| Thornhill et al (2022)23 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | N/A | Yes | Yes | Yes | Yes | Yes | Yes |
| Yadav et al (2022)24 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | N/A | Yes | Yes | Yes | Yes | Yes | Yes |
| Yinka-Ogunleye et al (2017)25 | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
Table 3 as given below includes the findings of the systematic review as well as information on the 14 studies that were selected for the review. The results of the meta-analysis (conducted using RevMan 5 software) are displayed in Figures 3, 4 and 5 below in the form of a forest plot that reflects and ranks all the studies included in this systematic review. After data on the sample size, variables analysed, and various components of the investigations selected for our systematic review were entered into the Revman 5 programme, a forest plot illustrating the risk ratio, odds ratio, and risk difference was obtained as part of the meta-analysis for our study.
| Author and year of study | Sample size and mean age | Study design | Study description | Study inference |
|---|---|---|---|---|
| Benslama et al (2022)12 | A 34 year old man | Case report | The patient had a 2-day history of fever and a mouth rash when they arrived. He also recorded mouth pain, swallowing issues, and headaches. He had a personal history of sexually transmitted diseases, particularly gonorrhoea and chlamydia genital infections. | Clinical examination revealed several mouth sores on the floor of the mouth and the top of the tongue, as well as bilateral laterocervical lymphadenopathy (figure). These oral lesions have a "cockade-like" pattern, with a white halo surrounding a central red ulcer. He didn't have any skin lesions when he arrived. Monkeypox was detected in swabs taken from lesions on the tongue using polymerase-chain reaction tests. Notably, the patient had not received a smallpox vaccination. |
| Girometti et al (2022)13 | 54 (all males); mean age 41 years | Observational study | Of the 54 people, 30 (55%) had lymphadenopathy, while 4 (7%) had oropharyngeal lesions. | In this study, oral mucosal enanthema were more frequently recorded among unvaccinated individuals than among those who had received vaccinations. This condition can occur in more than 70% of cases. |
| Iamaroon et al (2022)14 | - | Literature review | At the time this study was being written, the monkeypox outbreak in non-endemic areas had spread to at least 47 nations and more than 4,100 new cases. In contrast to central and western Africa, the clinical characteristics in non-endemic locations are unusual. Monkeypox is mostly diagnosed based on clinical manifestations and laboratory tests. | Before the rash appears on the face and other areas of the body, it may first present as mouth lesions since the oral mucosa is frequently affected by this condition. It is possible for oral symptoms to appear before skin eruptions, indicating that dental professionals should be well-versed in the disease's characteristics. |
| Martins-Filho et al (2022)15 | 3 (2 females); the females were 28 and 12 years respectively, with the male being 24 years old | Epidemiological case report | In a low-income area of Brazil, all 3 instances were identified between August 22 and August 26, 2022. Using real-time polymerase chain reaction, samples from skin lesions all tested positive for monkeypox DNA (RT-PCR). | Only the first of the three cases—involving a 28-year-old woman who saw the doctor 15 days after developing fever, asthenia, headache, and sore throat—was found to have early lesions in her oral mucosa. This case also had several pruritic, papulovesicular lesions on the limbs and trunk. |
| Noe et al (2022)16 | 2 males | Observational study | In order to emphasise the significance of recent advances for medical experts around the world and to share further observations regarding human-to-human transmission in these cases, this paper details the first two cases of monkeypox (MPX) infections in Germany. | One patient described having trouble swallowing and having white patches on his tonsils. These oral sores on the tonsils were supposedly the patient's first outward sign of MPX. Such oral lesions have been previously identified for MPX in animal models as a component of the lymphatic tissue's involvement. These oral symptoms were absent in the second patient. |
| Patel et al (2022)17 | 197 (all males); median age 38 years | Descriptive case series | Twenty seven participants presented with oral mucocutaneous manifestations without systemic symptoms. | Oral mucocutaneous lesions and systemic features were found to have a varied temporal relationship, which raises the possibility of a new clinical path for the illness. |
| Peters et al (2022)18 | 2 (both males); 38 and 30 year old respectively | Case report | One of the patients noticed a sore, sensitive, "pimple-like" nodule on the tip of his tongue that grew larger before he went to the emergency room. A well-defined, tan-grey ulceration of the front tongue that was around 1.0 cm in size was found. However, the second person did not have any reported oral lesions. | The differential diagnosis for monkeypox oral symptoms frequently includes more prevalent inflammatory and viral diseases. If the lesion appears as a tan-grey ulceration involving the anterior tongue, traumatised ulcerations from biting may be taken into consideration. |
| Piero-Mestres et al (2022)19 | 12 (all men); mean age 38.5 years | Clinical observation study | Real-time PCR was used to gather and evaluate 147 clinical samples from 12 individuals at various times. All instances had monkeypox DNA in their saliva, sometimes with high virus levels. | 11 out of 12 patients reported experiencing a generalised systemic syndrome, including fever, myalgia, general malaise, and mouth ulcers. Oral lesions were found in at least one area of the oral cavity in half of the individuals. |
| Samaranayake et al (2022)20 | - | Case review | The information was gleaned from the most recent literature, primarily from the databases of the World Health Organization and the Centres for Disease Control and Prevention, and covered the aetiology, modes of transmission, signs and symptoms, diagnosis, and management, as well as the risk of occupational transmission in dental settings. | Clinicians should be aware that the disease typically manifests as macules and ulcers on the oral mucosa before the characteristic skin lesions. Patients should be isolated and referred when necessary, especially when a local outbreak is present. Standard, contact, and droplet infection control measures should also be implemented. |
| Sukhdeo et al (2022)21 | 8 (all males); age range from 25-56 years | Observational study | In this study, clinical photos are provided to demonstrate the range of cutaneous and mucocutaneous lesions that eight patients with HMPX (whose diagnosis was supported by real-time polymerase chain reaction) presented with while receiving therapy in Toronto, Canada, between May and July 2022. | A primary tongue lesion was present in one patient. The 5 mm ulcer on the right tip of the tongue was painful, covered in purulence, and encircled by oedema. There was a localised swelling surrounding another ulcer on the left posterior part of the dorsal tongue, which is not visible in this image. The patient's initial clinical presentation consisted of these ulcers, which were a primary crop of lesions. On another patient's right upper lip, there was an ulcer. The 12 mm peripheral erythematous ulcer was not painful or pruritic. |
| Tarín-Vicent et al (2022)22 | 181 (all males); median age 37 years | Observational cohort study | Demographics, smallpox vaccination, HIV status, exposure to monkeypox, travel, mass gathering attendance, risk factors for STIs, sexual behaviour, signs and symptoms at initial presentation, virological results at multiple body sites, co-infection with other STIs, and clinical outcomes 14 days later were all outcomes assessed in all participants with a confirmed diagnosis. | Lesions in the oral and perioral area were present in 78 people (or 43%). 70 (39%) of the patients experienced difficulties that required medical attention, including 19 (10%) tonsillitis, 6 (3%) abscesses, and 8 (4%) exanthems. |
| Thornhill et al (2022)23 | 528 (all males) | International descriptive case series | 26 people reported that their first symptoms were oropharyngeal, including pharyngitis, odynophagia, epiglottitis, and oral or tonsillar lesions. | A variety of dermatologic and systemic clinical symptoms were present in monkeypox manifestations. The oral route (in the form of droplets), close or direct contact with skin lesions are the routes by which the monkeypox virus is spread. |
| Yadav et al (2022)24 | 2 (both males); 35 years and 31 years old respectively | Case report | One of the patients experienced several vesicular rashes in the lips and oral cavity, which led to an oedematous upper lip. None of the significant mouth lesions were visible to the other patient. | Oral lesions are an important diagnostic marker in cases of monkeypox. |
| Yinka-Ogunleye et al (2017)25 | 122 (84 males); median age 29 years | Epidemiological and clinical study | A form of oral vesicular rash appeared in all 122 confirmed or probable cases, and 45 (58%) of the 77 confirmed patients also experienced sore throats. | A rash on the tongue and mucous membranes, as well as any sores or lesions on the tongue, in the oral cavity, or on the corners of the mouth, are common early signs of monkeypox. |
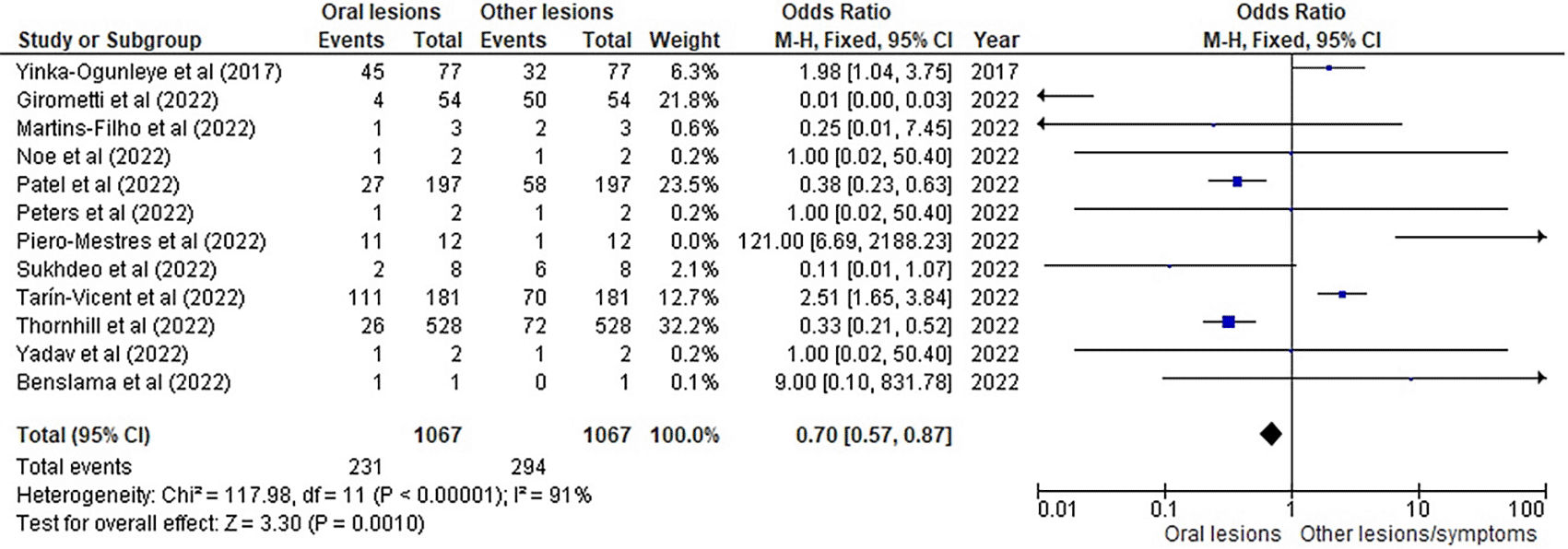
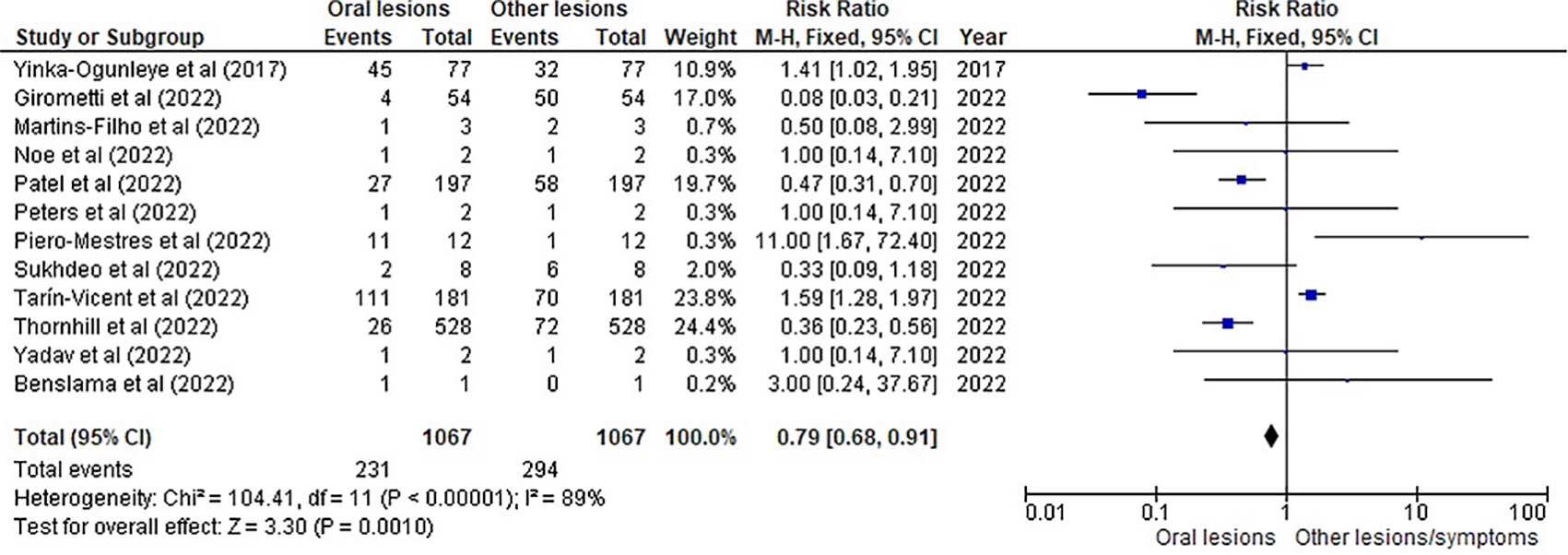
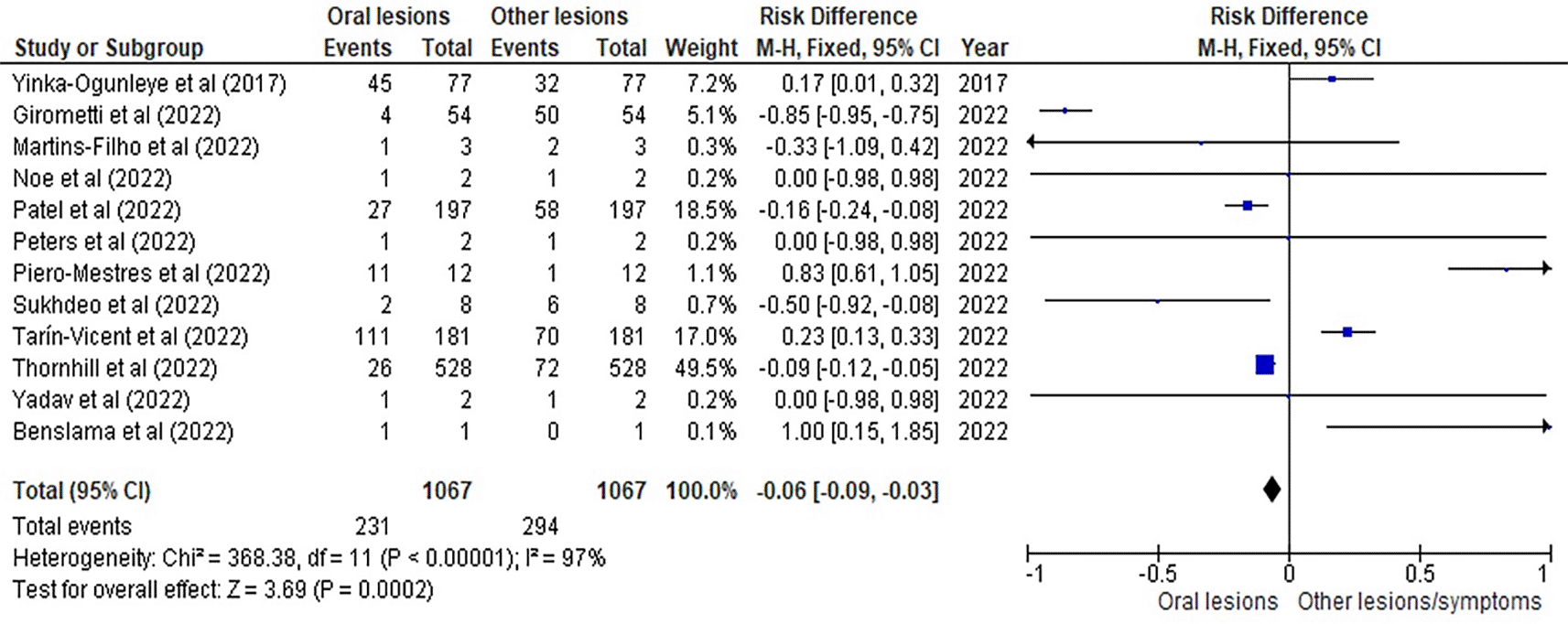
Figures 3, 4 and 5 represent the forest plots obtained after meta-analysis of the incidence of the oral lesions in patients of mpox that were a part of the studies that were selected for our systematic review.
Many diseases, including chickenpox, measles, bacterial skin infections, scabies, syphilis, and medication-induced allergies, share characteristics of the mucocutaneous lesions with mpox.26 Based solely on the clinical appearance, it might be challenging to discern between these disorders in some cases. When comparing mpox to chickenpox or smallpox, lymphadenopathy during the prodromal stage might be used to make the distinction. Healthcare professionals should gather a sample if mpox is suspected so that it can be further examined using polymerase chain reaction (PCR), a method that has strong specificity and sensitivity for finding the mpox virus (MPXV).27 The fluid from vesicles, pustules, or dry crusts should be collected, preserved in a dry, sterile tube without viral transport media, and kept cold. An optional method for making the diagnosis is a biopsy.28
As evident by the finding mentioned in Table 3 depicting the selected studies, oral lesions are primarily present at the start of the mpox affliction. For example, in the study by Tarín-Vicent et al,22 lesions in the oral and perioral area were present in 78 people (or 43%). In all the studies, oral lesions were present in some form or the other (as ulcers or vesicular rash most commonly around the tongue or lower lip area) during the prodromal stage of monkeypox which was accompanies by symptoms such as fever, myalgia, and general malaise and, in some cases, a pruritic rash. The case reviews by Iamaroon et al14 and Samaranayake et al20 also provide with literature evidence of oral lesions in monkeypox patients. One of the documented cases29 in these reviews mention of a male patient who was a returning tourist from Nigeria experienced right cervical lymphadenopathy, multiple 2-4-mm pustules, and central umbilication of the skin, particularly on the face, neck, and hands. The oral lesions were described as a single intact pustule on the lower labial mucosa and a few round 2-3 mm erosions on the mucosa, indicating that the original vesicles or pustules have already broken off. In the study by Girometti et al,13 oral mucosal enanthema were more frequently recorded among unvaccinated individuals than among those who had received vaccinations. This condition can occur in more than 70% of cases. This might represent the aspect of oral lesions occurring more in unvaccinated individuals which might prompt strengthening of the already stringent vaccination laws around the world especially in terms of traveling. Speaking of traveling, seven of the 14 studies that we selected for our review involved individuals who were traveling from one country to another which again warrants a separate investigation into the transmission of monkeypox or monkeypox-like infectious diseases within a particular defined area (such as a city, state or country) and how it might differ from the outcomes obtained in our investigation.
Figures 3, 4 and 5 represent the meta-analysis results of our investigation. Although the heterogeneity levels in the three different assessments are particularly high (91%, 89% and 97%), it is due to the fact that the studies that were selected had sample sizes that were lesser than what could be considered ideal and more importantly, the methodological differences in the studies selected contribute to the high levels of heterogeneity of the forest plots. The fixed effect model was employed in the odds ratio, risk ratio and the risk difference assessments so as to keep uniformity in terms of our interpretations and reduce bias that occurs resultantly after selection of studies with such variations in methodology and sample sizes.
The nasopharyngeal swab bio sample has been the gold standard for the detection of severe acute respiratory syndrome coronavirus 2 since the start of the COVID-19 pandemic (SARS-CoV-2). Saliva has recently become a practical and affordable bio fluid for COVID-19 diagnostics and may someday take the place of a nasopharyngeal swab.30 Saliva collection requires no specialised equipment, is non-invasive, and uses a straightforward approach. Since mpox infection frequently appears in the oral cavity, patient saliva may contain mpox virions and hence might be used as a bio sample to identify the virus. These patients will greatly benefit from further research to validate the use of saliva for mpox diagnoses.31 Vesicular or pustular lesions are the terms used to characterise mpox oral symptoms. The ulceration occurs following the vesicle or pustule rupture. Mpox patients’ lesions may mirror those caused by other viral diseases of the oral cavity, such as COVID-19, chickenpox, measles, measles-mumps-rubella, measles, and herpes zoster. Vesiculobullous lesions predominate in mpox oral lesions. The vesicles/bullae readily separate, resulting in numerous uncomfortable, superficial sores on the oral mucosa and lips. Recurrent lesions are more confined and frequently affect the keratinized oral mucosa and lip vermilion.32 Similar study findings imply that the virus can be carried in the saliva and spread via oral-skin and/or oral-anogenital contact.33
Fortunately, the majority of people with mpox infection recover on their own. To reduce gastrointestinal fluid losses, those with gastrointestinal symptoms (such as vomiting or diarrhoea) will need oral or intravenous rehydration. Several antivirals have been licenced for the management of smallpox based on animal models, but they may also be beneficial in treating mpox infections. Human dose studies for these medications have been carried out, but their effectiveness has not been fully explored. The first antiviral approved for the treatment of smallpox in adults and children weighing at least 3 kg is tecovirimat, and it is regarded as the preferred method of care.34 Dual therapy with tecovirimat and brincidofovir may be utilised in patients with advanced illness. By blocking the last steps in viral maturation and release from the infected cell, the viral envelope protein VP37—by which Tecovirimat functions—inhibits the transmission of the virus within an infected host.35 Although its effectiveness in treating mpox in people has not been investigated, investigations on animals treated with tecovirimat at various illness phases have shown better survival from lethal mpox virus infections in comparison to animals given with a placebo.36,37 The side-effect profile of the placebo was generally identical to that of tecovirimat in an enlarged safety study with 359 human volunteers given tecovirimat. Since June 2021, brincidofovir has also been authorised for the treatment of monkeypox in the US.38 An oral counterpart of the injectable medicine cidofovir, brincidofovir, may have a better safety profile than cidofovir, such as reduced renal damage.39 These medications function by preventing viral DNA polymerase.40 The effectiveness of brincidofovir against orthopoxvirus infections has been established, despite the paucity of studies examining its usage in treating mpox infections in animal models.41 The FDA has authorised the hyperimmune globulin known as Vaccinia Immune Globulin (VIG) for the treatment of specific vaccine-related side effects.42 These include vaccinia infections in people with skin disorders, aberrant infections brought on by the vaccinia virus, progressive vaccinia, severe widespread vaccinia, and eczema vaccinatum (except in cases of eye-related infections).42
As far as limitations go, our systematic review had a few to being with. For starters, the sample size observed in our selected studies were fewer than what would be considered ideal, but, since the number of articles which had documented the oral manifestations observed in patients suffering from mpox are very scarcely available, we selected the ones best suited for our objectives. Additionally, prior to the outbreak in May 2022, mpox had a limited clinical relevance, therefore it was frequently overlooked in the differential diagnosis. Other infectious illnesses could be in the differential diagnosis list. The countries of Central and Western African regions, where this disease is endemic, do not have a lot of data available on mpox due to the extremely limited number of studies performed, which as such necessitates the importance of documenting mpox and its effects observed in the form of symptoms such as the occurrence of oral lesions.
All the 14 articles selected in this review have reported the incidence of oral lesions in mpox sufferers, in at least the beginning stages of the disease, which have then blown up into full-fledged symptoms. As such, the identification and treatment of this illness may be aided by the seldom observed oral features of mpox infection. Acute onset oral signs (as seen in nearly all our selected studies) should be differentially diagnosed for mpox, especially in people who are more likely to experience this ailment. It is crucial to remember that recognising and identifying oral lesions in patients with mpox paves the way for additional research, effective care, and the prevention of cross-infection between patients and medical staff.
Conceptualization, HA, AD, FB, NM, FA, OM, KPS, MAS, HA, MKA; methodology, HA, AD, MKA; software, HA, AD, MKA; validation, HA, AD, MKA; formal analysis, HA, AD, MKA; investigation, HA, AD, MKA; resources, HA, AD, MKA; data curation, HA, AD, MKA; writing—original draft preparation HA, AD, FB, NM, FA, OM, KPS, MAS, HA, MKA; writing— review and editing. HA, AD, FB, NM, FA, OM, KPS, MAS, HA, MKA; visualization, HA, AD, MKA; project administration, HA, AD, MKA; funding acquisition, none. All authors have read and agreed to the published version of the manuscript.
Figshare. PRISMA flowchart. DOI: https://doi.org/10.6084/m9.figshare.23626704.v1
Figshare. PRISMA checklist. DOI: https://doi.org/10.6084/m9.figshare.23626695.v1
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
No
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: infectious diseases, public health
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: poxvirus immune evasion
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
References
1. Hernaez B, Muñoz-Gómez A, Sanchiz A, Orviz E, et al.: Monitoring monkeypox virus in saliva and air samples in Spain: a cross-sectional study.Lancet Microbe. 2023; 4 (1): e21-e28 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: poxvirus immune evasion
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: OMFS
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 01 Mar 24 |
read | read | |
|
Version 1 10 Aug 23 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)