Keywords
Fibroblasts, Fibroblast heterogeneity, Extracellular Matrix (ECM), Immunology, Macrophages, BigH3, Cthrc1
This article is included in the Cell & Molecular Biology gateway.
This article is included in the Advances in Fibroblast Research collection.
Fibroblasts, non-hematopoietic cells of mesenchymal origin, are tissue architects which regulate the topography of tissues, dictate tissue resident cell types, and drive fibrotic disease. Fibroblasts regulate the composition of the extracellular matrix (ECM), a 3-dimensional network of macromolecules that comprise the acellular milieu of tissues. Fibroblasts can directly and indirectly regulate immune responses by secreting ECM and ECM-bound molecules to shape tissue structure and influence organ function. In this review, we will highlight recent studies which elucidate the mechanisms by which fibroblast-derived ECM factors (e.g., collagens, fibrillar proteins) regulate ECM architecture and subsequent immune responses, with a focus on macrophages. As examples of fibroblast-derived ECM proteins, we examine Collagen Triple Helix Repeat Containing 1 (CTHRC1) and Transforming Growth Factor-β-inducible protein (TGFBI), also known as BIGH3. We address the need for investigation into how diverse fibroblast populations coordinate immune responses by modulating ECM, including the fibroblast-ECM-immune axis and the precise molecular mediators and pathways which regulate these processes. Finally, we will outline how novel research identifying key regulators of ECM deposition is critical for therapeutic development for fibrotic diseases and cancer.
Fibroblasts, Fibroblast heterogeneity, Extracellular Matrix (ECM), Immunology, Macrophages, BigH3, Cthrc1
Based on the reviewers’ comments, the authors have updated the text and figures providing additional written information regarding the relevance of fibroblast heterogeneity in tissue architecture and disease states; the contribution of metabolic factors to immune cell – fibroblast crosstalk; and diverse fibroblast contributions to wound healing. Moreover, additional information has been provided related to BIGH3, including its similarities to Periostin; the tissue specific functions of BIGH3; and the alternative mechanisms by which it regulates collagen deposition. Aligned with this, all figures and figure legends have been modified to reflect the updated text. These changes include the addition of fibroblast-derived metabolic factors to Figure 1; the representation of different fibroblast subsets and effect modifiers in Figure 2; the addition of alternative mechanisms for BIGH3-mediated regulation of ECM turnover in Figure 3; and a new graphical representation in Figure 4.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Fibroblasts orchestrate ECM deposition in steady state and disease: Fibroblasts are non-hematopoietic mesenchymal stromal cells which are found in all tissues, regulate tissue structure, and influence the phenotype and localization of tissue resident cell types.1–3 As a result, fibroblasts play a critical role in tissue homeostasis and tissue repair. Fibroblasts accomplish these functions in part by producing and secreting extracellular matrix (ECM) proteins, which alter the structure and composition of the composite tissue ECM.3 Here, the ECM refers to the diverse network of proteins that generates the three-dimensional structure of tissues.4–6 In addition, fibroblasts secrete a variety of molecules, including cytokines and chemokines, which can bind the ECM to influence immune cell function and recruitment.2,3,7
Under homeostatic conditions, fibroblast-derived ECM proteins provide essential support to resident cells and tissues. However, dysregulation of the ECM can contribute to pathogenic outcomes, including fibrosis. When tissues are injured, local tissue fibroblasts become activated and increase their contractility, secretion of inflammatory mediators, and synthesis of ECM components.3,8 These changes initiate the wound healing response.1 When damage is limited and non-repetitive, wound healing is efficient, a transient increase in the deposition of ECM components transpires to facilitate the restoration of functional tissue architecture.2 However, when injury is repetitive or severe such as in chronic inflammatory diseases, ECM components continue to accumulate, which can lead to structural alterations of the ECM, disruption of tissue architecture, organ dysfunction, and ultimately, organ failure.1,4 In these situations, fibroblasts produce excessive ECM, and changes to the fibrillar collagen network result in pathological tissue stiffness, loss of mechanical compliance, and loss of tissue function.2 As a result, fibrotic disease affects almost all organ systems and is a contributing factor in 45% of all deaths in high income countries.8,9 Therefore, an appreciation of the interconnectivity between fibroblasts, the ECM, and immune cells is required to understand the causality of fibrotic disease.3 Here we integrate the current understanding of the fibroblast-ECM-immune axis and review the evidence supporting current models defining fibroblast-immune interactions. These studies serve as the basis for future exploration into immune cell regulation by fibroblast-derived ECM molecules.
ECM form & function: The ECM is a dynamic 3-dimensional network of more than 300 different core proteins and matrix-modifying enzymes (often referred to as the matrisome).5,6,10 These components are produced and assembled by fibroblasts and may either be long lived or transient, making ECM dynamic and sensitive to local and systemic changes.2 Components of the ECM include collagens, proteoglycans (PGs), glycosaminoglycans (GAGs), elastin and elastic fibers, laminins, fibronectin, mucus and other proteins and glycoproteins, such as matricellular proteins.2,4,5 The diversity of proteins in these functional groups is immense. For example, there are approximately 28 types of collagens, molecules which provide structural support to tissues. These components may also combine for additional diversity. For example, fibril-forming collagens have abilities to form covalent cross-links and create matrix structures and include collagens I, II, III, V, and XI. These ECM components have a variety of disparate functions while providing architectural support to tissues. For example, collagens provide tissue strength and resilience, PGs form hydrated gels which cushion tissues, and mucus protects barrier surfaces.2,4 In addition, these components contribute to fundamental processes for tissue development, including cell proliferation, survival, migration, differentiation, autophagy, and angiogenesis.4 These concepts have been reviewed extensively elsewhere.4
There are a variety of other fibroblast-derived molecules and enzymes which modify ECM structure and regulate their degradation, such as matrix metalloproteinases (MMPs).1 Moreover, fibroblasts produce molecules which bind ECM components to alter the composition and function of ECM-resident cells. For example, fibroblasts produce and secrete cytokines, chemokines, and growth factors, which are an implicit part of immune cell regulation.2 It is also notable that immune cells are often connected to the unique matrix surrounding them, and every cell to varying degrees is coated in a glycocalyx, a complex network of sugar rich molecules either free or bound to proteins and/or lipids.2,11 As a result, the ECM regulates the signaling, functions, properties, and morphology of residing immune cells in addition to providing structural support for tissues.4
These properties may be altered in a tissue-specific manner.3 Specific ECM phenotypes configure the different tissues, including epithelial, muscle, connective, and more to meet the requirements for optimal tissue function.4,12 Here, the prevalence of different, tissue-specific heterogeneous fibroblast subsets with unique gene expression signatures can further alter the ECM and therefore tissue characteristics.13 Aligned with this, Muhl et al. used a scRNA-seq approach to reveal that fibroblast heterogeneity in ECM and matrisome production was a function of tissue specific differences in the heart, skeletal muscle, colon, and bladder.14 Finally, fibroblasts have unique functions depending on disease states. Fibroblasts that become activated due to repeated injury and chronic inflammation are typically referred to as myofibroblasts and have altered gene expression profiles that drive fibrotic disease. For example, Korunsky et al. performed cross-disease single cell RNA sequencing study of human inflammatory fibroblasts and demonstrated that two different myofibroblast subsets were preserved across Sjogren’s syndrome, interstitial lung disease, ulcerative colitis, and rheumatoid arthritis. Here, CXCL10+CCL19+ inflammatory fibroblasts localized with IFN-, TNF- and IL-1- producing T cells, whereas SPARC+COL3A1+ fibroblasts colocalized to a perivascular niche with elevated levels of TGF- and Notch ligands.15 Below, we highlight critical immune-facing functions of fibroblast- and myofibroblast-derived ECM components in steady-state and disease.5
Fibroblasts produce cytokines and chemokines which bind the ECM and are critical for immune cell recruitment and function in steady-state and disease2,16 ( Figure 1). For example, multiple in vitro and tissue-restricted in vivo studies have demonstrated that a relationship exists between fibroblasts and macrophages, an innate immune cell which orchestrates long-lived adaptive immune responses through phagocytosis, antigen presentation, and immunological mediator secretion.16 Fibroblasts and macrophages are found in almost all tissues and multiple studies indicate that fibroblast-derived CSF1, which can decorate the ECM, is a critical factor for macrophage survival. Using in vitro studies, Zhou et al. demonstrated that macrophages and fibroblasts form stable cell circuits that are resistant to perturbations and that cell-to-cell contact increased cell circuit survival because of local exchange of growth factors, including CSF1.17 In addition, in vivo studies have demonstrated that fibroblastic reticular cells (FRC) are required to establish the lymph node (LN) macrophage niche. D’Rozario et al. demonstrated that genetic ablation of FRC using a genetic tool in which Chemokine (C-C motif ) ligand 19 (Ccl19)-expressing cells could be depleted using diphtheria toxin resulted in rapid loss of monocytes and macrophages from LN in two separate in vivo models.18 Moreover, single-cell RNA sequencing (scRNA-seq) of murine brachial lymph nodes revealed that FRC subsets broadly expressed master macrophage regulator CSF1 and functional assays containing purified FRC and monocytes showed that CSF1R signaling was sufficient for macrophage survival.18 Comparative analysis demonstrated these effects were conserved between mice and humans.18
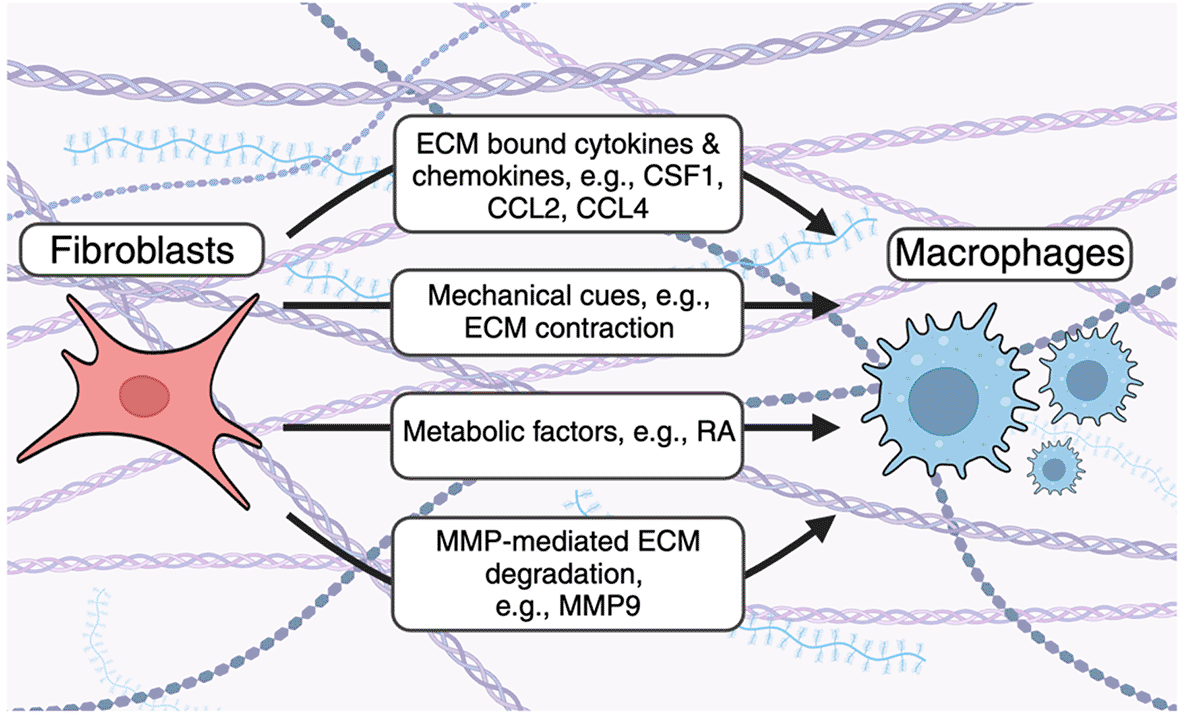
Fibroblasts use several mechanisms to recruit macrophages to the ECM. Fibroblast-derived cytokines and chemokines bind the ECM and enhance accumulation of ECM-associated macrophages. Fibroblast contraction remodels collagen fibers to enhance macrophage recruitment. Metabolic factors alter macrophage population dynamics and function. Fibroblasts secrete MMPs to cleave ECM- and ECM bound proteins to enhance macrophage accumulation in the ECM.
Monocytes, which can give rise to macrophages, have also been shown to require stromal cell derived CSF1. Emoto et al. demonstrated that CSF1 produced by sinusoidal endothelial cells was required for the survival of Ly6C- monocytes. Conversely, CSF1 produced by both endothelial and Lepr+ perivascular stromal cells were required for the survival of Ly6Chi monocytes.19 Taken together, these studies demonstrated that macrophage survival in specific tissues is regulated by stromal-derived CSF1. While all fibroblasts can generate CSF1, tissue-specific fibroblast subsets do exhibit different levels of CSF1 mRNA,20 so additional studies are required to delineate which subsets are critical for macrophage homeostasis.
Further investigation is required to determine the functional role of macrophages sustained by fibroblast-derived CSF1 in steady-state and disease. Previous work by Zhu et al. has demonstrated that a pharmacologically induced CSF1 blockade in mouse pancreatic tumour models results in increased antigen presentation and productive antitumour T cell responses.21 Despite this, the precise contribution of fibroblast-derived CSF1 in antitumour immunity is not well understood. Furthermore, the functional contributions of these macrophages in chronic inflammation and cancer also require subsequent study.
In addition to cytokines, activated fibroblasts, including myofibroblasts, produce ECM-binding macrophage chemoattractants indicating that fibroblasts are capable of recruiting monocytes and macrophages during injury, infection, and inflammation.22,23 For example, the bacterial ligand lipopolysaccharide (LPS), which activates toll-like receptor (TLR)4, has been shown to enhance expression of macrophage chemoattractants Ccl2 and Ccl4 in myofibroblast progenitor cells in a mouse model of liver fibrosis.22 As LPS-mediated TLR4 activation occurs widely in anti-bacterial immunity, this suggests that fibroblasts can respond to pathogen-associated molecular patterns (PAMP) to recruit immune cell subsets, including macrophages, in a variety of anti-infective contexts. However, the role of fibroblast-mediated macrophage recruitment in additional anti-infective immune responses, as well as chronic inflammation, requires future investigation.
Fibroblasts influence macrophage migration through mechanical cues. Previous studies have also demonstrated that fibroblasts can influence macrophage dynamics by regulating the physical mechanics of the ECM ( Figure 1). Hinz and colleagues showed that contractile myofibroblasts drive mechanical cues through local remodeling of collagen fibers resulting in increased macrophage migration directly towards myofibroblasts using 3D collagen gels in vitro.24 Migration occurred independently of chemotaxis and required macrophage-mediated attachment to collagen via the α2β1 integrin and stretch-sensitive ion channels.24 Similar observations have been described in several fibroblast-like cells,25–27 indicating the importance of these biomechanical mechanisms for cell communication and movement across tissues.
Fibroblast-derived matrix metalloproteinases degrade ECM proteins to enhance macrophage recruitment. Matrix metalloproteinases (MMPs) are a family of enzymes which degrade ECM proteins. These enzymes are either secreted or attached to cell surfaces, confining their activity to membranes, secreted proteins, or proteins within the extracellular space.28 MMPs have a complex role in regulating inflammation by acting on a multitude of immunological mediators, including antimicrobial host defense peptides, cytokines, chemokines, and ECM proteins as reviewed in detail here.28 In addition, matrix metalloproteinases are enhanced in stromal cells, including fibroblasts, in response to pro-inflammatory cytokines.29–31 As a result, modulation of the ECM during inflammation alters immune cell migration and ECM resident cell types, including macrophages ( Figure 1). For example, Shubayev et al. demonstrated that TNF-α-mediated MMP9 (also known as gelatinase B) production enhances macrophage recruitment in a model of peripheral nerve injury.32 Gong et al. demonstrated that mice deficient for the ECM-bound protease plasminogen (Plg) had decreased trans-ECM macrophage migration and decreased MMP9 activation.33 In addition, the authors demonstrated that MMP9 administration to Plg deficient mice resulted in increased macrophage accumulation in the ECM,33 suggesting that Plg activates MMP9 to increase ECM resident macrophages. Moreover, Tan et al. demonstrated that MMP9 can also cleave the ECM protein osteopontin (OPN) to enhance macrophage recruitment in a mouse model of renal fibrosis.34 Despite this, additional studies are required to understand how MMP-mediated degradation of other ECM components alters immune cell recruitment and fibrotic disease development.
In addition to cytokines, mechanical cues, and enzymatic means, fibroblast can regulate immune cell regulation through metabolic cues. Previous studies have demonstrated that metabolic factors are required for continuing collagen deposition during fibrotic disease. Schworer et al. demonstrated that the metabolic enzyme pyruvate carboxylase (PC) is required anaplerosis in tumour-associated fibroblasts for continuing collagen deposition in the glutamine and glucose scare tumour microenvironment (TME).35 Kay et al. also demonstrated that collagen deposition requires de novo synthesis of the amino acid proline, a major component of multiple collagens, from glutamine, by the enzyme pyrroline-5-carboxylate reductase 1 (PYCR1).36 Despite these findings, the impact that this had on immune cell, and specifically macrophage, infiltration into the TME was not investigated. To this end, some studies have investigated the interplay between metabolic factors and macrophage accumulation in various tissues. Buechler et al. demonstrated that Wt1+ stromal cells produce retinoic acid (RA) to maintain a population of GATA6+ Large Cavity Macrophages (LCM) in the peritoneal, pleural, and pericardial spaces.37 Similarly, Pucino et al. demonstrated that physiological concentrations of lactate in RA joints induces the IL-6 production in fibroblasts,38 which may enhance the differentiation of monocytes into macrophages.39 Taken together, these studies provide the rationale for future investigations on how metabolic factors alter the relationship between fibroblasts, ECM deposition, and macrophage accumulation.
In addition to fibroblast-derived components, immune-derived cytokines and growth factors play a critical role in fibrosis and fibrotic diseases by altering fibroblast heterogeneity and therefore ECM deposition. Below, we highlight the relationship between inflammation, fibroblast heterogeneity, and ECM deposition ( Figure 2). Moreover, we outline the role that modulators of growth factor signaling play in shaping ECM deposition and subsequent fibrotic disease.
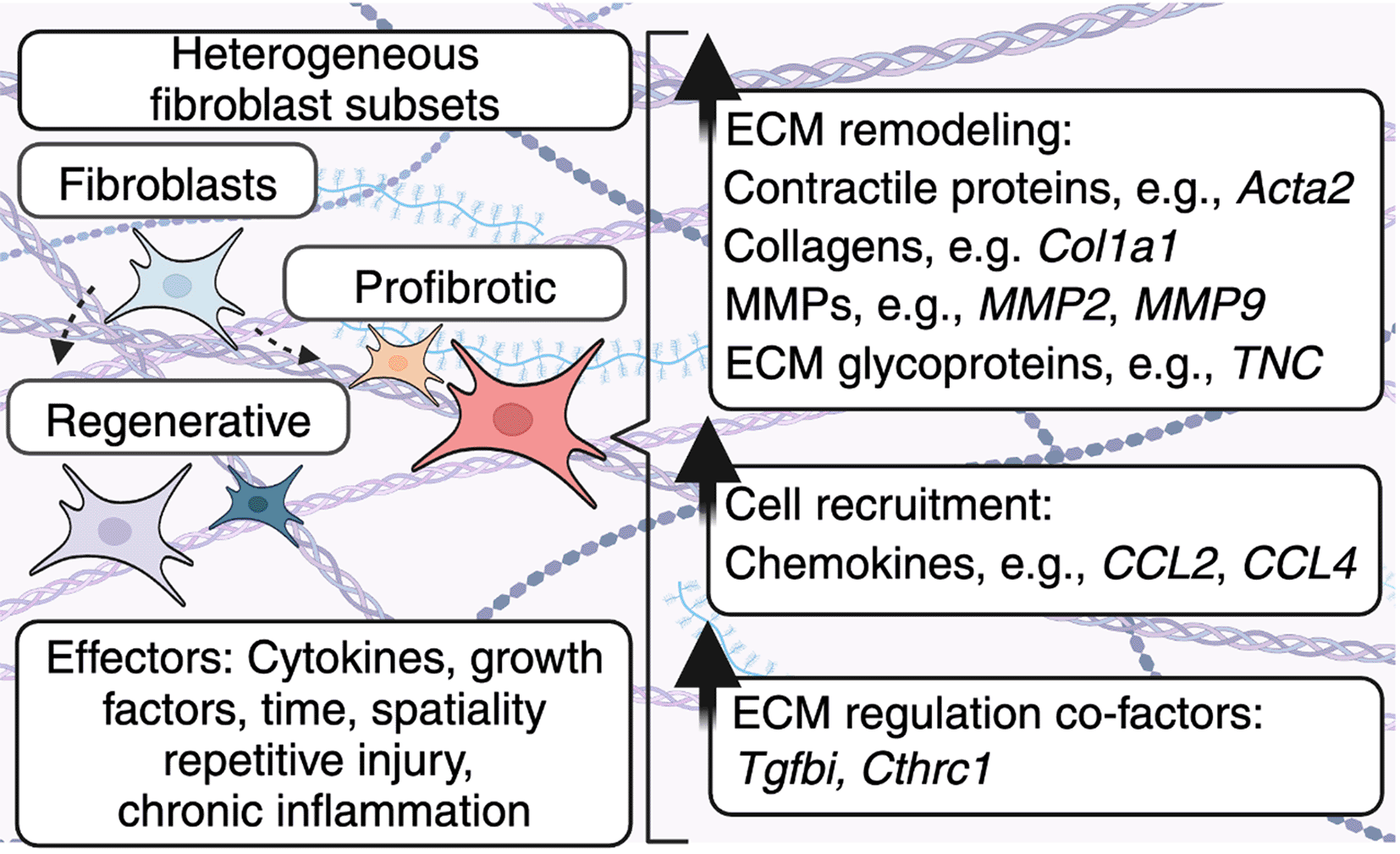
Fibroblasts become activated in response to cytokines and growth factors. These immune-derived signals are further modified by factors such as time, spatiality, repetitive injury, and chronic inflammation to adopt specific phenotypes, including profibrotic or regenerative, which alter the production of ECM components. As a result, these heterogeneous fibroblast subsets can alter the physical properties of the ECM, as well as ECM-resident cells. In addition, fibroblasts produce molecules, including TGFBI/BIGH3 and CTHRC1 which play multifaceted roles in regulating fibroblast-mediated ECM deposition.
ECM composition is regulated by diverse fibroblast subsets in health and disease. Here, pro-inflammatory cytokines, growth factors, and other ECM proteins can convert fibroblasts into myofibroblasts.40 Broadly, fibroblasts found in unperturbed tissues, myofibroblasts typically exhibit increased expression of contractile protein genes, such as alpha smooth muscle actin (Acta2)41 as well as other of ECM proteins.42
However, the full extent of fibroblast heterogeneity is much more extensive. Multiple single-cell -omics studies have demonstrated that fibroblasts are highly heterogeneous, especially within wounds. Guerrero-Juarez et al. highlighted the dynamic nature of fibroblast identities during wound healing and defined 12 different clusters/subtypes of fibroblasts that both coexisted within wounds and/or represented consecutive differentiation states with disparate expression profiles, including contractile and regenerative fibroblasts.43 Aligned with this Phan et al. also performed single-cell transcriptomic analysis in previously published wound-induced hair follicle neogenesis (WIHN) datasets and identified a population of regenerative fibroblasts marked by the expression of the retinoic acid binding protein Crabp1 which shared a gene signature with murine papillary fibroblast lineages which are required for hair follicle homeostasis.44,45 Similarly, Foster et al. used an integrated spatial multi-omics studies approach and highlighted the coexistence of different functional fibroblast subsets across space and time, including activated, responder, proliferator, remodeling, and mechanofibrotic fibroblasts which expressed Col1a1, Acta2, and Pdgfra.46
Identification of these subsets in wound healing is also translatable to identifying pathogenic fibroblast subsets across different disease states, such as cancer. For example, Wietecha et al. developed an in silico pipeline to identify genes and pathways to perform comparative analysis between kinetic healing and cancer transcriptomes. This comparison resulted in the identification of CAF in the inner tumour stroma similar to skin fibroblasts found in early wounds, which express collagen related genes, including Postn, Tnc, and Col12a1 regulated by the transcription factor RUNX2.47 These CAF were associated with increased severity in skin cancer, suggesting that understanding fibroblast heterogeneity and trajectories in wound healing may yield insights in other disease states.
The diversity of and trajectory of fibroblast subsets may be a function of immune-derived signals. Transcriptomic studies have demonstrated that macrophage:fibroblast crosstalk occurs in wound healing. Hu et al. performed in silico pathway analysis in a mouse model of wound healing to identify the macrophage-derived Oncostatin-M (OSM) pathway as a critical modulator of fibroblast function in early stages of wounds healing. Subsequent in vitro analysis demonstrated that OSM enhances the expression of Serpinb2, Serpine1, and Bnip3 in fibroblasts.48 Similar findings have been observed in lineage tracing studies. Building on previous experiments in mice which demonstrated that embryonic mesenchymal precursors expressing Engrailed (En1) or Delta-like homolog 1 (Dlk/Pref1) generate skin fibroblast and adipocyte lineages, Shook et al. demonstrated that the predominant population of ECM-producing myofibroblasts in the skin are adipocyte precursor cells (AP) derived from En1-lineage traced fibroblasts.49 In addition, this study demonstrated that wound bed myofibroblasts upregulated Acta2 and Col1a1 mRNA expression compared to fibroblast populations from uninjured skin.49 Further, the authors compared the transcriptional profiles of 2 major populations of fibrotic mesenchymal cells that are enriched in skin wound beds: APs and CD29high cells. APs had greater expression of ECM components/regulators including collagens Col5a2, Col14a1, as well as matrix metalloproteinases Mmp2, Mmp3, Mmp23, Mmp27. Conversely, CD29high fibroblasts expressed elevated levels of Col6a3, Col7a, Mmp13, and Tnc. Moreover, platelet-derived growth factor C (Pdgfc) secreted by CD301b-expressing macrophage triggered the proliferation of APs, but not other myofibroblasts, highlighting the role of immune-derived growth factors in shaping fibroblast heterogeneity. Similar findings related to macrophage:fibroblast crosstalk have been observed in human studies. For example, Ng et al. demonstrated that MERTK+ macrophages inhibit the inflammatory phenotype of capsular fibroblasts via integrin-mediated extracellular matrix remodeling.50 However, more human studies are required to determine the role of macrophages in altering wound healing and other disease contexts.
In addition to altering wound healing by secreted soluble cytokines and growth factors, macrophages promote the differentiation of regenerative or fibrotic fibroblast subsets by phagocytosing transcription factors. Gay et al. demonstrated that late stage wound macrophages phagocytize Wnt inhibitor SFRP4 to promote fibrogenesis over regeneration.51 Taken together, these studies highlight the diverse contributions of fibroblast subsets to wound healing by way of ECM deposition. Although select studies have elucidated how cytokines or growth factors alter fibroblast-mediated ECM deposition and therefore their respective fibrotic or regenerative phenotypes future work is required to define the precise mechanisms by which ECM deposition is regulated.
Transforming growth factor beta (TGF-β) is a major immune-derived growth factor which drives the expression of myofibroblast-associated genes. TGF-β is produced by a variety of cells, including macrophages, under inflammatory conditions and drives myofibroblast activation.16 In the canonical TGF-β pathway, Latent TGF-β is activated by av integrins in the ECM and binds to TGF-β receptor 2 on fibroblasts to drive the activation of intracellular kinases, including SMAD2/3, co-SMAD, and SMAD4 to upregulate ECM and myofibroblast-associated genes, such as Col1a1, Fn1 and Acta2.52,53 In addition, TGF-β can also signal through non-canonical signaling pathways and activate all three mitogen-activated protein kinase (MAPK) pathways and proteins, including extracellular signal-regulated kinase (ERK), p38 MAPK, and c-Jun-N-terminal kinase (JNK).52 TGF-β-mediated signaling through these pathways may occur in either a Smad-dependent or -independent fashion.52 TGF-β also activates Rho GTPase signaling and the PI3 kinase/Akt pathway.52 As TGF-β is crucial for myofibroblast development and ECM production, modulators of TGF-β-signaling may be co-factors that affect myofibroblast phenotypes and ECM production from these cells. We define co-factors as proteins that are required for optimal signaling of another protein, such as TGF-β. We propose that identifying co-factors to TGF-β-driven myofibroblast activation and pro-fibrotic ECM deposition may represent potential targets for therapeutic development. In the following sections, we highlight two potential immune-derived co-factor candidates: CTHRC1 and BIGH3.
Collagen triple helix repeat containing 1 (CTHRC1), a secreted ECM protein, has recently been identified as a critical modulator of ECM protein modulation and wound healing.54,55 Human CTHRC1 contains a N-terminal 30 amino acid hydrophobic signal peptide secretory domain, a short collagen triple helix repeat (CTHR) domain consisting of 12 repeats of the Gly-X-Y motif, and a highly conserved C-terminal domain with similar structure of the globular C1q domain of Collagen VIII.56,57 CTHRC1 is expressed in macrophages, myofibroblasts, endothelial cells as well as mesenchymal-derived cells58 and as a result, CTHRC1 expression occurs in multiple tissues, including the bone,59 the lung,60 and tumours.61
Previous studies suggest that CTHRC1 modulates TGF-β signaling ( Figure 3A). For example, Cthrc1 modulates TGF-β signaling by promoting the degradation of canonical signaling intermediates, including Smad2/3, and has been suggested to influence Wnt and b-integrin signaling to affect cancer cell proliferation and metastasis.58 Guo et al. showed that Cthrc1 modulated β-integrin signaling by enhancing phosphorylation of Focal adhesion kinase (FAK) to promote metastasis of epithelial ovarian cancer (EOC) cells.62 In fibroblast and smooth muscle cells, CTHRC1 levels are associated with increased cell migration in vitro.63 Other reports suggest that CTHRC1 may negatively regulate TGF-β-mediated signaling pathways. For example, Leclair et al. demonstrated that overexpression of CTHRC1 in smooth muscle cells reduced levels of phosphorylated Smad2/3, which is required for TGF-β-mediated gene expression and collagen production.64 As Cthrc1 expression is enhanced in response to TGF-β in vitro,63 mechanistic evidence suggests that TGF-β and CTHRC1 may act in a negative-feedback loop to limit TGF-β-induced ECM deposition. Due to conflicting reports on the role of CTHRC1 in a disease context, additional studies are required. Future queries delineating the precise relationship between CTHRC1 in TGF-β-mediated signaling and the role of CTHRC1 in a disease context must focus on cell- and tissue-specific mechanisms.
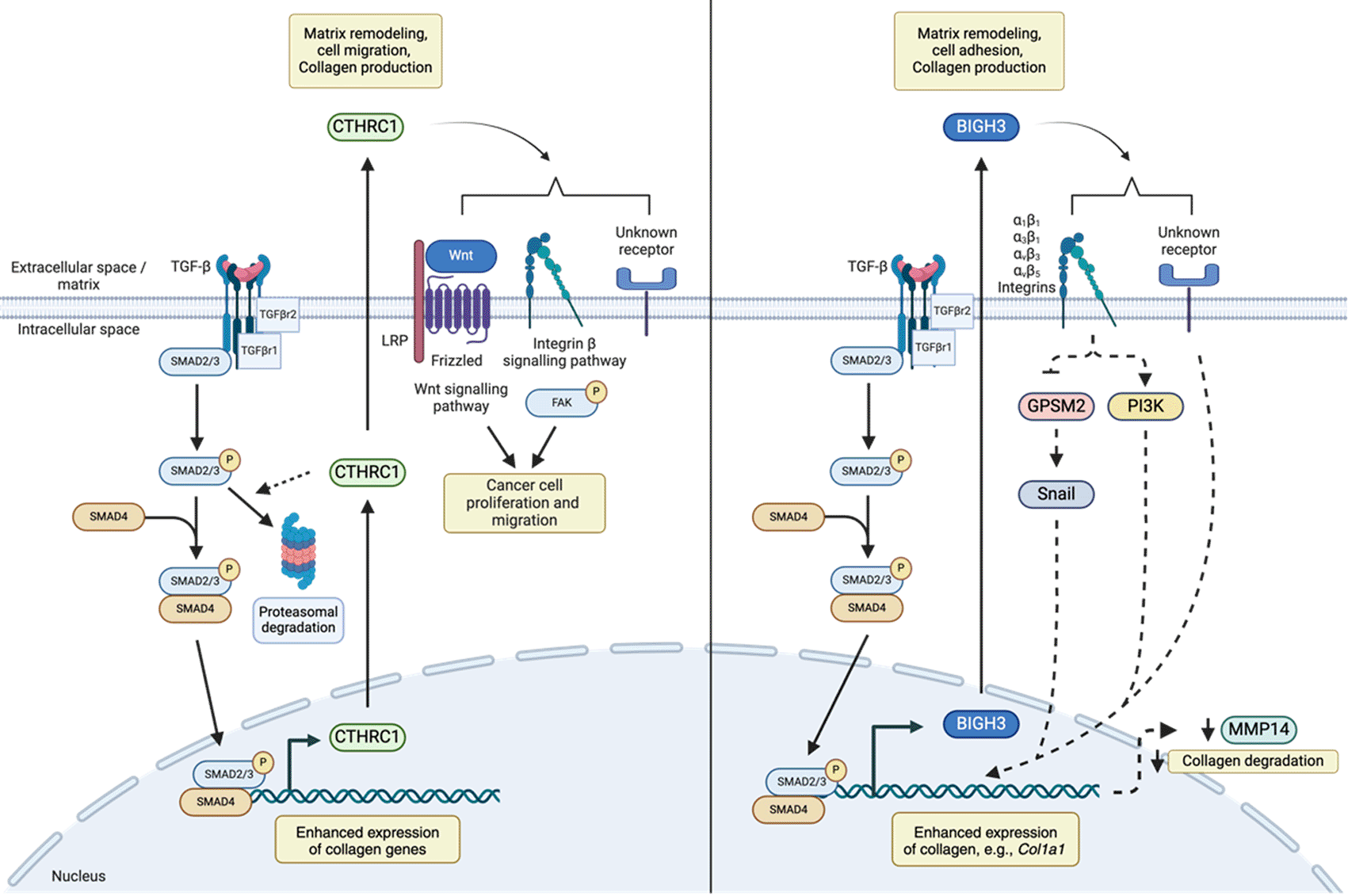
(Left) TGF-β signaling enhances production of extracellular matrix proteins, as well as CTRHC1, resulting in negative feedback loop limiting its own production. Secreted CTHRC1 can bind to collagens and integrins and has been implicated in cancer cell proliferation and migration by influencing Wnt and integrin β signaling pathways. (Right) TGF-β signaling enhances BIGH3 production which binds integrin receptors. Proposed pathways of BIGH3-mediated collagen production include activation of Snail and/or PI3K, transcription factors which influences collagen production as well as suppression of MMP14, which promotes collagen turnover. Legend: dashed lines represent prospective/predicted interactions.
CTHRC1 has been shown to mark lung myofibroblasts in the context of models of lung fibrosis in murine systems and idiopathic pulmonary fibrosis (IPF) in humans.20,60,65–67 Cthrc1 expression may also distinguish two disease-associated myofibroblast clusters across tissues.20 One disease-associated myofibroblast cluster is marked by the simultaneous expression of Lrrc15 (leucine-rich repeat 15) and Cthrc1 expression, whereas the other is only marked by Cthrc1 and not Lrrc15 expression.20 scRNA-seq-based characterization of collagen-producing cells in fibrotic mouse lungs demonstrated that the Cthrc1+ fibroblast cluster expresses the highest levels of ECM proteins, including Col1a1 and Col3a1.60 Tsukui et al. used transgenic mice to track and ablate Cthrc1-expressing fibroblasts in lung fibrosis. Lineage tracing using Ctrhrc1-CreER mice crossed to Rosa26-tdTomato mice demonstrated that Cthrc1-expressing lung fibroblasts expand in a mouse model of bleomycin-induced pulmonary fibrosis. Cthrc1-Cre-ER-labeled cells showed elevated expression of ECM genes, including Col1a1 and Postn (periostin). Further, depletion of Cthrc1-expressing lung fibroblasts using Cthrc1-CreER mice crossed to Rosa26-tdTomato and Diphtheria toxin subunit A expressing mice attenuated hydroxyproline, a marker of collagen deposition following administration of bleomycin.66 Taken together, these results suggest that Cthrc1 expressing cells drive ECM deposition and may promote fibrosis.
Conversely, different studies have demonstrated that CTHRC1 may limit ECM deposition and fibrosis. Global Cthrc1 knock-out (KO) resulted in increased hydroxyproline levels and tissue remodeling in the lungs of mice following bleomycin administration.65 In a mouse model of vascular fibrosis, Cthrc1 was transiently expressed by adventitial fibroblasts following injury and was associated with decreased collagen deposition in cross-sections of carotid arteries.63 The discrepancies between these studies may highlight the cell-, tissue-, and disease-specific role of Cthrc1. As such, further studies are needed to determine the precise role of CTHRC1 in diverse fibrotic diseases. In addition, specific genetic tools, including fibroblast-specific Cthrc1 KO mice or fluorescently-labelled Cthrc1-expressing cells are required to properly assess the pathogenicity of Cthrc1+ myofibroblasts in different tissues and disease models.
Transforming growth factor beta inducible protein (TGFBI), is also known as keratoepithelian,68 and is referred to here as beta-inducible growth hormone 3 (BIGH3) is produced by fibroblasts and macrophages.69 BIGH3 was discovered in 1992 as a gene highly upregulated by A594 lung adenocarcinoma cells following TGF-β stimulation.70 The structure of BIGH3 has not been solved; however, early characterization of this protein using cDNA sequence analysis suggested that it contains a secretory signal peptide, four fasciclin 1 (FAS1) domains that mediate binding to extracellular matrix proteins, such as collagens, and a RGD motif that enables the binding of this protein to integrin receptors.70–72 Due to structural similarities, BIGH3 has been hypothesized to be a paralog of Periostin (Postn),73 which drives collagen production in the presence of TGF-β.74 BigH3 has been shown to be expressed by fibroblasts in response to stimulation with TGF-β75 and by macrophages in ‘M2’ polarization conditions in vitro60,61,76 or following ingestion of apoptotic cells.69 Upon secretion by fibroblasts and macrophages, BIGH3 binds ECM components, such as collagens,77 and subsequently binds the integrin avb578 and promotes adhesion and migration of mesenchymal and ectodermal-lineage cells, including fibroblasts, chrondrocytes, osteoblasts, keratinocytes, and endothelial cells.79–81
Studies investigating the role of BIGH3 in disease indicated tissue-specific functions. In the central nervous system, Peng et al. demonstrated that BigH3 is elevated in the serum and cerebrospinal fluid (CSF) of glioblastoma patients and that TGFBI-expressing macrophages promoted the survival of glioma stem cells by avB5-Src-Stat3 signaling.78 In the heart, Schwanekamp et al. demonstrated that BigH3 was enhanced in response to myocardial infarction. Despite this, cardiac-specific deletion of BigH3 did not alter cardiac disease or fibrosis post-myocardial infarction.82 In the colon, TGF-β signalling plays a major role in promoting fibrotic lesions in inflammatory bowel diseases (IBD).53 Aligned with this BigH3, as well as ECM genes COL1A1, COL1A2, and COL3A1 are upregulated in patients with fibrotic IBD, suggesting that combined BIGH3 and TGF-β signalling may accelerate colon fibrosis.53,83 In the lungs, studies by Yang et al. and Xu et al. have independently demonstrated that BIGH3 is elevated in patients with interstitial lung disease.84,85 Ahlfeld et al. demonstrated that BigH3 is elevated in response to lung injury and BigH3 KO mice at early ages had documented lung developmental abnormalities, lack of elastin-positive tips, reduced proliferation, and abnormally persistent aSMA myofibroblasts, which resolve by adulthood.86 The authors also demonstrated that lungs in BigH3 deficient mice had reduced elastic recoil and gas exchange efficiency.86 It remains to be seen if adult animals exhibit these defects or compensatory effects mask phenotypes seen in younger animals.
In addition to impacting lung development, in vitro studies have demonstrated that BIGH3 can promote collagen deposition in lung fibroblasts.87 Merl-Pham et al. demonstrated that TGF-β enhances the production of BigH3 in primary human lung fibroblasts.88 Yang et al. found that siRNA-mediated knockdown (KD) of BigH3 in human lung fibroblast cultures resulted in decreased TGF-β-mediated collagen 1 (Col 1) and aSMA production, demonstrating that BIGH3 is necessary to produce these ECM proteins,87 potentially through autocrine stimulation. Despite these studies, the molecular mechanism by which BIGH3 mediates Col1a1 deposition downstream of TGF-β is unclear. It has been posited based upon in vitro studies using lung fibroblast cell lines that BIGH3 mediates its effects via a multi-part mechanism. Briefly, TGF-β first elicits BIGH3 expression, then BIGH3 binds a multitude of integrin receptors, including a1b1,89 a3b1,90,91 avb379 or avb579 integrin. BigH3 then drives activation of PI3K-signaling91 and increased Snail expression, encoded by Snail1, via downregulation of a Snail negative regulator G-protein signaling modulator 2 (GPSM2).79,87 Taken together, these studies revealed that BIGH3 is both necessary and sufficient to induce collagen production in lung fibroblasts. Therefore, mechanistic studies indicate that BIGH3 modulates TGF-β-mediated signal transduction pathways ( Figure 3B).
Alternative mechanisms for BIGH3-mediated regulation of collagen deposition in the lung have also been proposed. Zhang et al. demonstrated that BigH3 deficient mice had stunted growth in vivo, as well as enhanced proliferation and cyclin D1 expression ex vivo, suggesting that BIGH3 may limit cellular proliferation.92 Nacu et al. demonstrated that primary lung fibroblasts stimulated with BIGH3 had increased Col 1 protein abundance in the absence of transcription, and subsequently demonstrated that BigH3 suppresses the transcription of MMP14, which degrades collagen.69 These results suggest that BIGH3 reduces collagen turnover by decreasing ECM degradation. As a result of these conflicting reports, further study is required to characterize the full scope of ECM deposition changes in response to BIGH3. In addition to studies defining the tissue-specific nature of BIGH3 and the mechanisms of its effect on ECM deposition, further investigation is required into additional roles of BIGH3 in the context of fibroblasts and macrophage spatiality, including mechanical sensing, and/or monocyte/macrophage chemotaxis. As such, cell- and tissue- restricted BIGH3 KO models paired with high-resolution techniques, such as scRNA-seq are required to examine the role that BIGH3 plays in modulating ECM deposition, development, and disease.
The interface between fibroblasts, the immune system, and the ECM is wide and complex. As a result, an appreciation that fibroblasts and the immune system act in a finely tuned and integrated system for ECM deposition is required for understanding the biology of fibroblasts, as well as their roles in ECM deposition and fibrotic disease. Current research focuses on the relationship between fibroblasts and macrophages. As a result, additional studies are required to determine whether the relationships between fibroblasts and other immune cell subsets can shape ECM deposition or if macrophages are required as an intermediate. Despite this, targeting the fibroblast-ECM-immune axis may provide opportunities to prevent fibrotic disease. For example, defining the mechanism of action of immune-derived ECM modulators, including CTHRC1 and BIGH3, may provide an opportunity to modulate disease-associated myofibroblast development and/or prevent excessive fibrosis.
Indeed, an attractive approach to ameliorate TGF-β related fibrosis may be to target potential co-factors or effect modulators within the TGF-β signaling pathway, such as CTHRC1 or BIGH3. It is possible that fibrotic deposition by fibroblasts is akin to accessing your online bank account using two-factor authentication: TGF-β representing the PIN and cofactors representing the code sent to your phone ( Figure 4). Here, TGF-β alone is a potent inducer of ECM-deposition in fibroblasts, whereas cofactors such as CTHRC1 or BIGH3 do not induce fibrosis alone. In combination, these cofactors increase TGF-β-mediated ECM deposition synergistically. To this end, we envision that intervention strategies specifically targeting TGF-β signalling cofactors (or the macrophage and myofibroblasts that produce them) may limit fibrotic disease and prevent off-target effects that are common among current therapies.
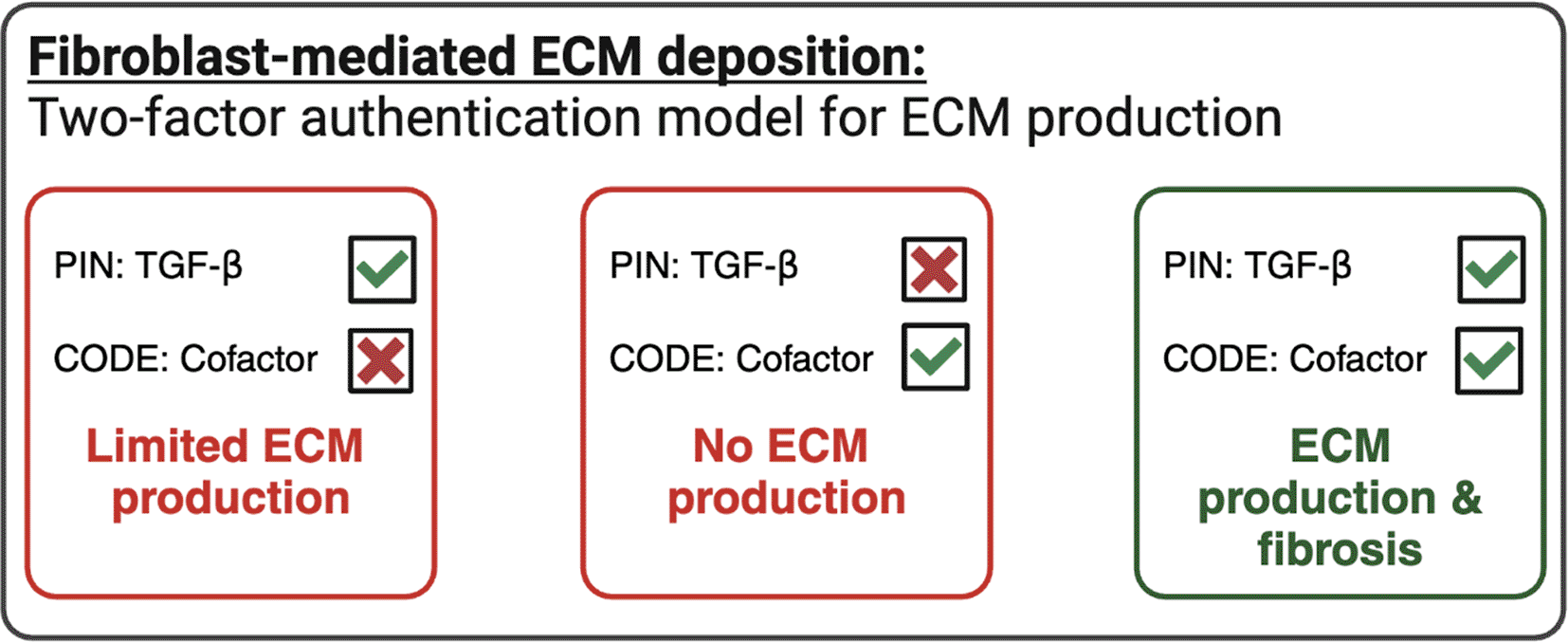
The role, mechanism, and source of cofactors, including BIGH3 and CTHRC1 in health and fibrotic disease are not well defined. Here, we speculate that fibroblasts are required for enhanced ECM deposition downstream of TGF-β, but cofactors alone are not sufficient, akin to accessing your online bank account using two-factor authentication: TGF-β representing the PIN and cofactors representing the code sent to your phone. Further studies are required to determine key aspects of BIGH3 and CTHRC1 biology and how this drives ECM deposition and fibrotic disease in combination with other pro-fibrotic mediators.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the topic of the review discussed comprehensively in the context of the current literature?
Partly
Are all factual statements correct and adequately supported by citations?
Partly
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Partly
References
1. Ramachandran P, Dobie R, Wilson-Kanamori JR, Dora EF, et al.: Resolving the fibrotic niche of human liver cirrhosis at single-cell level.Nature. 2019; 575 (7783): 512-518 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Inflammatory diseases, Intestinal fibrosis, Colon cancer
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Immunology, Stromal Immunology, Virology, Respiratory infections, Innate immunity
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Immunology, Immunometabolism, fibroblast biology, T cell biology, autoimmunity.
Is the topic of the review discussed comprehensively in the context of the current literature?
Partly
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Partly
References
1. Gauthier V, Kyriazi M, Nefla M, Pucino V, et al.: Fibroblast heterogeneity: Keystone of tissue homeostasis and pathology in inflammation and ageing.Front Immunol. 2023; 14: 1137659 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Immunology, Immunometabolism, fibroblast biology, T cell biology, autoimmunity.
Is the topic of the review discussed comprehensively in the context of the current literature?
Partly
Are all factual statements correct and adequately supported by citations?
Partly
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Partly
References
1. Guerrero-Juarez CF, Dedhia PH, Jin S, Ruiz-Vega R, et al.: Single-cell analysis reveals fibroblast heterogeneity and myeloid-derived adipocyte progenitors in murine skin wounds.Nat Commun. 2019; 10 (1): 650 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: wound healing, fibroblasts, bioinformatics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 2 (revision) 05 Dec 24 |
read | read | read | |
|
Version 1 19 Feb 24 |
read | read | ||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)