Keywords
Systemic Lupus Erythematosus, SLEDAI 2K, BAFF, methylprednisolone pulse dose
Systemic Lupus Erythematosus, SLEDAI 2K, BAFF, methylprednisolone pulse dose
Systemic lupus erythematosus (SLE) is a chronic multisystem autoimmune rheumatic disease characterized by autoantibody production towards nuclear antigen.1 Almost all patients with SLE have antinuclear antibodies (98%), and some also have autoantibodies against double-stranded DNA (dsDNA) (76%), hypocomplementemia (71%), and antibodies to Ro (SSA) (35%). SLE has a wide range of clinical symptoms and the potential to affect nearly all organs and tissues.2
In the 1970s, a pulse dose of 500-1000 mg/day for 1 to 3 days of MEP was established to treat life-threatening complications associated with SLE.3 Pulse-dose MEP is the fastest immunosuppression therapy for life- or organ-threatening SLE.3 It is effective for renal involvement and other severe symptoms such as pulmonary hemorrhage and neuropsychiatry.3 Conventional treatment with intravenous MEP can reduce activity and complement activation in lupus nephritis.3 However, some patients continue to develop increasing renal impairment, and even those with favorable responses are at risk for relapse. Currently, objective measures to estimate the rate of response to pulse-dose MEP are unavailable, and the safety profile for the increased risk of infection is high.
Sixty to seventy percent of SLE patients have elevated blood BAFF levels, which positively correlate with SLEDAI 2k and anti-dsDNA levels.4 In addition, the BLISS-52 clinical trial demonstrated that belimumab, an inhibitor of BAFF, can reduce serology activity, prevent flares, and reduce corticosteroid use.5 A study by Milz et al. showed that blood BAFF levels were elevated compared to healthy controls in different autoimmune diseases, such as Idiopathic Thrombocytopenic Purpura (ITP), and that Glucocorticoid (GC) therapy dramatically reduces serum BAFF levels.6 This may be related to GC receptor binding in the promoter region.6
Interferon-gamma-induced protein 10 is a member of T-helper 1 lymphocyte chemokines produced and released in response to stimulation by infiltrating monocytes, macrophages, and endothelial cells.7 These cells subsequently bind to the T-lymphocyte-expressed CXCR3 receptor, essential for T-lymphocyte trafficking into SLE patients’ afflicted organs.8 IP-10 levels are significantly higher in the acute phase of SLE.9 Additionally, SLE treatment modalities such as prednisolone, mycophenolate, and hydroxychloroquine significantly lower serum IP-10 levels.10
The goal of this study is to use clinical manifestations, routine laboratory examinations, and more specific protein examinations such as sBAFF, BAFF-R, and IP-10 to predict SLEDAI 2k score following pulse-dose MEP, given that the spectrum of clinical manifestations and response to treatment varies from patient to patient.
This study is a prospective cohort observational analysis that examines data on patients with systemic lupus erythematosus (SLE) who met the criteria of the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) 2019 or had class III or IV lupus nephritis (LN) according to World Health Organization (WHO) criteria. These patients underwent a follow-up period after receiving a daily dose of 500 mg MEP pulse for three consecutive days, during which their progress and response to the treatment were closely monitored and assessed.
The study included patients between the ages of 15 and 50 years who were willing to participate and did not have comorbidities such as pneumonia, tuberculosis, viral, fungal, parasitic, or protozoal infections, sepsis, diabetes mellitus, Graves’ disease, cardiovascular disease, or cancer. Additionally, patients were excluded if they had overlap syndrome with systemic sclerosis, rheumatoid arthritis, or dermatomyositis inflammation. Subjects were withdrawn from the study if they died or developed sepsis during the observation period. Patients who met the inclusion and exclusion criteria were informed about the study’s purpose, process, and potential risks and benefits, and asked to sign a consent form to participate. The explanation also highlighted the advantages and disadvantages of participating, allowing patients to make informed decisions about their involvement.
The determination of our study’s sample size was guided by the “rule of thumb” for multivariate analysis, which stipulates that a minimum of 15 samples per predictor is needed to ensure robust statistical analysis. Since we aimed to investigate five predictor variables in this study, the minimum required sample size was calculated as 75. To account for potential dropouts and missing data, which are common occurrences in research studies, we increased this number by an additional 10%. This precautionary measure resulted in a total minimum sample size of 83. By adhering to this method, we ensured that our sample was statistically adequate for the multivariate analysis of the five predictor variables.
Before administering the pulse dose of MEP, all information on the SLE Disease Activity Index 2K (SLEDAI 2K) form was completed, and the SLEDAI 2K score was recorded.11 Blood samples, typically ranging from 5 to 10 milliliters, were carefully collected from each patient using a standard venipuncture procedure. The samples were then processed and analyzed for the levels of sBAFF, IP-10, and BAFF-R. Specifically, the concentrations of sBAFF and IP-10 were measured using enzyme-linked immunosorbent assay (ELISA) techniques, while BAFF-R expression was analyzed through flow cytometry. Following the 3-day pulse dosage of MEP, blood samples were collected again using the same procedure, and these parameters were re-analyzed to assess and compare the changes in response to the treatment.
Measurement of sBAFF and IP-10 were measured using ELISA
S BAFF was examined in PRODIA LABORATORIES, CAP certified, using Microplate Reader Biorad model 680Bio-rad Laboratories inc, CA, USA. Reagent kit used in this study was Quantikine® HS ELISA Human BAFF/BLyS/TNFSF13B (R&D Systems, Inc., Minneapolis, USA) according to standard protocols. The results were measured in pg/ml.
Measurement of R BAFF and B cells (CD19) using flowcytometry
R BAFF and B cells (CD 19+) was examined in Clinical Pathology Laboratories of Gadjah Mada University Faculty of Medicine, Nursing and Public Health, ISO 17025 certified, using BD FACS Canto II 8 color Flowcytometry. Reagent used was PE anti human CD19 antibody (CD 19+) and CD 45 PerCP cy5.5 according to standard protocols. The results were measured in Mean Fluoresence Index (MFI), percentage of B cells CD 19+ to total lymphocyte, percentage of R BAFF to B cells CD 19+.
The Urine Protein Creatinine Ratio (UPCR) was determined at baseline, prior to the administration of MEP, by dividing the level of spot urine protein by the level of spot urine creatinine. Urine protein and urine creatinine were estimated by ERBA CHEM 5 V PLUS. In this study, the cutoff for normal UPCR is less than 150 mg/g and more or equal to or less than 500 mg/gr, consistent with diagnostic criteria for LN according to EULAR/ACR classification criteria.
All statistical analyses were performed using R Studio v. 4.1.1.1, employing the tidyverse and gtsummary packages.12,13 This study employed various statistical methods, including descriptive statistical analysis, univariate and multivariate logistic regression, and visualization using boxplots. Moreover, Pearson correlation analysis was conducted to evaluate the association between continuous variables by computing the Pearson correlation coefficient and its corresponding p-value, which provided insight into the magnitude and significance of any linear relationship.
In this study, we minimized potential biases through several strategies. To mitigate selection bias, clear inclusion and exclusion criteria were established, ensuring a representative study group. Measurement bias was addressed by using standardized assessment tools and established laboratory test methods. Multivariate analyses were conducted to control for confounding variables, such as age and sex. Attrition bias was tackled by carefully tracking all dropouts and withdrawals, and their potential impact on the study’s findings was thoroughly assessed. Additionally, all data and code were made openly available to promote transparency and reproducibility.
The sampling for this study was conducted from June 2021 to September 2022, after obtaining ethical approval from the Gadjah Mada University Faculty of Medicine, Nursing and Public Health ethics committee for human research (approval number: KE/FK/0359/EC/2021). The study was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent prior to their participation in the study.
In adherence to ethical guidelines, the inclusion of minor participants, aged between 15 to 17 years, in this study necessitated informed consent. In these cases, the consent was obtained through a two-step process: firstly, the minor’s assent was sought, which involved explaining the study’s purpose, procedures, potential risks, and benefits in an age-appropriate manner. Secondly, informed consent was also obtained from a parent or legal guardian. It was ensured that both the minor and their guardian fully understood the study before consent was provided. It was clarified that participation was voluntary, and they could withdraw from the study at any time without any negative consequences.
A total of 100 SLE patients who met the inclusion and exclusion criteria were enrolled in this study, conducted from May 2021 to September 2022. Out of the 100 samples, 20 were excluded from the analysis due to the discontinuation of MEP, owing to side effects such as infection and elevated blood sugar levels, patient death prior to the completion of the procedure, disease activity assessment using the MEX-SLEDAI index, and the attending physician postponing the procedure due to discrepancies in calculating the SLEDAI-2K score between the researcher and the attending physician (Figure 1).
In this study, 80 individuals were eligible to participate, with a majority of female participants (97.5%).21 The average age of participants was 28 years, with a standard deviation of 9.5 years. Before receiving MEP treatment, the median SLEDAI 2K score for all patients was 28 (22, 38), indicating a high level of disease activity. In addition, all of the other variables including the median elapsed time between the onset of initial symptoms and the study’s completion, C-reactive protein (CRP), Erythrocyte Sedimentation Rate (ESR), leukocyte, thrombocyte, Neutrophil-to-lymphocyte (NLR), targeted protein level for sBAFF, proportion of BAFF R to B cell CD19 (BAFF R (%)), median fluorescence intensity (MFI) for BAFF R, percentage of B cells CD19 to lymphocytes (CD19 %), and serum IP-10 were also measured pre-treatment with MEP. Specifically, CRP, ESR, leukocyte, thrombocyte, and NLR levels were found to be 7 (5, 28) mg/dl, 62 (32, 100) mm/hour, 6,100/L (4,300, 9,250), 200,737/L (109,179), and 5.1/L (2.9, 9.3) respectively, while the targeted protein level for sBAFF was 1,584 pg/ml (860, 2,892), the proportion of BAFF R to B cell CD19 (BAFF R (%)) was 87% (66, 94), the MFI for BAFF R was 3,404 (2,024, 5,556), the percentage of B cells CD19 to lymphocytes (CD19 %) was 19%, and the serum IP-10 was 157 pg/ml (69, 526) (Table 1).
| Variables | N = 801,2,3 |
|---|---|
| Age (years) | 28.08 (14.10) |
| Sex, Male (%) | 4 (5.0) |
| CRP mg/dL | 7 (5, 28) |
| ESR mm/hour | 62 (32, 100) |
| Leucocyte (cells/μL) | 6,100 (4,300, 9,250) |
| Thrombocyte (cells/μL) | 200,737 (109,179) |
| NLR | 5.1 (2.9, 9.3) |
| sBAFF (pg/mL) | 1,584 (860, 2,892) |
| BAFF R (%) | 87 (66, 94) |
| MFI BAFF R | 3,404 (2,024, 5,556) |
| CD19 (cells/μL) | 19 (10) |
| Seizure (% yes) | 12 (15%) |
| Psychosis (% yes) | 8 (10%) |
| Organic brain syndrome (OBS) (% yes) | 20 (25%) |
| CND (% yes) | 2 (2.5%) |
| Lupus headache (% yes) | 20 (25%) |
| CVA (% yes) | 5 (6.2%) |
| Vasculitis (% yes) | 7 (7.5%) |
| Rash (% yes) | 37 (46%) |
| Alopecia (% yes) | 46 (57%) |
| Oral or nasal mucosal ulcers (% yes) | 31 (39%) |
| Arthritis (% yes) | 51 (64%) |
| Myositis (% yes) | 12 (15%) |
| Pleurisy (% yes) | 21 (26%) |
| Pericarditis (% yes) | 3 (3.8%) |
| Fever (% yes) | 17 (21%) |
| UPCR (mg/mmol) | 1,814 (638, 5,358) |
| Hematuria/hpf | 17 (5, 123) |
| Cast urin/μl | 2.2 (0.5, 8.0) |
| Leucocytouria (cells/mL) | 16 (6, 39) |
| Anti dsDNA (IU/mL) | 175 (45, 200) |
| C3 (mg/dL) | 45 (26, 66) |
| C4 (mg/dL) | 9 (4, 20) |
| IP10 (pg/mL) | 157 (69, 526) |
| Disease duration (years) | 20 (5, 48) |
| SLEDAI 2K pre-treatment | 28 (22, 38) |
| SLEDAI 2K post-treatment | 18 (14, 22) |
Among the neuro-psychiatry descriptors listed on the SLEDAI 2K form, seizures, psychosis, organic brain syndrome, visual disturbance, cranial nerve disorder (CND), lupus headache, and new onset stroke (CVA) were observed in 12 (15%), 8 (10%), 20 (25%), 2 (2.5%), 20 (25%), 5 (6.2%) of participants respectively. Mucocutaneous symptoms such as vasculitis, rash, alopecia, and oral ulcers were found in 7 (7.5%), 37 (46%), 46 (57%), and 31 (39%) of participants, respectively. Arthritis and myositis were observed in 51 (64%) and 12 (15%) musculoskeletal patients, respectively. Pleurisy and pericarditis were observed in 21 (26%) and 3 (3.8%) participants, respectively. Overall, 17 (21%) of individuals had a fever.
The median concentration of UPCR was 3,648.86 mg/g. 64% of individuals had a UPCR of 500 mg/gr. Median hematuria, cast cylinder, and leucocyturia values were 17/hpf (5, 123), 2.2/uL (0.5, 8.0), and 16 cells/mL (6, 39), respectively. Immunology parameters revealed a mean anti-dsDNA concentration of 175 U/mL (45, 200). The median concentrations of C3 and C4 were 45 mg/dL (26, 66), and 9 mg/dL,4,14 respectively.
A correlation analysis revealed significant positive correlations between SLEDAI 2K scores, IP10 levels, sBAFF levels, and UPCR before treatment with response to treatment measured by SLEDAI 2K scores post-treatment (p < 0.05). Additionally, there is a significant negative correlation between C3 levels before treatment and SLEDAI 2K scores post-treatment (Table 2 and Figure 2).
| Variables | Coefficient correlation (r)1 | p |
|---|---|---|
| Age (years) | -0.013 | 0.546 |
| SLEDAI 2K pre-treatment | 0.51 | 0.0001 |
| NLR | 0.029 | 0.4 |
| IP10 (pg/mL) | 0.11 | 0.164 |
| sBAFF (pg/mL) | 0.27 | 0.008 |
| MFI BAFF R | 0.14 | 0.115 |
| BAFF R (%) | 0.25 | 0.23 |
| CD19 (%) | -0.16 | 0.926 |
| Anti dsDNA (IU/mL) | 0.18 | 0.053 |
| C3 (mg/dL) | -0.13 | 0.879 |
| C4 (mg/dL) | -0.076 | 0.749 |
| Neutrophil (cells/μL) | 0.076 | 0.252 |
| Lymphocyte (cells/μL) | 0.023 | 0.421 |
| Leucocyte (cells/μL) | -0.067 | 0.722 |
| Hemoglobin (g/dL) | -0.1 | 0.814 |
| Thrombocyte (cells/μL) | -0.15 | 0.909 |
| CRP mg/dL | 0.098 | 0.193 |
| ESR mm/hour | -0.033 | 0.614 |
| Hematuria/hpf | -0.02 | 0.57 |
| Leucocytouria (cells/mL) | 0.098 | 0.193 |
| Cast urin/μl | 0.18 | 0.0508 |
| UPCR (mg/mmol) | 0.2 | 0.0381 |
| Disease duration (years) | -0.12 | 0.855 |
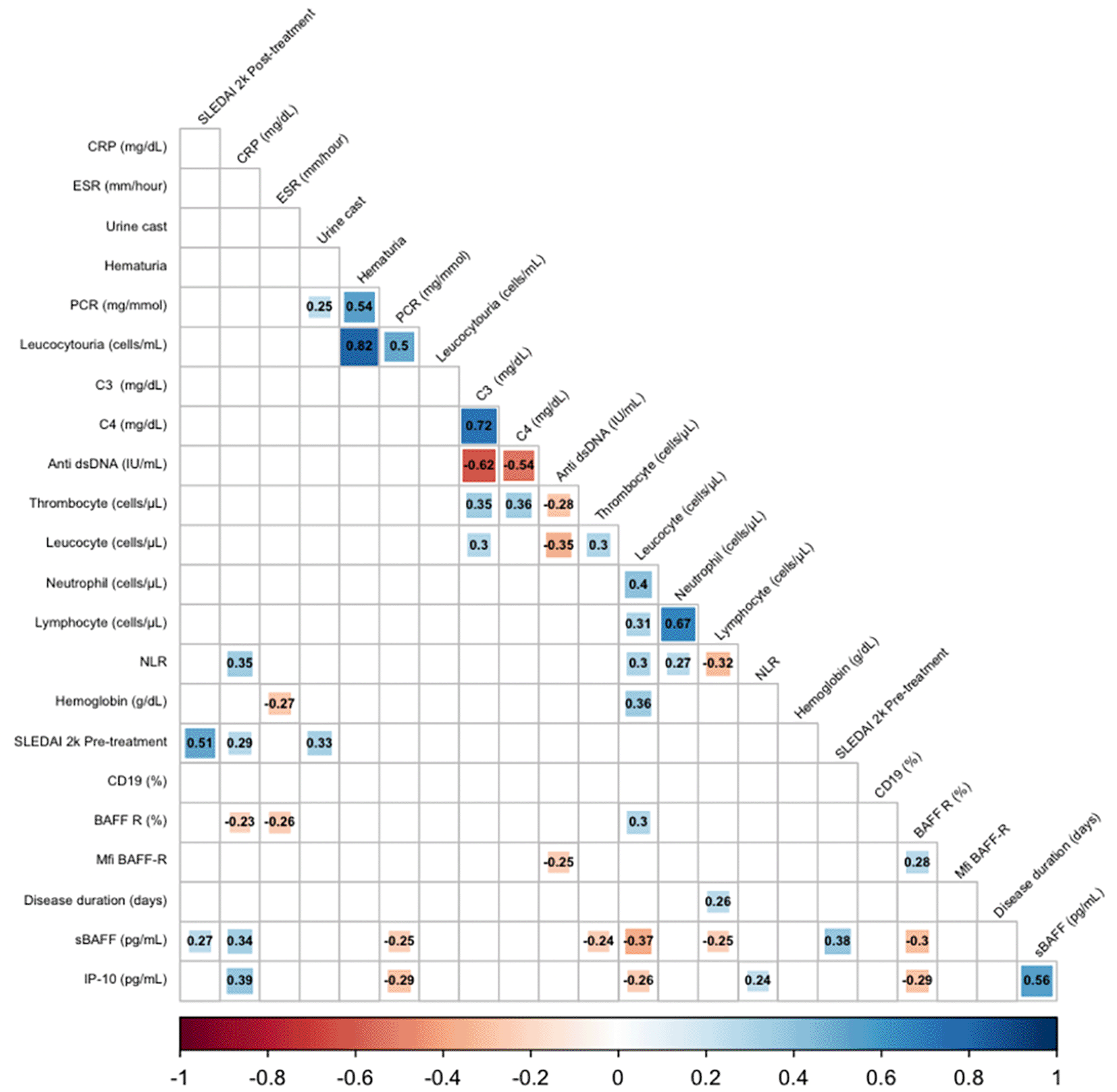
CRP: C-reactive protein; ESR: Erythrocyte Sedimentation Rate; PCR: Protein Creatinine Ratio; C3: Complement component 3; C4: Complement component 4; NLR: Neutrophil-to-lymphocyte ratio; SLEDAI 2K: Systemic Lupus Erythematosus Disease Activity Index 2000; CD19: CD19-positive B cells; BAFF R: B cell activating factor receptor; Mfi BAFF-R: Median fluorescence intensity of BAFF receptor; SBAFF: Soluble B-cell activating factor; IP-10: Interferon gamma-induced protein 10.
In our study, we performed a receiver operating characteristic (ROC) analysis to examine the relationship between various explanatory variables and the post-treatment SLEDAI-2K score. Our analysis determined that the optimal cutoff values for anti-dsDNA were 193.4 u/ml, for an NLR greater than 16.74/L, and a BAFF-R percentage greater than 48.8%.
We also conducted an independent t-test analysis, which revealed significant mean differences in SLEDAI-2K scores post-treatment based on NLR values greater or less than 16.74/L (p < 0.05). However, our analysis found no significant association between SLEDAI-2K scores and dsDNA levels greater or less than 193.4 u/ml or R BAFF levels more significant or less than 48.8% (as shown in Figure 3).
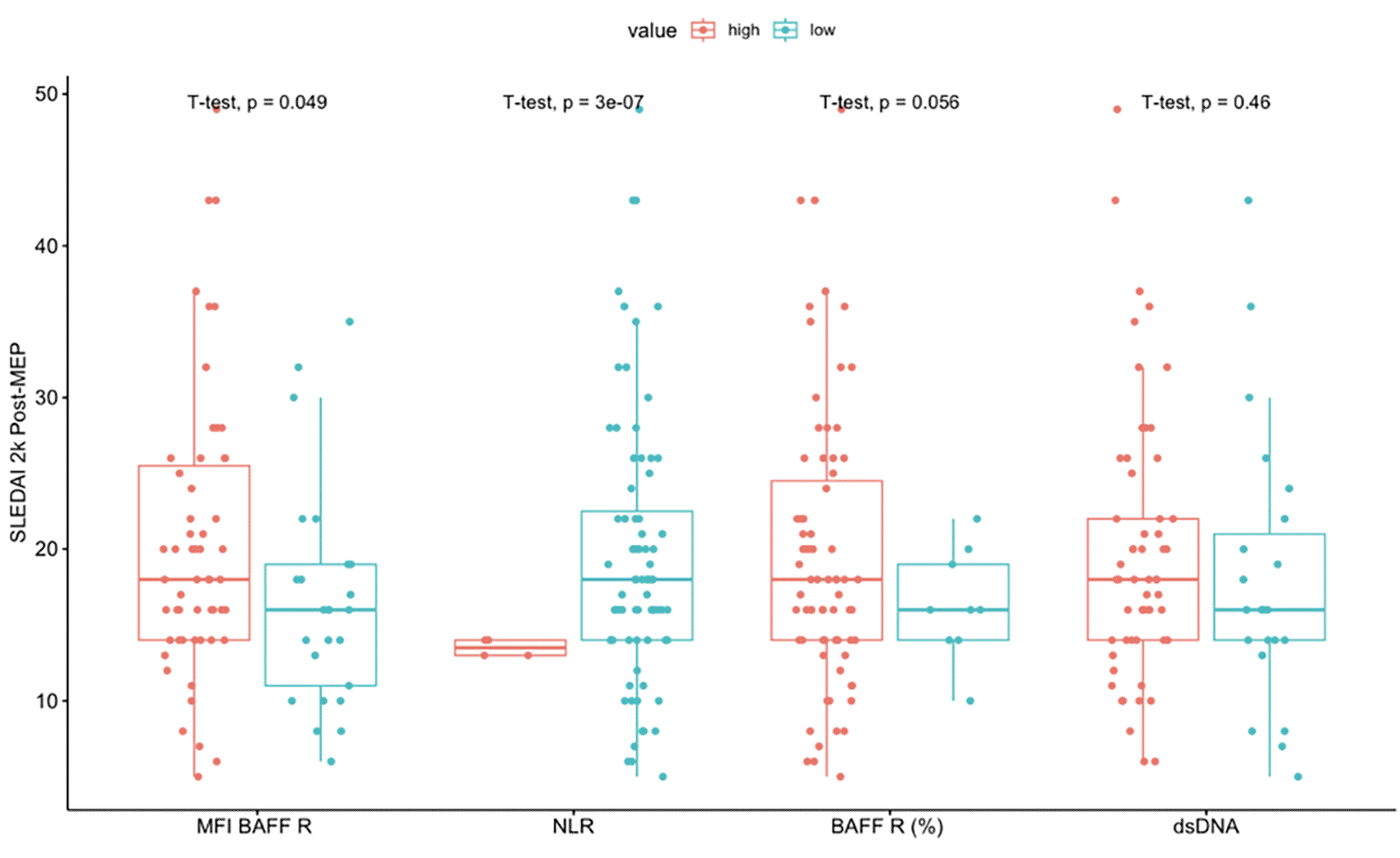
NLR: Neutrophil-to-lymphocyte ratio; BAFF R: B cell activating factor receptor (BAFF R (%)); MFI BAFF R: Median fluorescence intensity of BAFF R on B cells; Anti dsDNA: antibodies against double-stranded DNA (IU/mL).
We conducted a linear regression analysis to identify the predictive impact of each pre-treatment MEP parameter on the SLEDAI 2K therapeutic score. The results showed a significant correlation between SLEDAI 2K and sBAFF (pg/mL), oral or nasal mucosal ulcers, and alopecia. We then used Stepwise analysis to determine the top five predictive characteristics: UPCR, CD19 (%), sBAFF, vasculitis, and rash.
A substantial positive correlation study between pre-treatment factors and SLEDAI 2K scores post-MEP pulse revealed that the decline in each level of these variables, as indicated by the pre-post analysis, cannot achieve normal values. This may explain why the higher the level of pre-treatment variables, the greater the post-treatment SLEDAI 2K score. A daily dose of 500 mg MEP for three days can reduce the values of each variable but not below normal. The same rationale applies to the negative correlation between post-treatment C3 levels and SLEDAI scores, as a low C3 level suggests a more active disease.
We observed substantial mean differences in clinical symptoms such as arthritis, rash, alopecia, oral ulcer, positive hematuria, and proteinuria in this study. This may be attributable to the high prevalence of these factors across all study participants: 63%, 46%, 57%, 38.8%, 73.8 %, and 80%, respectively.
Our study found a strong correlation between the SLEDAI 2K pre-dose MEP and various markers of disease activity, including CRP levels, urine casts, and renal activity parameters. An increase in CRP levels and the presence of urine casts were both indicative of high disease activity. Additionally, we observed a positive correlation between Protein Creatinine Ratio, casts in the urine, hematuria, leukocyturia, and renal involvement. Immunology tests also revealed correlations, such as a positive connection between C3 and C4 levels and a negative connection between anti-dsDNA and C3 and C4 levels. We also found a substantial positive correlation between hemoglobin and leucocyte levels, as well as between thrombocyte and leucocyte levels, regarding hematological involvement. However, we discovered a significant negative correlation between anti-dsDNA and C3, C4 levels, thrombocytes, and leukocytes. Additionally, we observed a significantly negative relationship between ESR and hemoglobin levels.
Our study found a significant positive correlation between the NLR and leucocytes, neutrophils, and CRP levels. This suggests that as the NLR level increases, leucocytes, neutrophils, and CRP levels also increase. On the other hand, we observed a significant negative correlation between the NLR and lymphocyte levels, indicating that the lymphocyte level decreases as the NLR level increases. This is in line with the calculation of the NLR, which is determined by dividing the neutrophil count by the lymphocyte count.
In the presence of systemic inflammation, it is common for leukocyte numbers to decrease and for there to be alterations in circulating leukocytes, with lymphopenia and neutrophilia being typical features. Despite this, the NLR is a more stable indicator of inflammatory processes than leukocytes. This is because leukocyte numbers can be more readily impacted by factors such as dehydration or overhydration, excessively dilute specimens, or the influence of the blood collection procedure.15
The study by Zaki et al. aimed to investigate the association between BAFF R levels and SLE activity markers. The study revealed a negative association between BAFF R levels and SLEDAI, ESR, CRP, and anti-dsDNA levels.12 Conversely, the study found a positive correlation between BAFF R levels and complement protein C3 and C4 levels (p < 0.001).12 Additionally, the study observed a rapid reduction in BAFF R levels with the progression of the disease, with the lowest expression frequency observed in the group with the most active disease.16 Similar to this study, we also found a strong negative correlation between BAFF R levels and disease activity markers such as CRP, sBAFF, and ESR. In our study, the MFI of BAFF R levels showed a significant positive correlation with leucocyte count. At the same time, MFI BAFF R, but not BAFF R (%), had a significantly negative association with anti-dsDNA. This study found that sBAFF levels correlated positively with SLEDAI pre- and post-dose MEP, demonstrating that sBAFF levels increased during severe SLE flares and remained elevated following a pulse dose of MEP. Conversely, the study found a significant negative connection between sBAFF and proteinuria, contrasting with another study that found a positive correlation between sBAFF and proteinuria.17 The authors noted that the significant chronicity of lupus nephritis (LN) in the study population may have influenced proteinuria results. Moreover, the accuracy of actively proliferative LN in the study is questionable, as the classification of LN was based on World Health Organization criteria and not by biopsy.
Another study by Suso et al. observed high glomerular expression of BAFF in the inflammatory infiltrate of patients with class II mesangial and class III lupus nephritis in their histopathological analysis of LN.18 The authors noted that the high expression of BAFF was strongly associated with activity scores but not chronicity ratings, making this one of the most intriguing findings of their study.18
There was a significantly positive correlation between IP 10 and CRP, NLR, and s BAFF. Qin et al. found significant positive correlations between IP 10 and CRP, NLR, and sBAFF.15 Specifically, there was a positive correlation between NLR and CRP (r = 0.471, p < 0.01), ESR (r = 0.610, p < 0.01), and SLEDAI (r = 0.471, p < 0.01). These findings suggest that IP 10 levels may be associated with markers of disease activity in systemic lupus erythematosus (SLE).
This study found that IP-10 levels were significantly negatively correlated with leucocyte, C-reactive protein (PCR), and BAFF R (%). This finding was consistent with the study by Hrycek et al., which showed a negative correlation between IP-10 levels and leucocyte (r = -0.423), polymorphonuclear cells (PMN, r = -0.303), lymphocyte (r = -0.386), and monocyte (r = -0.365) levels.19 However, the inverse association between IP-10 levels and PCR levels was inconsistent, as active lupus nephritis is expected to have higher PCR levels than inactive lupus nephritis.
A meta-analysis confirmed this finding by Puapatanakul et al., which showed that serum IP-10 levels in active non-renal lupus did not differ significantly from inactive lupus nephritis and non-renal systemic lupus erythematosus (mean difference 22.6 pg/mL, p = 0.83).20 Another study found that IP-10 was a poor biomarker for predicting renal involvement.14 However, other investigations have reported conflicting findings. This discrepancy may be related to the varying levels of CXCR receptor expression on kidney-infiltrating cells. The current study’s authors hypothesized that the infiltration of IP-10 into renal tissue could increase its correlation with proteinuria in urine relative to serum levels.
The results of the univariate correlation test as shown in Table 3 indicated a strong positive association between the SLEDAI 2k, IP 10, and BAFF S variables prior to therapy and the post-therapy SLEDAI 2k score. This suggests that the post-therapy SLEDAI 2k score is proportional to the value of these variables prior to therapy. Additionally, the study found a non-significant negative connection between C3 levels and the post-therapy SLEDAI 2K score. This indicates that a lower C3 level is associated with a higher post-therapy SLEDAI 2K score. The correlation between the pre-and post-therapy SLEDAI 2K levels of CD19, C4, Hb, and platelets was modest and not statistically significant.
| Variables | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| N | OR | 95% CI1 | p-value | OR | 95% CI1 | p-value | |
| UPCR (mg/mmol) | 80 | 0.00 | 0.00, 0.00 | 0.076 | 0.00 | 0.00, 0.00 | <0.001 |
| CD19 (%) | 80 | -0.14 | -0.33, 0.05 | 0.15 | -0.18 | -0.35, -0.02 | 0.031 |
| sBAFF (pg/mL) | 80 | 0.00 | 0.00, 0.00 | 0.016 | 0.00 | 0.00, 0.00 | 0.029 |
| Vasculitis (yes) | 80 | 4.3 | -2.7, 11 | 0.2 | 7.2 | 1.1, 13 | 0.021 |
| Rash | 80 | 5.9 | 2.1, 9.6 | 0.003 | 7.5 | 4.0, 11 | <0.001 |
| CRP mg/dL | 80 | 0.03 | -0.04, 0.09 | 0.4 | |||
| ESR mm/hour | 80 | -0.01 | -0.04, 0.03 | 0.8 | |||
| Cast urin/μl | 80 | 0.23 | -0.05, 0.50 | 0.10 | |||
| Hematuria/hpf | 80 | 0.00 | 0.00, 0.00 | 0.9 | |||
| Leucocytouria (cells/mL) | 80 | 0.00 | -0.01, 0.02 | 0.4 | |||
| C3 (mg/dL) | 80 | -0.04 | -0.10, 0.03 | 0.2 | |||
| C4 (mg/dL) | 80 | -0.06 | -0.23, 0.11 | 0.5 | |||
| Thrombocyte (cells/μL) | 80 | 0.00 | 0.00, 0.00 | 0.2 | |||
| Leucocyte (cells/μL) | 80 | 0.00 | 0.00, 0.00 | 0.6 | |||
| Disease duration (years) | 80 | -0.03 | -0.08, 0.02 | 0.3 | |||
| IP10 (pg/mL) | 80 | 0.00 | 0.00, 0.01 | 0.3 | |||
| Seizure (yes) | 80 | 3.9 | -1.6, 9.3 | 0.2 | |||
| Psychosis (yes) | 80 | -2.3 | -8.9, 4.3 | 0.5 | |||
| OBS (yes) | 80 | 3.2 | -1.3, 7.8 | 0.2 | |||
| Lupus headache (yes) | 80 | 1.2 | -3.4, 5.7 | 0.6 | |||
| CVA (yes) | 80 | 3.6 | -4.6, 12 | 0.4 | |||
| Oral or nasal mucosal ulcers (yes) | 80 | 4.4 | 0.48, 8.4 | 0.029 | |||
| Arthritis (yes) | 80 | 4.0 | 0.00, 8.1 | 0.050 | |||
| Fever (yes) | 80 | 1.4 | -3.4, 6.3 | 0.6 | |||
| Visual disturbance (yes) | 80 | 5.6 | -3.5, 15 | 0.2 | |||
| CND (yes) | 80 | 10 | -2.5, 23 | 0.11 | |||
| Myositis (yes) | 80 | 2.8 | -2.7, 8.3 | 0.3 | |||
| Alopecia (yes) | 80 | 4.5 | 0.60, 8.4 | 0.024 | |||
| Pleurisy (yes) | 80 | 3.4 | -1.0, 7.9 | 0.13 | |||
| Pericarditis (yes) | 80 | 4.6 | -5.8, 15 | 0.4 | |||
| MFI BAFF R (high) | 80 | -3.9 | -8.1, 0.32 | 0.069 | |||
| NLR (high) | 80 | 6.0 | -3.0, 15 | 0.2 | |||
| BAFF R (%) (high) | 80 | -3.3 | -9.5, 3.0 | 0.3 | |||
| Anti dsDNA (high) | 80 | -1.7 | -6.0, 2.7 | 0.5 | |||
The study also analyzed the difference in mean post-SLEDAI 2K scores between clinical manifestation variables that were present and absent, including seizures, psychosis, SOO, visual disturbances, cranial nerve disorders, lupus headaches, CVA, vasculitis, arthritis, myositis, rash, alopecia, mouth ulcers, fever, hematuria, and proteinuria. The results revealed a significant difference in the mean post-therapy SLEDAI 2K score for arthritis.
A prediction model was generated using multivariate analysis with multiple linear regression after eliminating certain variables that were found to be correlated with each other (Table 3). This was done to ensure that the assumption criteria of multivariate linear regression analysis were met. The purpose of developing a formula that can predict SLEDAI 2K scores after MEP pulse dose is because SLE patients sometimes present with complicated conditions, the most common being flare-ups and infections. By predicting the SLEDAI score post-treatment, we can consider whether it will be beneficial or even worsen the infections before administering MEP pulses.
The resulting model was used to predict the response to MEP 500 mg (pulse) therapy for three days based on pre-therapy variables. The prediction formula was derived as follows: 13.41 + (0.0008542 * x1) - (0.1829338 * x2) + (0.0008776 * x3) + (7.1801728 * x4) + (7.5429676 * x5), where x1 is the UPCR (Urine Protein to Creatinine Ratio) prior to therapy, x2 is the CD19 (%) value prior to therapy, x3 is the sBAFF (B-cell activating factor) value prior to therapy, x4 represents the status of vasculitis prior to therapy (presumably coded as a binary value), x5 indicates the presence of a rash prior to therapy (also presumably coded as a binary value). This model is designed to predict the post-therapy SLEDAI 2K (Systemic Lupus Erythematosus Disease Activity Index 2000) score. The SLEDAI 2K score is a commonly used tool for assessing disease activity in patients with systemic lupus erythematosus. It incorporates a wide range of clinical and laboratory findings, and the prediction from the model above will be a crucial aspect of personalized treatment strategies for patients undergoing MEP 500 mg (pulse) therapy.
In conclusion, the study found that several variables, including the SLEDAI 2k, IP 10, and BAFF S prior to therapy, strongly correlate with the post-therapy SLEDAI 2k score. The study’s results can be used to predict the response to MEP 500 mg therapy for three days based on pre-therapy variables.
We construct the following formula to predict the SLEDAI 2k score after the MEP pulse: 13.41+ (0.0008542 * x1) + -0.1829338 * x2) + (0.0008776 * x3) + (7.1801728 * x4) + (7.5429676 * x5), where x1 is the UPCR prior to therapy, x2 is the CD19 (%) value prior to therapy, x3 is the sBAFF value prior to therapy, x4 is the status of vasculitis prior to therapy, x5 is the rash prior to therapy. Further validation is required for clinical use. Additional validation is required for usage in clinical practice.
Figshare: main dataset (sheet1), ip10 (sheet 2), baff (sheet 3). https://doi.org/10.6084/m9.figshare.22179919.v4. 21
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The author expresses their deep gratitude to the Boards of Directors of Sardjito General Hospital, Panti Rapih Hospital, and Karyadi General Hospital in Semarang for their approval and support in using patients in this study. The author extends their heartfelt appreciation to all participants who contributed to the success of this research. The author would also like to extend their thanks to Prodia Laboratory and the Clinical Pathology Laboratory of the Faculty of Medicine, Nursing, and Public Health at Gadjah Mada University for their invaluable support in conducting the laboratory examinations. The author acknowledges Mrs. Dewi and Mr. Adi for their assistance and support throughout the research.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Autoimmune Diseases
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Rheumatology, Autoimmunology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 16 Aug 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)