Keywords
Endocrine disruptor, Non-ionizing radiation, Thyroid disorder, Electromagnetic fields
Endocrine disruptor, Non-ionizing radiation, Thyroid disorder, Electromagnetic fields
Thyroid abnormalities, including functional (hypothyroidism/hyperthyroidism) and anatomic (benign nodules/thyroid cancer) disorders, had affected 200 million of the world population in 2013.1 This situation is demonstrated by incidences of hypothyroidism and hyperthyroidism that have reached 226/100,000 population per year (5% prevalence) and 51/100,000 population per year (2% prevalence), respectively.1 Thyroid nodules are found in 12% of the global general population in which the incidence rises among older population.2 At least 50% of individuals have one thyroid nodule at 60 years old, where 90-95% of them are benign.2 There are 3.2 million patients with thyroid cancer worldwide, generally dominated by women, with an incidence of more than 560,000 new cases per year.3 Some of the triggering factors for thyroid abnormalities are iodine, selenium, vitamin D deficiency, smoking, pathogen infection, drugs, chemicals, and external radiation.4 It has been suggested that an increase in thyroid dysfunctions is due to radiation exposure.5
Radiation is categorized into ionizing and non-ionizing. Ionizing radiation (i.e. X-ray or gamma-ray exposure) breaks molecular bonds due to its high frequency, whilst non-ionizing radiation (i.e. infrared, microwave, or radiofrequency waves) damages cells by altering chemical reactions and inducing mechanisms related to heat and electricity. Owing to its frequent use, non-ionizing radiation is likely to cause thyroid abnormalities.6 A study suggested that increased cell phone use is a contributing factor to higher incidence rate of thyroid cancer in Sweden and other Nordic countries.
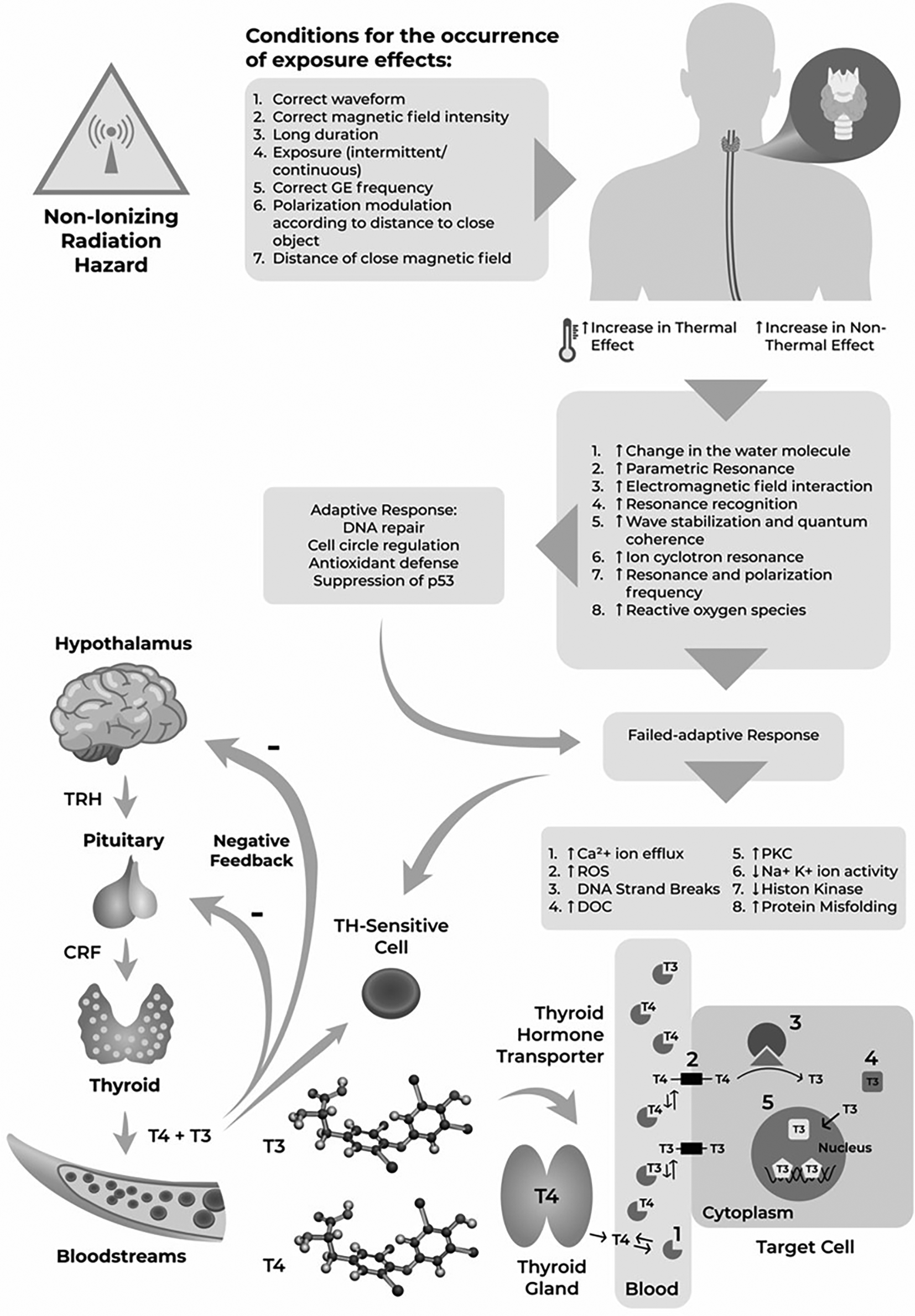
The exposure to non-ionizing electromagnetic fields (EMF) in human cells may lead to several events, including cyclotron ion resonance, parametric resonance, electromagnetic field interaction with electrons, wave stabilization and quantum coherence, resonance and polarization frequency, resonance recognition, electron excitation in water molecules, and increase of free radical concentration, which can affect the body.7–9 The aforementioned events are followed by an increase of calcium (Ca2+) efflux, formation of reactive oxygen species, deoxyribonucleic acid (DNA) strand breaks, increased activities of ornithine decarboxylase (ODC) and protein kinase (PKC), disturbances in Na+/K+ phosphatase activities, decreased levels of melatonin, impaired histone kinase activities, and increased protein misfolding if adapted responses do not occur.10 These events will eventually cause genotoxic and carcinogenic effects under certain conditions.11–14
The Oceania Radiofrequency Scientific Advisory Association (ORSAA) is an organization composed of scientists from various academic disciplines who are investigating the scientific research that relates to the effects of artificial EMF radiation on humans, animals and the environment. ORSAA database contains all information on the effects of non-ionizing EMF radiation on human, animals and the environment from 2015 to present. The importance of this database is derived to the fact that the number of mobile phone users has reached 7 billion people or 97% of the world's population. In Southeast Asia alone, the number of cellular phones used is more than twice the population, reaching 600 million in 2014.15 The thyroid gland plays an important role in the growth process and metabolism; however, it has a high risk of being exposed to non-ionizing radiation because of its superficial and close location to the cellular phone when in use.16,17 The exposure may cause abnormalities in thyroid gland morphology and thyroid hormone functions.16,17 Abnormality of thyroid hormones are associated with some diseases.16,18 In addition, it is also suspected to affect thyroid hormone actions, either the transporters or genomic and non-genomic actions, which in turn will disrupt the physiological response.15,17,19–21
This systematic review examined data from experimental research (both in vivo and in virtro), observational studies, reviews, and medical hypotheses published in the database to map and identify the relationship between non-ionizing EMF radiation and thyroid dysfunctions. This systematic review aimed to answer the following research questions:
All studies containing information on the effect of non-ionizing EMF exposure on thyroid cells and their functions were collected from the ORSAA database. The ORSAA database is a comprehensive database of bio-effects from non-ionizing radiation by ORSAA, an organization consisting of researchers and science practitioners, that investigates the effects of electromagnetic radiation on humans, animals, and the environment. The members also continuously collect all articles published in other databases such as PubMed, Google Scholars and others that specific on the effects of bio-effects from non-ionizing radiation. The organization was founded in 2015 by a group of academics and researchers in Australia more information can be found here. The sources of ORSAA database are from all articles from PubMed database, Google Scholars, the Australian Radiation Protection and Nuclear Safety Agency (ARPANSA) technical series documentation and their monthly literature survey on electromagnetic radiations, and the EMF-Portal of Rheinisch-Westfälische Technische Hochschule (RWTH) Aachen University. The ORSAA database was used because it is comprehensive and it covers all studies published in other databases but specific on effects of electromagnetic radiation on humans, animals, and the environment. This ensured that all articles related to the topic were covered but in smaller number of datasets. In addition, the database easy to search, unbiased, and accessible online free of charge.
The searches were conducted using keyword “thyroid” on ORSAA database between 20 March and 1 April 2021. All data from the database were included without year restriction. All cross-sectional, prospective or retrospective studies in humans were considered eligible. Only articles written in English were included. In this article, reviews, commentaries and hypothesis were considerate not eligible. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) to provide the workflow for the search strategy for each step.73
Bibliometric data including the author, year of publication, title, journal and other important information were transferred to EndNote version X9 (Thompson Reuters, Philadelphia, PA, USA). All articles were reviewed based on title and abstract based on inclusion and exclusion criteria manually. Those article who did not meet the criteria were excluded from the EndNote library. Full-text of article that met the inclusion criteria were read and critical data were extracted and summarized in the tables manually. For observational and experimental studies, extracted data included the number and type of subjects, exposure parameter (i.e., power density, frequency duration, distance), thyroid-related variables, and results. As for the observational studies, the aim of the study was also summarized in the table.
The ORSAA database has 3,914 publications related to the exposure of non-ionizing radiation as of 1 April 2021. Out of the total, 3,871 were excluded because they were not relevant (the studies did not consist the word of “thyroid”) leaving 43 articles that were related the exposure of non-ionizing radiation on thyroid (Figure 1). After reading the full-texts of the 43 articles, additional 18 articles were excluded with reasons: not related to thyroid (n=2),22,23 different study purposes (n=6)24–29 and full-text unavailable (n=5).30–34 In addition, four review articles (two discussing the effect of non-ionizing EMF on thyroid gland abnormalities,35,36 while the two others discussing the effect of non-ionizing EMF on thyroid hormone functions)37,38 and one hypothesis article elaborated the impact of non-ionizing radiation on the likelihood of thyroid cancer39 were also excluded (Figure 1). A total of 25 studies consisted of 15 experimental40–54 and 10 observational,6,55–63 respectively, were included in this systematic review (Figure 1).
Detailed extracted data from the included literature reporting non-ionizing EMF effects on human thyroid are presented in Table 1 (for experimental studies) and Table 2 (for observational studies). There were 15 experimental studies40,42,43,45–49,51–54 included with five studies examining the effect of non-ionizing EMF on thyroid hormone functions,42,45,46,52,54 nine studies examining the changes of the histocellular morphology,40,43,47–49,51–53 and one study examining both parameters44 (Table 1). The observational studies consisted of ten studies; four of them assessed the effect of non-ionizing EMF on thyroid hormone function,55,56,58,63 five studies assessed the association with thyroid cancer incidence,6,39,57,59,61 and one study examined the impact both on the thyroid gland morphological changes and the thyroid hormone functions60 (Table 2). Most of the experimental research publications were from Sweden49,51,52 which predominantly published in 200546,49 (see Table 1). As for the observational studies, the highest number of publications was recorded in 201657,60 with most of the authors were from the United States59,61,62 (see Table 2).
| Author, Year | Sample | Real/simulation | Exposure duration | Freq | Power density | Specific absorption rate | Distance (cm) | Variable | Result |
|---|---|---|---|---|---|---|---|---|---|
| De Seze et al., 200141 | 20 H | R | 2h 5d 4w | 900 MHz | Ua | Ua | Ue | TSH | No significant effect |
| De Seze et al., 200141 | 30 H (18-40 yrs) | R | 1 d | 900 MHz | Ua | Ua | Ue | TSH | No significant effect |
| Koyu et al., 200546 | 30 male Spraque–Dawley rats (12 weeks) | Si | 1/2 h 5d 4w | 900 MHz | 1±04 W/cm2 | 2 W/kg (whole body) | Ue | T3, T4, TSH | Significant effect: decreased serum levels of T3, T4. TSH |
| Djeridane et al., 200842 | 20 H Male (20-32 yrs) | R | 2h 5d 4w | 900 MHz | Ua | 0,3 W/kg (Temporal brain) | Ue | TSH | No significant effect |
| Sinha et al., 200854 | 20 male Charles Foster rats (4-5 wks) | Si | 2h 7d 3w | 2450 MHz | 16.5 uW/cm2 | 3.6 uW/gm parallel to the E plane and 0.98 uW/gm parallel to the H plane (whole body) | 23 | T3.T4, TSH | Significant effect: decreased serum T3 and increased T4 |
| Jin et al., 201345 | 240 Sprague Dawley Rats (120 male 120 female-8 weeks) | Si | 3/4h 5d 8w | CDMA 848.5 MHz | Ua | 4.0 W/kg CDMA | Ue | T3, T4, TSH | No significant effect |
| WCDMA 1950 MHz | 2.0 W/kg CDMA and 2.0 W/kg WCDMA (whole body) | ||||||||
| Rajkovic et al., 200549,50 | 24 male Wistar rats (2 mts) | Si | 4h 7d 4w | 50 Hz | Ua | Ua | 12 | MC, parafollicular cells, and nerve fibers | Partial Significant effect: On thyroid vasculature, (increased number of NPY-containing nerve fibers) |
| Silva et al., 201553 | 96-well plates of Primary thyroid cell (20-60 years old, mostly female) | Si | A: 3 | A: 900 MHz | A: 80 uW/cm2 | A: 0.082 W/kg | 40 | Cell proliferation, genomic instability (DNA ploidy of cell’snuclei), ROS HSP 70 | No significant effect |
| B: 16 | B: 900 MHz | B: 80 uW/cm2 | B: 0.082 W/kg | ||||||
| C: 65 | C: 895 MHz | C: 210 uW/cm2 | C: 0.170 W/kg | ||||||
| Rajkovic et al., 200549 | 24 male Wistar rats (2 months) | Si | 4h 7d 4w | 50 Hz | Ua | Ua | 12 | Number and volume density of MC, serotonin MC, histamine MC | Partial significant effect: Certain alterations of thyroid MC in rats exposed to EMF (increased numerical and volume density of intact type A MC in thyroid) |
| Rajkovic et al., 200651 | 30 male Wistar rats (2 months) | Si | 4h 7d 4w | 50 Hz | Ua | Ua | 12 | Histological and cellular alterations | Significant effect: Increased volume density of follicular epitheliuma and interfollicular tissue, volume density of capillaries, thyroid activation index. Decreased volume density of colloid. Follicular cells in the EMF group revealed the frequent finding of several colloid droplets within the same thyrocyte with the occasional presence of large-diameter droplets Alterations in lysosomes, granular endoplasmic reticulum and cell nuclei |
| Eşmekaya et al., 201043 | 30 male Wistar rats (2 months) | Si | 1/3h 7d 3w | 900 MHz | Ua | 1.35 W/kg | 10 | Pathology of the gland, structural alterations, and apoptosis activity | Significant effect: altering the gland structure, including hypotrophy cells, and enhancing caspase-dependent pathways of apoptosis |
| Rajkovic et al., 201052 | 72 male Wistar rats (25-35 days) | Si | 4h 7d 4w | 50 Hz | Ua | Ua | 12 | Histological alterations | No Significant effect: But histological analysis showed predominance of micro follicles and columnar thyrocyte, reduced interfollicular space |
| Agustiño et al., 201240 | 54 female Sprague Dawley rats | Si | 1/2h | 2.45 GHz | Ua | P: 1.5 W = 0.046±1.10-3 W/kg (mean thyroid) | Ue | HSP-90, HSP-70, lesion in the gland, antiapoptotic activity and integrity chromatin condensation and nuclear fragmentation in the thyroid cells | Partial Significant effect: the thyroid gland is sensitive to 2.45 GHz RF, a transitory decrease in the values of HSP-90 and HSP- 70, no signs of lesion or apoptosis in the glandular structure |
| P: 3 W= 0.104±5.10-3 W/kg (Mean thyroid) | |||||||||
| P: 12 W = 0.482±12.10-3 W/kg (Mean thyroid) | |||||||||
| Agustiño et al., 201247 | 96 adult female Sprague-Dawley rats | Si | 1/2h | 2.45 GHz | Ua | P: 1.5 W = 0.046±1.10-3 W/kg (Mean thyroid) P: 3 W = 0.104±5.10-3 W/kg (Mean thyroid) P: 12 W = 0.482±12.10-3 W/kg (Mean thyroid) | Ue | HSP-90, HSP-70, lesion in the gland, antiapoptotic activity and integrity chromatin condensation and nuclear fragmentation in the thyroid cells | Partial Significant effect: the thyroid gland is sensitive to 2.45 GHz RF; a transitory decrease in the values of HSP-90 and HSP-70, recovery of HSP-90 basal levels is slower at the 3 W power setting, there are no signs of lesion or apoptosis in the glandular structure |
| Hajioun et al., 201444 | 2 groups of 40 Wistar rats male and female (80-90 days) | R | 2h (12 × 10 min) 7d 4w | 900 MHz | Ua | Ua | Ue | Histological alterations | Significant effect: a reduction in the number of cubic cells and disorder in threated groups, as well as a reduction in the amount of follicular fluid and follicular diameter in groups exposed to radiation and received garlic extract |
| T3, T4, TSH | Significant effect: Decreased T4 and T3 increased TSH | ||||||||
| Agustiño et al., 201548 | 56 adult female Sprague Dawley rats | Si | A: 1/2h (Se) | 2,45 GHz | Ua | P: 3W = 0.102±12.10–3 W/kg (Mean thyroid) | Ue | HSP-90 and morphological changes in thyroid gland tissues | Significant effect: glandular hypertrophy in relation to the SAR and/or number of exposures, modification of the distribution of HSP-90 associated with membranes and parafollicular cells |
| B: 1/2h (Se) | P: 12 W = 0.429±12.10–3 W/kg (Mean thyroid) | ||||||||
| C: 1/2 h (10×) 2w | P: Repeated 3W = 0.107±6.10–3 W/kg (Mean thyroid) |
| Author, Year | Aim of study | Sample | Exposure | Exposure Duration | Freq | Specific Absorption Rate | Distance (cm) | Variable | Results |
|---|---|---|---|---|---|---|---|---|---|
| Bergamaschiet al., 200456 | Evaluating the effects of mobile phone use on thyroid function and to analyze the role of to analyze the role of occupational stress on TSH secretion disorders | 1355 men and 1243 women employees (Age: 28.96 ± 6.18 years old for males and 27.84 ± 6.10 years old for females | Real Mobile Phone | <19h/month, 19h and 33h/month, >33h/month conversation time. | Ua | Ua | Ue | TSH | Significant effect: There was a greater prevalence of subjects with low TSH values among the 192 employees with more than 33hrs./month conversation time. On the basis of our data, it is not possible to establish whether this result is determined by exposure to EMF'S from mobile phones or by the stress of using these instruments. |
| Eskander et al., 201258 | Assessing the role of exposure to radio frequency radiation (RFR) emitted either from mobiles or base stations and its relations with human's hormone profiles. | 14–22 and 25–60 years old male and female (115 participants) | Real Mobile Phone and RFR emitted from base stations | 1 year, 3 years and 6 years for hormonal analysis | GSM-950 MHz | Ua | 20-100 m, 100-500 m and >500 m from base station | Total T3, T4 | Significant effect: Persons of ages 14–22 years and 25–60 years who were exposed, for time intervals extended to 6 years, to RFR either from mobile telephones or from base stations suffered high significant decrease in their serumT3 and T4 levels |
| Mortavazi et al., 200963 | Investigating the effects of EMF induced GSM mobile phones on the TSH and thyroid hormones in humans. | 23 male and 54 female healthy university student (19-29 years old) | Real Mobile Phone | Those whose average daily use of their mobile phones in talk mode was 5-20 minutes (group 1), >120 minutes (group 2), those who had not used mobile phones before the study, In group 1, the minimum and maximum period of mobile use was 6 months and 5 years In group 2, the minimum and maximum periods of mobile use were 1 and 10 years | Ua | Ua | Ue | The levels of T3, T4 and TSH | Significant effect: A higher than normal TSH level, low mean T4 and normal T3 concentrations in mobile users were observed. It seems that minor degrees of thyroid dysfunction with a compensatory rise in TSH may occur following excessive use of mobile phones |
| Kato et al., 201559 | Examining the association between electric blanket use and thyroid cancer incidence in a prospective cohort study. | 89,527 Women (50–79 years old) | Electric Blanket | Years of use: <1: 9, 1-4: 18, 5-9: 16, 10-19: 12, 20+: 18 | Ua | Ua | Ue | Thyroid cancer | No significant effect: No association was found between use of electric blankets and subsequent risk of thyroid cancer (HR = 0.98). Duration of electric blanket use measured in years, months, or hours had no effect on risk. |
| Milham and Morgan, 200862 | Investigating the cancer incidence in the teachers, and its cause. | 137 teachers | Cumulative exposure to high frequency voltage transients on the classroom’s electrical wiring | < 3 years (1.52 years), 3-14 year (7.48 years), ≥15 years (16.77 years) The average latency time between start of employment at the school and diagnosis for all cancers was 9.7 years. The average latency time for thyroid cancer was 3.0 years | The G/S meter measures the average rate of change of the high frequency voltage transients between 4 and 150 KHz. | Ua | Ue | Thyroid cancer | Significant effect: The cancer incidence in the teachers at this school is unusually high and is strongly associated with high frequency voltage transients, which may be a universal carcinogen, similar to ionizing radiation. |
| Carlberg et al., 201657 | Studying the incidence of different types of thyroid cancer, data was obtained from the Swedish Cancer Register for the time period 1993–2013 (earlier data is not available). | 11,513 men and 10757 women (0-80+ years old) | Real Mobile Phone | The number of total minutes of out-going mobile phone calls in million minutes for the time period 2001–2013 | Ua | Ua | Ue | Thyroid cancer | This study has shown an increasing incidence of thyroid cancer in Sweden and the Nordic countries. Increased use of CT and PET-CT for medical examinations has elevated the population’s exposure to the ionizing radiation and should be considered as a risk factor. |
| Kunt et al., 201660 | Examining the effects of exposure to an EMF on thyroid nodule formation, serum FT3, FT4 of electrical workers | The study group included 47 electrical workers, The control group was created from 47 healthy individuals (29 and 52 years: and 28 and 52 years) | High-voltage transmission lines and transformers | Mean working period of the study group was determined as 15.9±6.72 years Mobile phone usage time control group:17.21±6.64 years 537.46±8.47 minute/month Mobile phone usage time Study group 15.89±6.72 Years 504.55±7.69 Minute/month | Ua | Ua | Ue | Serum concentrations of thyroid- TSH, FT3, FT4 and Thyroid Gland | Partial significant effect: Although thyroid function tests (FT3 and TSH) were lower in the study group, they were not statistically significant. The FT4 level was detected significantly lower in the study group than the control group. Furthermore, anteroposterior diameter measures of the thyroid gland of the study group increased when compared with the control group according to the morphometric measurement by USS; however, the result was not statistically significant. Left anteroposterior diameter measure of the thyroid gland was found significantly higher in the study group. There was not any significant difference between the groups in terms of nodule and parenchyma. |
| Baby et al., 201755 | Investigating the effects EMFs induced by the global stem for mobile communications mobile phones on the TSH in active cell phone using medical students | 83 Participant (49.4% males and 50.6% females aged 18-25 years old) | Real Mobile phone | < 1 h: 41 1-2 h: 24 2-3 h: 6 > 3 h: 8 Missing data: 4 | SAR values for commonly used Apple iPhones and Samsung Galaxy S Phones | Ua | Ue | TSH | Significant effect: There was a significant correlation between total radiation exposure and an increase in TSH among both groups –in those with and without family history of thyroid illness. |
| Luo et al., 201961 | Investigating the association between cell phone use and thyroid cancer. | 462 histologically confirmed incident thyroid cancers (papillary, follicular, medullay, anaplastic) with 498 population-based controls. 21-84 years old Female 375 male 87 (case) Female 344 and male 154 (control) | Real Mobile phone | Daily use hour (≤1, 1-2, >2), daily phone call (≤3, 3-6, >6), phone use year (≤12, 12-15, >15 years), age at first use (≤20, 21-50, >50) cumulative use hour, cumulative phone call. Cases and controls each had a median of 5 calls per day, Years of use were also similar, ranging from 1 to 35 years with median of 13 for cases and from 2 to 33 years with a median of 13 for controls. Median duration of daily usage was 1 hour for both cases and controls with a range of less than 1 hour to 10 h/d for cases and less than 1 to 15 h/d for controls. | Ua | Ua | Ue | Thyroid Cancer | No significant effect: This study found no significant association between cell phone use and thyroid cancer. A suggestive elevated risk of thyroid microcarcinoma associated with long-term and more frequent uses warrants further investigation. |
| Carlberg, et al. 20206 | Studying the incidence of thyroid cancer using the Swedish Cancer Register and NORDCAN for the Nordic countries | women 5047 men aged 0-80+ years old | Real Mobile phone | Ua | Ua | Ua | Ue | Thyroid Cancer | Cancer incidence has been steeply increasing in Sweden and all Nordic countries during the 21st century. Use of the handheld mobile phone is increasing, in particular, the smartphone gives high RF radiation exposure to the thyroid gland. It is postulated that this might be a causative factor for the increasing incidence supported by human epidemiology that has shown an association between mobile phone use and thyroid cancer. |
Out of fifteen experimental studies included,40–54 ten studies assessed the morphological and histocellular changes of thyroid gland40,43,44,47–53 and six studies assessed hormonal function (T3, T4, and thyroid-stimulating hormone (TSH)).41,42,44–46,54 Out of ten experimental studies that assessed the effect of EMF exposure on morphological and histocellular changes,40,43,44,47–53 some indicators were used to assess the effects of EMF exposure including changes in the number of NPY-containing nerve fibers, changes in thyroid mast cells,50 changes in volume density of the follicular epithelium and interfollicular tissue, capillary volume density,51 thyroid activation index, and alterations in colloid volume density.49 The presence of several EMF-exposed colloid droplets in the thyroid follicular cells on the same thyrocytes with some large-diameter droplets was also reported as a parameter.51 Moreover, the evaluation based on the alterations of lysosomes, granular endoplasmic reticulum, and cell nuclei have been used.51 A study also included the changes in glandular structures, including those deriving from cell hypotrophy and increased apoptosis via caspase-dependent pathways.43 Other parameters such as the changes of predominance follicles and thyrocytes, changes of thyroid interfollicular space,52 the level of heat shock protein (HSP-90) and HSP-70,47 recovery rate of HSP-90 basal level,40 the number of cubic cells on thyroid gland,44 and the fluid volume and follicular diameter have been used.44 The methods of the studies are presented in Table 1.
Out of ten experimental studies that assessed the effect of EMF exposure on morphological and histocellular changes,40,43,44,47–53 eight of them showed significant effects,40,43,44,47–51 while the remaining two studies showed no effect.52,53 Studies that demonstrated significant changes of thyroid gland reported the alterations of thyroid vasculature (increased number of neuropeptide Y-containing nerve fibers),50 increased the number and volume density of mast cells,49 increased the density of follicular and interfollicular tissue, capillaries, and thyroid activation index,51 increased the number of hypotrophy cells and cell apoptosis,43 decreased the level of HSP-90 and HSP-70,40,47 reduced the normal follicular diameter,44 and induced the glandular hypertrophy.48 Detailed of results from all studies are presented in Table 1.
Among six studies assessing hormone function, three showed significant effects, with hypothyroidism being the most found condition.44,46,54 One study showed the decreased serum levels of T3, T4, and thyroid-stimulating hormone (TSH) (central hypothyroidism).64 Another study reported low T3 syndrome indicated by an increased level of T4 and a normal level of TSH.54 A study reported an increase in TSH but a decrease in T3 and T4 levels, hence the primary hypothyroidism.44 Detailed of results from all studies are presented in Table 1.
There were 12 in vivo studies in animal models,40,43–52,54 where most of them (n=10) used mice model40,43–49,51,52,54 with the number of animals ranged from 2054 to 24045 and age ranged from 25 days52 to 90 days/12 weeks.44,46 Some studies used male mice,43,46,49,51,52 female mice40,47,48 or both male and female mice.44,45 One study used male rat as model.50 Two studies were conducted on human subjects with number of samples of 20 and 30 individuals and aged between 18 and 40 years.41,42 Only one study was in vitro.53
There are various types of EMF exposure measured in experiment studies. Three studies used real mobile phone as the source of EMF exposure,41,42,44 while others used EMF exposure produced from various simulation devices.40,43,45–54 Those who used the real phone, investigated the radiation transmitted from the European GSM-standard commercial cellular phones, namely Motorola,41 Motorola 8200,42 and Nokia 1200.44 In the two studies using human subjects, the cell phone was made in ‘calling’ modes.41,42 Another study using the mice exposed the cellular phone transmission through various modes (i.e. calls, unanswered calls, and connected calls (no talking)).44 The simulation devices with various antenna were used as sources of radiation in 12 studies,40,43,45–54 of which three studies carried the experiment in gigahertz transverse electromagnetic (GTEM) chambers,40,47,48 one study in reverberation chamber,45 one study in an 5% CO2 incubator,53 and seven others in an open experimental arena.43,46,49–52,54 In four studies, the antenna was replaced with a single coil of the solenoid as a radiofrequency source.7,49,51,52 In one study, the antenna was placed on top of the animal holders and in another study was placed on the ceiling of the incubator above the plate.53 In two studies, the cage was placed above the midline of horn antenna43 and above the dipole antenna.46
Most studies continuously exposed their subjects with the radiation for 30 minutes40,47,48 though there was a study employing 65-hours continuous exposure.53 In the case of the exposure intermittent duration, it is varied across studies (from 20 minutes43 to 4 hours49,51,52). Based on the frequency, most studies performed the exposure up to four hours per day and seven days a week for four weeks (n=4).49,51,52
Out of fifteen experimental studies included,40–54 only nine studies included specific absorption rate (SAR) as a considered variable.40,42,43,45–48,53,54 However, only four studies provided the formula of how SAR value was calculated (either using Equation 140,47,48 and Equation 243):
Where, SARE is the predicted experimental SAR value, SARS is the value obtained from the simulation, WS (g) is the mouse model’s weight, and WE (g) is the weight of the experimental mouse. ERMS is the root mean square value of the electric field (V/m), σ is the mean electrical conductivity of the tissues in Siemens/meter (S/m), and ρ is the mass density (kg/m3). Apart from those equations, studies that used various simulators as the source of EMF exposure, estimated the SAR values using computational simulations, namely finite difference time domain40,47,48,54 and RF simulation techniques.53
Power density43,46,53,54 and distance43,49,51–54 between the EMF source and the subjects were among other considered experimental parameters. Meanwhile, radiation wave frequencies were mentioned in all the experimental studies. A study determined the average value of power density on initial exposure using a specific reference point.43 The power decreased uniformly with distance from the antenna axis.43 The studies used frequencies varying from low (50 Hz)49,51,52 to ultra-high (2.45 GHz),40,47,48 with a majority used 900 MHz (n = 6).41–44,53 Studies had the distance of 10 cm43—40 cm,53 where most of them employed 12-cm distance.49,51,52
Six studies investigated the effects of non-ionizing EMF radiation on the morphology and histocellularity of the thyroid gland6,57,59–62 (Table 2). A disagreement was found among the studies; two studies reported an association,60,62 two studies – no association.,59,61 while two others required further research for conclusive findings.6,57 A study reported a significant increase in the left anteroposterior diameter of the thyroid gland associated with the exposure of high-frequency voltage transient.60 A link between RF-EMF exposure and thyroid cancer incidence among Swedish and Nordic populations was suggested by two studies.6,57
Five observational studies revealed significant relationships between EMF exposure and alteration of thyroid functions with common occurrence of hypothyroidism.55,56,58,60,63 A study among 2598 employees of EMF-related workplaces found that the workers who having frequent cellular phone conversations more than 33 hours/month had chance to had TSH level less than 0.4 U/I compared to those with cellular phone conversations less than 19 hours/month.56 A six years observational study revealed that the decrease in T3 and T4 levels were more pronounced in subjects using cellular phone frequently or those living near a phone tower.58 Similarly, a decrease in FT4 among electrical workers who worked in high-voltage electric transmission lines (HVETL) compared to controls who did not work on the workplace60 and an increase in TSH levels were observed significantly more prevalent in frequent cellular phone users, regardless their family histories of thyroid disease.55 None of observational studies assessed the actions of thyroid hormone, either related to transporters or genomic and non-genomic actions. Furthermore, the mechanism of how EMF affects the thyroid gland remained vague.
The highest number of subjects included in observational studies were 89,527 participants,59 and the least were 77 participants63 with an age range of 1458—80+ years old.6,57 The indicators can be total hours of cellular phone use in conversation mode per month,56 duration of daily call mode or mobile phone use,55,61,63 or cumulative cellular phone use for years.57,58 Parameters including radiation frequency56,58,62 and SAR (based on the mobile phone type) were scarcely considered in the studies.55 In a study, SAR values from each of the cell phones used by participant were retrieved to calculate total radiation exposure.55
Out of total studies included, some examined the effects of EMF exposure to the morphology and histocellularity of the thyroid gland6,57,59–62 and some examined its effects on hormone functions.55,56,58,60,63 However, no study focused on the effects of EMF exposure on thyroid hormone transporters, as well as genomic and non-genomic actions of thyroid hormones. None of the studies describe the conditions that may trigger the effects and the thyroid's adapted response mechanisms.
A group led by Mancini discovered the effect of electromagnetic wave exposure from cellular phones on estrogen receptors, including an estrogen receptor α (ERα) gene methylation.65 The exposure is also associated with an absence of adapted responses, namely DNA repair, cell cycle regulation, antioxidant formation, and p53 suppression in colonic cells.65 This finding stresses the importance of studying the effect of EMF on thyroid hormones since they also act on nuclear receptors, the same way as estrogen.66
The power released by non-ionizing radiation when making a call using a cellular phone also depends on the level of coverage received from the base station.67 Cellular phones or base stations are designed to transmit the minimum amount of power to maintain calls.67 The technology used to establish communication between the cellular phone and the base station depends on the system used by the cellular phone operator.67 Studies identified ROS as a dominant pathway and used HSP-70, HSP-90, and ROS as markers.47,48,53 A study showed a significant transient reduction of HSP-90 and HSP-70 after exposure to EMF and slower recovery of HSP-90 actions at 3 W exposure power.47 The result is consistent with the theory that transcription and translation in several genes (JUN, HSP 70, and MYC) are caused by excessive ROS production in tissues due to exposure to ionizing/non-ionizing radiation.68
Several experimental studies have described the mechanism of thyroid abnormalities due to EMF exposure.44,46,54 EMF can affect iodine uptake in the thyroid gland, increase the pituitary gland temperature,46 and trigger a rise in serum cortisol levels, inhibiting the conversion of T4 to T3.44 It may also cause glandular tumors due to a rise in ornithine carboxylase.54 The deleterious effect of EMF on thyroid follicles is also associated with increased caspase-dependent apoptotic pathways.44
A study demonstrated that thyroid cell apoptosis in exposed mice occurs through the induction of caspase-4 and caspase-9-dependent apoptotic pathways and can be observed by the loss of mitochondrial cristae. The anti-apoptotic function of HSP-90 is observed in regions close to protein kinases, in follicular and parafollicular cells, and also between lobular and capsular membranes.48 The mechanism for the disruption of thyroid gland morphology and thyroid hormone functions due to non-ionizing exposure has been described in previous experimental studies;43,48 however, full explanations are still needed including the biomolecular pathways. Moreover, the effects on thyroid hormone transporters and genomic and non-genomic actions are not well understood. A previous review has suggested that non-ionizing EMF may act as endocrine disruptors (EDs) and might interfere with thyroid hormone at any level, including its functions, actions, and structural abnormalities of the gland.69
Non-ionizing EMF can interact with the thyroid at certain levels and affect the thyroid gland structure and the functions and actions of thyroid hormones.13,63,70,71 EDs affect processes, such as thyroperoxidase iodine symporter, hepatic uridine diphosphate-glucuronosyltransferases (UPDGTs), and deiodinase, causing changes in serum T3 and T4 levels and a decrease in TSH. The foregoing hormones’ level alterations could lead to thyroid hyperplasia/tumors. In addition, EDs affect cellular transporters' action and decrease the T4-TTR binding and thyroid receptors, resulting in T3 tissue changes and ultimately disrupting growth and development processes (congenital disorders).72
Various EDs mechanisms and their effects on the thyroid have been described, such as competition/blocking of the sodium/iodide symporter (NIS) and inhibition of thyroid peroxidase (TPO), leading to a decrease in the synthesis of T3 and T4.72 Inhibition of sulfotransferases (SULTs) can also decrease peripheral T3 synthesis.72 Additionally, EDs disrupts hepatic catabolism through upregulation of glucuronosyltransferases or SULTs (via CAR/PRX or AhR) and hepatic transport through upregulation of organic anion transporting polypeptides (OATPs) or monocarboxylate transporters (MCTs) (via CAR/PRX or AhR). The mechanisms eventually increase the biliary elimination of T3 and T4.72 EDs also induce impaired binding to serum transport proteins, but their effect on the thyroid remains unknown since no studies are available. EDs can also interfere with the binding between thyroid hormone receptor and thyroid response element (TR-TRE), both directly and indirectly, resulting in gene transcription-dependent thyroid hormone activation disruptions. Summary of the impacts of non-ionizing EMF exposure on the thyroid has been presented in Figure 2.
This systematic review has its strengths and limitations. It is the first comprehensive review regarding the effects of EMF on the thyroid. The previous reviews only discuss the overall effect of EMF on human organs, while the specific impact on the thyroid has not been well emphasized. The limitations include an absence of non-English studies, unrepresentative results due to the heterogeneity of the studies, and the low number of studies on the specific topic and low number of studies originally searched as a curated database was used rather than searching the entirety of existing literature. Thus, future studies should focus more on the above aspects.
A number of studies have found significant effects of non-ionizing EMF radiation on thyroid gland and its hormones, but disagreements remained present. Each study has varying standards or procedures related to the instrument, sample size, dosimetry, and wave parameters, such as frequency, distance, and duration of exposure. Currently, there is no valid and standardized method or procedure for assessing the effect of non-ionizing radiation exposure on the thyroid when, in fact, EMF is frequently used daily. Such variations have lead to disputable results.
To date, studies examining the effects of non-ionizing radiation exposure in thyroid hormone transporters and genomic and non-genomic actions is limited. However, further studies with an adequate control group, high sensitivity methods in detecting hormone levels with better protocols are required. Some of the highlighted suggestions for future research are a larger sample size, longer exposure durations, and adjustments of different frequencies. Due to a lack of data, studies explaining the effect of EMF and its relationships with other elements, such as thyroid hormone transporters, genomic and non-genomic actions, and several compensatory mechanisms, require further investigation. Accurate predictive models, such as selection of biomarkers, development of predictive models that can estimate compensatory mechanisms, exposure to external materials, minimization of methodological bias, and other complex factors related to experiments in humans and animals, should be considered in future research.
All data underlying the results are available as part of the article and no additional source data are required.
Figshare: Do electromagnetic fields significantly affect thyroid cells and their functions? – a systematic review from ORSAA database, https://doi.org/10.6084/m9.figshare.21587394.v4. 73
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)