Keywords
Antioxidant activity, Flavonoids, Geopropolis, Hepatoprotection, Stingless bees, Ecology and conservation, Biochemistry and chemical ecology, pollination
Antioxidant activity, Flavonoids, Geopropolis, Hepatoprotection, Stingless bees, Ecology and conservation, Biochemistry and chemical ecology, pollination
Treatment of liver diseases is presently one of the major medical challenges, and the development of alternative therapies is of paramount importance, with emphasis on the investigation of the potential liver protection from natural products (Cogliati et al., 2016). Propolis has great relevance in Brazilian popular medicine for the treatment of various diseases. The interest of the scientific community is growing and a wide variety of scientific experiments are in course, providing evidence that propolis contains bioactive compounds, such as phenolic compounds, which are known to exert antioxidant activity and thus protection against oxidative stress, besides anti-inflammatory, antineoplastic, immunomodulatory, and wound-healing properties (Silva et al., 2014; Bonamigo et al., 2017).
Melipona subnitida Ducke (“jandaíra”; Meliponinae, Apidae) is a stingless bee endemic from the northeast Brazil, where it is important for the pollination of species of the caatinga, an exclusive dry forest ecosystem of Brazil. Melipona subnitida geopropolis has bioactive substances with antioxidant and anti-inflammatory activity (Souza et al., 2018). It is hypothesized that it may also prevent or minimize damages to liver cells subjected to hepatotoxic substances. Paracetamol, also known as acetaminophen, is widely used as an analgesic and antipyretic in Brazil. Prolonged use or even the ingestion of high doses of this drug may cause hepatotoxicity in humans and animals, due to increased lipid peroxidation in the liver, causing membrane disintegration and hepatocellular necrosis (Brayner et al., 2018).
Acetaminophen intoxication is a useful experimental model capable of causing severe liver damage after acute administration, resulting in severe histological lesions and changes in the serum biochemical profile (Junapudi et al., 2018). The present study aimed to evaluate the effectiveness of geopropolis produced by M. subnitida as a hepatoprotector and preventive of liver damage experimentally induced in Wistar rats by acetaminophen.
The experimental protocol was submitted to the Animal Ethics Committee (CEUA) of the University of the State of Rio Grande do Norte (UERN), under protocol number 006/15. We declare that all phytosanitary and environmental efforts were taken, aiming at the welfare of the animals involved in the research. We also emphasize that no effort was spared so that the animals were admitted for euthanasia in the most humane way possible, according to the National Council for the Control of Animal Experimentation (2015) Euthanasia Practice Guideline.
Geopropolis produced by stingless bees M. subnitida Ducke was collected from apiaries in the city of Mossoró, Rio Grande do Norte, located at 37°20′39″ West and 05°11′15″ South. The climate of the region is warm semi-arid tropical type, with annual average temperature 27°C, average air humidity 50% and annual average rainfall 500 mm.
Portions of geopropolis were collected from several hives and combined to make up a mass of 100 g. The pooled geopropolis was powdered with a blender and transferred to a flat bottom balloon. The extraction was performed by adding 300 mL of 80% ethanol and leaving the flask standing at room temperature in the dark, for 10 days (GARCIA et al., 2004). The extract obtained was then concentrated at 40°C under reduced pressure. The concentrated extract was dried in a ventilated oven at 50°C.
The dried hydroethanol geopropolis extract (HPE) was diluted in methanol HPLC-degree to a concentration of 1 mg/mL, then filtered through a 0.45 μm filter and an aliquot of 10 μL of the solution injected into a HP 1260 (Agilent Technologies) HPLC chromatograph equipped with diode array detector (DAD) using a Zorbax Eclipse Plus C18 column (4.6 × 150 mm, 3.5 μm) at 40°C. The solvents used were 0.1% acetic acid (A) and MeOH (B). The following gradient was used, based on the concentration the solvent B: 0-10 min, 10-20%; 10-20 min, 20-40%; 20-30 min, 40-50%; 30-38 min, 50-60%; 38-48 min, 60-65%; 48-55 min, 65-75%. The flow was set at 1,0 mL/min and the column temperature at 40°C. The DAD detector was adjusted for detection at 210 and 400 nm.
The constituents of HPE were characterized by the HPLC-DAD-ESI-MS/MS technique, according to Ferreira et al. (2017a). A Shimadzu DADSPD-M10AVP chromatograph was used, equipped with a degasser, two LC-20AC pumps, CTO-20A furnace column, SIL 20AC auto-injector and SPD-20A diode array detector, adjusted to 210 and 400 nm. The chromatograph was coupled to an Esquire 3000 Plus Bruker Daltonics mass spectrometer, equipped with a quadruple ion capture mass analyzer and hardware components controlled by the CBM-20A software. The analyses were performed with a Phenomenex Gemini C-18 reverse phase column (4.6 × 150 mm, 3.5 μm), protected by a guard column. The same gradient program, solvents and flow rate were applied, as described above.
The full mass acquisition was obtained by electrospray ionization in the positive and negative modes in the m/z interval 100-1200. Helium was used as collision and nitrogen as nebulizing gas, with assistance of a coaxial nitrogen coating gas, at a pressure of 27 psi. The desolvation was assisted using a counter-current nitrogen flow fixed at 7.0 L min-1 flow and capillary temperature of 320°C. Data dependent MS/MS events were performed on the most intense ions detected at full MS scan. The maximum time of ion capture accumulation and the MS numbers to obtain the mean MS spectra were set at 30 and 3 MS, respectively.
To evaluate the HPE antioxidant activity, the in vitro photocolorimetric method of the free radical DPPH (2,2-diphenyl-1-picryl-hydrazil) was used. A set of tubes containing ethanol solutions of HPE at concentrations: 100, 50, 40, 20, 5, and 2 ppm, and free radical DPPH at 220 μg/mL. After 20 min in the dark, absorbances were read at 518 nm, using a Shimadzu UV 1601 UV-vis spectrophotometer. All readings were performed in triplicate, and the percentages of antioxidant activity were calculated, using the formula: %AA = 100 - [(Aa - Ab) × 100]/Aa, where: %AA = percentage of antioxidant activity; Aa = absorbance of the DPPH solution; Ab = absorbance of the solution containing HPE plus DPPH (MENSOR et al., 2001).
The research was carried out in partnership with the State University of Rio Grande do Norte (UERN), developed in the Central Animal Facility, using 24 rodents of the species Rattus norvegicus Berkenhout, 1769, Wistar strain, from the institution’s own bioterium, the animals chosen were healthy, with 60 days of age and weighing approximately 200 g. The animals were divided into 04 groups and conditioned in boxes (06 animals per box) made of polycarbonate, lined with wood shavings and kept in a room with a 12 hour light cycle, controlled aeration, exhaustion and acclimatization (23°C), receiving balanced feed (23% crude protein, 4% total lipids, 5% fiber and 12% minerals) and water ad libitum.
The choice of sample size was made using as a basis the work of Tzankova et al. (2019), who also worked on hepatoprotective activity in this same animal model.
The animals were randomly distributed in four experimental groups with six animals each. In order to avoid potential confounding factors in the results, animals of the same age and weight range were used. In addition, raffles were carried out to compose the experimental groups, randomizing the animals to one of the intervention groups or the control group. GI was treated neither with HPE nor acetaminophen (negative control); G2 received 50 mg/kg HPE; G3 received 50 mg/kg HPE and 600 mg/kg acetaminophen; G4 received 600 mg/kg acetaminophen (control).
The control and identification of the animals was done by separating the groups into boxes, identifying them by numbering, so that each one contained a different group. Aiming at daily handling, other boxes were used to separate the animals from each group, ensuring safety in administering the substances to the animals, avoiding repetition. This control was under the supervision of the researchers responsible for the study.
The administrations of water, HPE, and acetaminophen occurred by gavage once a day on the laboratory premises. The methodology for the application of the acetaminophen and extract was based on Said (2001). For eight consecutive days, drinking water and HPE were administered, and on the eighth day acetaminophen was administered one hour after drinking water and HPE administration. The animals were divided into 04 groups consisting of 06 animals: G1 (Negative Control): drinking water; G2: 50 mg/kg of geopropolis hydroethanolic extract; G3: 50 mg/kg of geopropolis hydroethanolic extract, together with the last administration (1 hour after), received a dose of 600 mg/kg of acetaminophen (200 mg/mL, Paracetamol, EMS S/A, Brazil); G4: (Positive control): drinking water, next to the last administration (1 hour after), they received a 600 mg/kg dose of acetaminophen (200 mg/mL, Paracetamol, EMS S/A, Brazil). For administration of the hydroethanolic extracts of M. subnitida D., a pool was made with the six extract samples at a concentration of 45 mg/mL.
The animals were weighed on the first and last day of administration and their physical characteristics were monitored daily.
The animals were anesthetized with a dose of Ketamine (10 mg/kg, i.p.) and Xylazine (10 mg/kg, i.p.) 48 hours after the last administration of HPE and acetaminophen. Once the anesthetic procedure was completed, celiotomy was performed on the alba line to access the hepatic vein, 3 mL of blood from each animal was collected and transferred to tubes without ethylenediaminetetraacetic acid (EDTA) addition.
The material was packed in thermal boxes and sent for biochemical analysis, which was used to determine blood markers of liver damage: alanine aminotransferase (TGP), aspartato aminotransferase (TGO), albumin (ALB), urea (URE), total protein (TP), glucose (GLC) and total triglycerides (TRIG). The biochemical analyzes were performed in the clinical pathology laboratory of the Federal Rural University of the Semi-Arid - UFERSA, using commercial reagents (VIDA Bioteconologia®) and spectrophotometric analyses.
The animals were induced into deep anesthesia using ketamine (10 mg/kg) and xylazine (10 mg/kg) intraperitoneally, and after that they were euthanized by exsanguination according to the National Council for the Control of Animal Experimentation (2015) Euthanasia Practice Guideline. Upon death, the animals were submitted to necropsy using the technique proposed by Vasconcelos (1996). The livers were submitted to macroscopic examination, and observation of possible changes in size, color, consistency, contours, presence of hemorrhages, and necrosis.
The livers were washed with 0.9% NaCl solution, fixed in 10% formalin buffered solution and routinely processed for histology after paraffin inclusion and attainment of cuts 5 μm thick and hematoxylin-eosin (HE) staining. The histological sections were examined for the presence of vacuolar degeneration of the cells that compose the hepatic parenchyma, infiltration of inflammatory cells and hepatocyte necrosis. The macroscopic and microscopic analyses were performed by two experienced and independent evaluators.
The data obtained from serum biochemical parameters (TGO, TGP, albumin, urea, glucose, triglycerides and TP) were expressed as mean values ± standard deviation, as well as minimum and maximum values using SAS software statistics version 8.0. The influence of geopropolis concentrations (dry matter) on antioxidant activity, total phenols and flavonoids was determined by Spearman correlation. After analysis of parametric assumptions, statistical differences between the different experimental groups were obtained by analysis of variance (One-Way ANOVA) followed by Tukey’s test. The percentage data had an arc-sine transformation √x. When the Gaussian distribution was stopped, the values were compared by the Kruskal-Wallis test. Values of p < 0.05 were considered significant.
HPLC-DAD-ESI-MS/MS analysis revealed that HTP has a great diversity of constituents, most of which belong to the class of phenolic compounds. such as chalcones (Isoliquiritigenin, 4,5′,6′-Trihydroxy-2′,4′-dimethoxy chalcone, 4,5′,6′-Trihydroxy-2′,4′-dimethoxy chalcone, 2′,4′-Dihydroxy-4-methoxy chalcone, 4′-Methoxy-4,2′,6′-trihydroxy chalcone, Mandelic acid acetyl-rhamnoside), flavones (Apigenin, Chrysoeriol, 5,3′,4′-Trihydroxy-6,7-dimethoxy flavone, 5,4′-Dihydroxy-6,7-dimethoxy flavone, Naringenin, 3-O-Methyl galangin), flavonols (Quercetin, Kaempferide, Rhamnetin, Myricetin-3,7,3′-trimethyl ether, Quercetin-dimethyl ether, Kaempferol dimethyl ether, Kaempferol dimethyl ether, Quercetin-dimethyl ether, Quercetin-3,7,3-trimethyl ether, Kaempferol methyl ether coumaroyl-arabinoside. The following non-flavonoid phenol compounds have also been identified (4-Demethyldeoxypodo-phyllotoxin-4-O-glucoside, 4-Methoxy-3-prenyl benzoic acid acetyl-glucoside, 4-O-Vinyl-3,5-diprenyl coumaric acid, 4-O-Ethoxy-3,5-diprenyl coumaric acid, 4-Methoxy-3-prenyl benzoic acid acetyl-rhamnoside).
Biochemical analysis showed that the values of the enzymes TGO and TGP and urea increased significantly in groups G3 and G4 compared to groups G1 and G2. However, the levels of these biochemical parameters were lower in G3 compared to G4 (Table 1).
HPE: hidroethanol geopropolis extract from Melipona subnitida Ducke.
| Parameters1 | Experimental groups2 | Reference values3 | |||
|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | ||
| TGO | 108 ± 23.01c | 111.8 ± 26.4c | 189 ± 32.32b | 267.75 ± 56.72a | 81-180 U/L |
| TGP | 70.67 ± 9.81c | 78.17 ± 10.55c | 113.6 ± 26.52b | 174.0 ± 51.59a | 74-143 U/L |
| Albumin | 7.36 ± 0.32a | 7.63 ± 0.19a | 7.7 ± 0.24a | 7.0 ± 0.25a | 3.4-4.8 g/dL |
| Urea | 58.2 ± 9.47b | 55.17 ± 3.6b | 56.0 ± 5.13b | 83.02 ± 9.36a | 30-42 mg/dL |
| Glucose | 96.6 ± 17.02a | 99.0 ± 15.82a | 93.29 ± 10.58a | 100.67 ± 10.35a | 79-144 mg/dL |
| Triglycerídes | 51.67 ± 12.95a | 55.75 ± 29.67a | 58.0 ± 23.13a | 59.50 ± 24.56a | 42-160 mg/dL |
| TP | 6.93 ± 0.65a | 7.47 ± 0.21a | 7.97 ± 0.58a | 7.73 ± 0.48a | 5.5-10.4 g/dL |
3 Based on Melo et al. (2010).
The rats in groups G1 and G2 had morphologically normal livers with no anatomical changes (Figure 1A and B). In the livers of the rats in the G3 group, focal pale areas and small spots of hemorrhage in the capsule were observed (Figure 1C). The rats in the G4 group had diffusely pale livers with marked hemorrhage in the capsule and a friable consistency (Figure 1D).
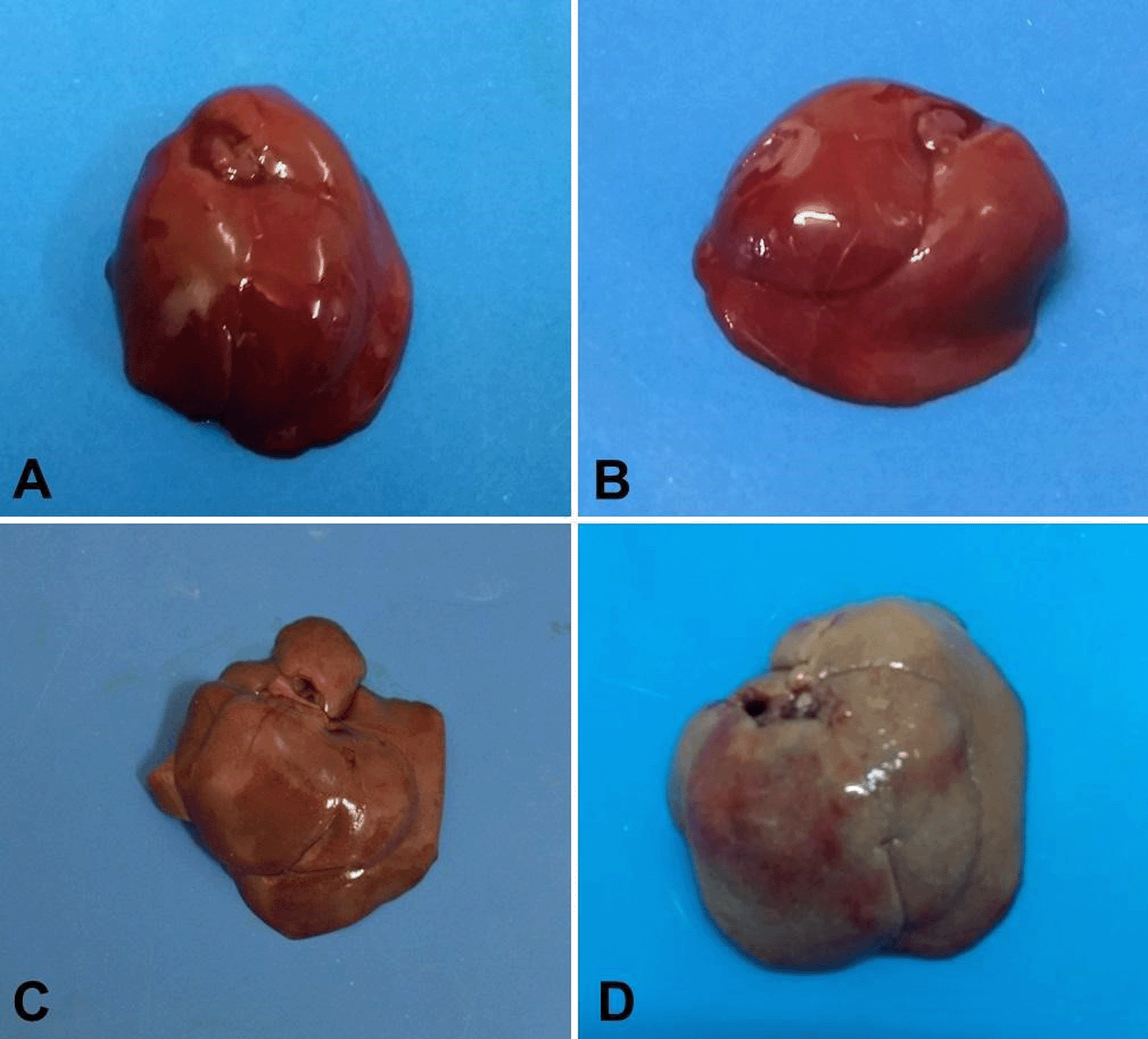
Normal morphologic aspect is noted on livers A (G1) and B (G2); C (G3) has pale brown color and discrete hemorrhage; D (G4) is markedly pale and hemorrhagic suffusions are seen on the capsule.
Histopathological evaluation showed that the liver tissue of G1 and G2 showed no changes and had normal histopathological aspects (Figure 2A and B). In G3, the histopathological examination revealed foci of swelling, vacuolization (hydropic degeneration) and necrotic hepatocytes, as well as mild infiltration of neutrophilic and monocytic inflammatory cells. Moderate dissociation of hepatocyte strands and sinusoidal congestion was also found (Figure 2C). Histological changes were more intense in the livers of G4, with loss of the architectural pattern of the organ, evidenced by diffuse degeneration and necrosis of hepatocytes, several foci of hemorrhage, marked dissociation of hepatocyte cords, and intense infiltration of inflammatory cells such as neutrophils, monocytes, and macrophages (Figure 2D).
The composition of HPE is similar to the composition of the geopropolis of Scaptotrigona aff. depillis (Ferreira et al., 2017b), in that both contain methoxylated flavonols and chalcones as predominant constituents. The present study represents the first report of the presence in geopropolis produced by the bee Melipona subnitida Ducke of constituted with lignan 4-desoxypodophyllotoxin 4-O-glucopyranoside. The literature reports that this chemical constituent presents antineoplastic activity (Zilla et al., 2014).
The antioxidant capacity of HPE, with IC50 48.0 μg/mL, represents high protection against oxidative stress. According to Melo et al. (2010), IC50 values below 50 μg/mL are considered highly active. Geopropolis of other species of Melipona also have shown high antioxidant activity; for example samples of geopropolis of Melipona fasciculata from the State of Maranhão (northeast Brazil) exhibited IC50 values in the range 2.24 μg/mL - 44.24 μg/mL (Silva et al., 2016).
The evaluation of serum biochemical parameters, as well as the macroscopic and histological aspects of the livers of Wistar rats showed that the HPE analyzed exerted a hepatoprotective effect by inhibiting hepatotoxicity induced by acetaminophen, a drug known to cause hepatocellular necrosis and elevation of serum levels of TGO and TGP enzymes (Lopes and Matheus, 2012). In the livers of G3 animals, it was possible to observe a reduction in the intensity of degenerative and necrotic lesions occurring in hepatocytes, as well as decreases in the mean values of serum levels of TGO, TGP and urea enzymes. The liver enzymes TGP and TGO can be measured in the blood and reflect the status of liver function. In cases of hepatocyte lesions, increases in their serum levels occur (Jesus et al., 2014).
The results of this investigation corroborate the previously reported results. Said (2001) demonstrated the hepatoprotective effect of the hydroethanol extract of Apis mellifera propolis. In this study, the author used acetaminophen to induce liver lesions in Swiss rats. The author found that the group of rats that received the extract at doses of 50-400 mg/kg showed no lesions in the hepatocytes and the liver tissue showed histological pattern within normality.
The macroscopic and histological evaluation of the livers of G2 animals shows that the oral administration of HPE does not exert hepatotoxicity, indicating that the product is safe at 50 mg/kg. A study using isoniazid and rifampicin (100 mg/kg, i.p.) for induction of hepatic lesion in albino rats revealed no toxicity after five days of treatment with ethanol extract of propolis of A. mellifera (200-440 mg/kg, orally). The experimental group of rats that received the extract showed no serum biochemical changes, as well as no histological changes on the liver (Wali et al., 2015).
Among the constituents of propolis that have proven medicinal effects are flavonols, such as quercetin and kaempferol, which were detected as constituents of the propolis evaluated in the present study. These compounds, in joint action, demonstrate high anti-inflammatory potential by modulating the action of cellular components involved in the mechanism of inflammation (Vaher and Koel, 2003). Chalcones is another chemical constituent found in HPE that should also be mentioned for its biological activity. Chalcones have been shown to exert a variety of cytoprotective and modulatory functions as well as beneficial roles in inflammatory processes (Kontogiorgis et al., 2008).
According to Lahsasni et al. (2014) chalcones are among the promising substances for use as pharmaceuticals. Kontogiorgis et al. (2008) demonstrated that chalcones exert a variety of cytoprotective and modulating functions as well as inflammatory activity. The results obtained in the present study suggest that the observed hepatoprotective and anti-inflammatory effects are associated with HPE flavonoids.
After analysis it was observed that the hydroethanolic extract of geopropolis produced by M. subnitida showed hepatoprotective activity in the liver of Wistar rats, indicated by a decrease in the severity of acetaminophen-induced macroscopic and microscopic tissue alterations, in addition to a decrease in the level of TGO, TGP and urea enzymes. Additionally, HPE showed a great variety of phenolic compounds including chalcones, flavones and flavonols besides excellent antioxidant activity. In this sense, in order to improve the methods of use of geopropolis in the treatment of liver diseases caused by different agents, further studies on this subject should be conducted to evaluate the safety and viability of the methods used, so that later the geopropolis produced by M. subnitida can be used in the treatment of humans and other animal species, after undergoing clinical trials.
Figshare: Seric biochemical parameters, https://doi.org/10.6084/m9.figshare.23596476.v1 (Paiva et al., 2023a).
The project contains the following underlying data:
• Seric biochemical parameters.docx [Seric biochemical parameters (means ± SD) obtained with Wistar rats (Rattus norvegicus Berkenhout, 1769).]
Figshare: Photomicrographs of livers of Wistar rats, https://doi.org/10.6084/m9.figshare.23600130.v1 (Félix et al., 2023a)
The project contains the following underlying data:
• Photomicrographs of livers.docx [Photomicrographs of livers of Wistar rats (Rattus nervegicus Berkehout) stained with hematoxylin and eosin.].
Figshare: Macroscopic aspect of livers of Wistar rats, https://doi.org/10.6084/m9.figshare.23600103.v1 (Félix et al., 2023b).
The project contains the following underlying data:
Repository: ARRIVE checklist for ‘Composition and hepatoprotective effect of geopropolis of Melipona subnitida’, https://doi.org/10.6084/m9.figshare.23620488.v1. (Paiva et al., 2023b).
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Medicinal chemistry, Pharmacy
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neuroscience, natural and alternative medicine, neurosurgery
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 17 Aug 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)