Keywords
Calcium Hydroxide (Ca(OH)2), Gelatin-Chitosan-Tetraethyl Orthosilicate-Calcium Hydroxide (G-CH-TEOS-Ca(OH)2) Composite, capping material, COX-2, PGP 9.5, TNF-α, neutrophils
Calcium hydroxide (Ca(OH)2) is the material of choice for pulp therapy. However, Ca(OH)2 has drawbacks such as toxicity, poor sealing, and tunnel defect formation. Alternative materials have been developed to provide more biocompatible materials with better dentin formation ability. The objective of this study was to evaluate the effect of composites containing gelatin (G), chitosan (CH), tetraethyl orthosilicate (TEOS), and Ca(OH)2, namely G-CH-TEOS-Ca (OH)2 (Extended data) on inflammation of the dental pulp (expression of COX-2, PGP 9.5, TNF-α, and neutrophil number).
A total of 16 Wistar rat models of acute pulp injury were prepared and divided into two groups, treatment and control, 8 with each. In the treatment group, we applied a pulp-capping material using G-CH-TEOS-Ca (OH)2 and Ca(OH)2. On the 1st and 3rd days, rats were sacrificed. Tissue samples from 4 rats in each group were processed for histological preparation. COX-2, PGP 9.5, and TNF-α were observed using immunohistochemical (IHC) staining, and neutrophil numbers were observed using hematoxylin-eosin staining. Image analysis of COX-2, PGP 9.5, and TNF-α expression was performed using ImageJ software.
The results showed a decrease significantly in COX-2 expression while PGP 9.5 and TNF-α expression were significantly higher than those in the control group. Neutrophil numbers were lower in the treatment group than in the control group, but the difference was not statistically significant.
The G-CH-TEOS-Ca (OH)2 composite material may have potential as an exposed pulp medicament by reducing mediator of inflammation (COX-2 expression) and increasing the regeneration factor (TNF-α expression) and nerve (PGP 9.5 expression).
Calcium Hydroxide (Ca(OH)2), Gelatin-Chitosan-Tetraethyl Orthosilicate-Calcium Hydroxide (G-CH-TEOS-Ca(OH)2) Composite, capping material, COX-2, PGP 9.5, TNF-α, neutrophils
In this manuscript, changes in the author's affiliation Bidhari Pidhatika.
We revised the manuscript according to the reviewer's suggestion in the Abstract Conclusion by removing the number of neutrophils.
In the introduction, an explanation has been added regarding the composition of the G-CH-TEOS-Ca (OH)2 material in accordance with previous studies.
The results of the study have been added to the explanation of the statistical analysis of homogeneity, normality and ANOVA analysis. Changes are also made to the contents of Table 1 which previously contained the results of the Post hoc LSD analysis to the results of the ANOVA analysis, while the results of the Post hoc LSD analysis are shown in Figure 6.
Figure 7 which previously displayed the graph of the number of neutrophils has been removed. The graph of the number of neutrophils has been moved and combined with Figure 6.
See the authors' detailed response to the review by Angela Quispe-Salcedo
See the authors' detailed response to the review by Euis Reni Yuslianti
See the authors' detailed response to the review by Caroline Anselmi
Dental caries is one of the most common chronic illnesses worldwide. Tooth decay is a complex illness involving tooth structure, oral bacteria, and dietary carbohydrates. It dissolves the mineral content of the teeth and is mostly dependent on several important contributing factors.1 Small surface roughness or subsurface demineralization is the first symptom, followed by cavitation, pulp involvement, swelling, abscesses, and systemic symptoms.2
Severe toothache can be incapacitating, and infection and sepsis resulting from caries spreading to the dental pulp can sometimes cause major systemic consequences (e.g., spreading local infection and, very rarely, treatment-related death due to anesthesia-related complications) in addition to tooth loss.3 An inflammatory reaction in the pulp is called pulpitis.4 occurs primarily due to cariogenic causes and less frequently as a result of trauma, dental abnormalities, and restorative procedures. Pulp hyperemia occurs when inciting conditions are present. Pulp diseases, including acute pulpitis, chronic pulpitis, pulp degeneration, and pulp necrosis, are dental disorders that originate in pulp tissue. Dental caries that are not treated appropriately may later evolve into pulp disease.5
Neutrophils are the most abundant white blood cell in humans. Neutrophils are immunological cells with unique biological characteristics that exhibit strong antibacterial activity. These cells are highly effective in phagocytosing prokaryotic and eukaryotic species, leading to their death. Their role as first responders to acute inflammation, aiding in the healing of injured tissues and removal of pathogens, is well established. Crucially, the antimicrobial activity of neutrophils is not limited to their capacity to combat bacteria within a restricted intracellular space. Neutrophils also release effectors into the extracellular space where their killing apparatus can survive and continue to operate long after death.6,7
An important evolutionary enzyme that is involved in a variety of physiological and pathological processes is COX-2. It controls the expression levels of numerous serum proteins and plays a crucial role in viral infection. This enzyme significantly affects proinflammatory cytokines, and its inhibition or lack by itself does not impair the immune system’s ability to fight illness.8
Protein gene product 9.5 (PGP 9.5) is a neuronal protein with a specific biological purpose. It is expressed in axons and is used to map individuals with neurodegenerative diseases. It functions as a carboxyl-terminal hydrolase and ligase. Significantly, while PGP 9.5, a highly neuron-specific protein that is frequently employed as a generic neuronal marker, has also been utilized to quantify the neural protein recovered from pulpal tissue7 and to detect nerve fibers in the dental pulp. It has been shown to be helpful in identifying the A-delta and C fibers of dental pulp.9 It has been hypothesized that processes leading to nerve degeneration are connected to the early loss or lack of expression of this protein. The nerve structures of the dental pulp were assessed using PGP 9.5. A potential diagnostic marker for identifying nerve fibers in inflamed tooth pulp is PGP 9.5 expression.10
In response to bacterial endotoxins, such as lipopolysaccharide (LPS), macrophages preferentially release tumor necrosis factor α (TNF-α).9 Numerous lines of evidence suggest that TNF-α is responsible for maintaining chemotaxis in fibroblasts and inflammatory cells.11 Inflammatory cytokines, such as TNF-α and interleukins, play a crucial role in the first 48 h of normal pulp tissue healing. They not only attract inflammatory cells and stem/progenitor cells but also trigger a series of events that lead to tissue regeneration, reparative dentin formation, or both.12
Traditionally, calcium hydroxide has been the preferred material for vital pulp therapy. At pH 12, calcium hydroxide (Ca(OH)2) is an alkaline substance that has been shown to have bactericidal effects and the capacity to stimulate the growth of hard tissue in human teeth. Ca(OH)2 is widely used in vital pulp treatment. Nevertheless, Ca(OH)2 has a number of drawbacks, such as superficial necrosis of the pulp and surrounding tissues, poor sealing and adherence to dentin, unpredictable dentinal bridge formation, and tunnel defects in these bridges that could serve as possible entry points for bacteria. Because of this weakness, scientists are searching for substitute materials with antimicrobial properties that can be mixed with calcium hydroxide.13,14
In the past two years, Tetraethyl Orthosilicate (TEOS), Carbonated Hydroxyapatite (CHA), chitosan (CH), and gelatin (G) have been used to create composite materials. A silane-based crosslinker that can establish bonds with both inorganic and organic materials is called TEOS. The inorganic mineral Ca(OH)2 found in this study’s composite material, gelatin, chitosan, TEOS, and Ca(OH)2, bonds to polymer chains in a naturally occurring organic polymer matrix (gelatin and chitosan) by the action of TEOS, a crosslinker. Silicon (Si) found in TEOS binds as siloxane to form a silica core that can prevent the spontaneous release of Ca(OH)2. This substance can be used for medication delivery with good stability.15 In this study, the composition of the G-CH-TEOS-Ca(OH)2 material is in accordance with previous studies. The results of previous study indicated that G-CH-TEOS-Ca(OH)2 has a positive effect on Nestin expression.16
This study aimed to determine the effect of composite materials with gelatin (G), chitosan (CH), Tetraethyl Orthosilicate (TEOS), and Ca(OH)2 on inflamed dental pulp. The research was done outside on mice. Evaluation was carried out on the expression of inflammatory factors, such as COX-2 expression and number of neutrophils, pulp vitality (PGP 9.5 expression), and pulp repair process (TNF-α expression).
The Ethics Commission of the Faculty of Dentistry at Universitas Gadjah Mada approved the study procedure through the issuance of ethical clearance number No. 58/UN1/KEP/FKG-RSGM/EC/2023 on May 9, 2023. The Integrated Research and Testing Laboratory (LPPT) Unit IV, Universitas Gadjah Mada, provided experimental animals. At the Integrated Research Laboratory, Faculty of Dentistry, Universitas Gadjah Mada, the gelatin-chitosan-TEOS-Ca (OH)2 composite material was prepared in accordance with our earlier study.16
Sixteen male Wistar rats (Extended data) were used in the experiment and were divided into two groups. Eight rats in each group were divided into four groups on the first and third observation days following treatment. Before treatment and termination to Wistar rats, all rats were anesthetized using Ketamine HCl (0.1 ml/100 g body weight) (Ketamil, CV. Karebet Karya Persada, Indonesia, catalog No. 2960000999-2HH-100335784) to reduce animal suffering.38 Ketamine HCl (0.1 ml/100 g body weight) was used intramuscularly to anesthetize the experimental animals, and cavities were then prepared. The maxillary first molars were prepared on the occlusal surface to pulp depth using a diamond round bur No. 010 driven by a micromotor that rotated at 35,000 rpm. The treatment group was treated with Gelatin, Chitosan, TEOS, and Ca(OH)2 composite,37,38 whereas the control group was treated with Ca(OH)2 (Brazilian Biodinamica brand powder). The application was performed on the base of the cavity before temporary filling was used to fill it ( Figure 1).36
The rats were administered ketamine HCl (0.1) and xylene ml/100-gram body weight) intramuscularly on the thigh to induce anesthesia on the first and third days following treatment. The jaws were fixed for four weeks at 4°C in 10% buffered formalin and decalcified with 10% EDTA (pH 7.4) (Extended data). The next step involved tissue cutting, histological preparation, and the creation of paraffin blocks. Using a microtome (Accu-Cut® SRMTM Rotary Microtome, Sakura Finetechnical Co., Ltd, Japan), paraffin blocks were sliced into 4 μm thick pieces for hematoxylin-eosin and immunohistochemistry (IHC) staining.
The paraffin sections were dewaxed three times for five minutes apiece using xylene, and the rehydration process was then carried out using 100% alcohol for five minutes, and 95%, 90%, 80%, and 70% for three minutes each. An immunohistochemistry kit (Elabscience, USA) was used for IHC staining. To remove endogenous peroxidase activity, samples were incubated with 3% H2O2 for 10 min. The samples were then washed thrice in PBS for two minutes. After adding Normal Goat Blocking Buffer (Elabscience, USA), the samples were incubated for 30 min at 37°C (Extended data). The samples were shaken to remove excess liquid. Primary antibodies against COX-2 (Rabbit Polyclonal antibody COX-2, NB100-689, Novusbio, USA), TNF-α (Rabbit Polyclonal Antibody TNF-α, bs2081R, Bioss Antibody, USA), and PGP 9.5, (Monoclonal Mouse Antibody PGP 9.5, MAB60072, R&D Systems, USA) were added at a proper dilution ratio of 1:100 in PBS. Overnight Incubation was performed at 4°C. The next day, the cells were washed three times with PBS washes for two minutes. Polyperoxidase-anti-Mouse/rabbit IgG (Elabscience, USA) was added to the samples, which were then incubated for 20 min at room temperature. PBS was used for washing three times for two minutes. After adding 3,3′-diaminobenzidine (DAB) to the samples, the DAB coloring process was closely monitored until the color of the samples turned brown, indicating a positive result. The chromogenic reaction was stopped by washing the sections in deionized water, after which hematoxylin was used for counterstaining.
Using a light microscope at 200× magnification, COX-2 expression, PGP 9.5, and TNF-α were identified as varying degrees of brown staining, primarily found in the cytoplasm and nuclear membrane of the positive cells. Three fields were observed in the prepared cavities ( Figure 1).36 ImageJ software (National Institutes of Health) free access, version 154, was used for the image analysis of COX-2, PGP 9.5 and TNF-α expression. Method of Counting: Three distinct ocular fields were used to count neutrophil cells in the cavity preparation area from HE staining. Expression of COX-2, PGP 9.5 and TNF-α were performed using ImageJ software on a Windows-based platform, with the aid of an additional plug-in available for free download from https://sourceforge.net/projects/ihcprofiler/. Data were analyzed using post hoc LSD and ANOVA (IBM SPSS Statistics, USA).
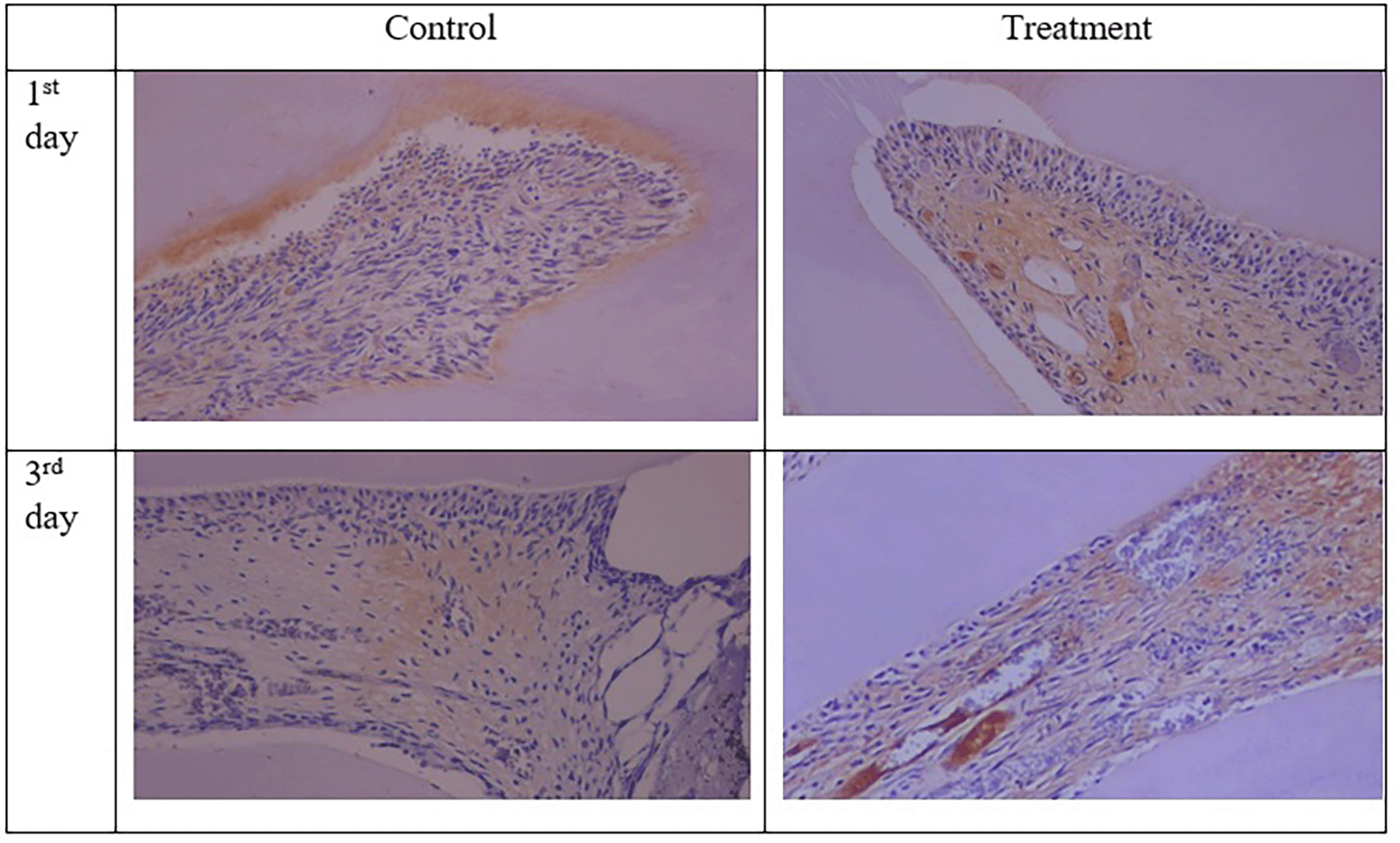
The results of the normality test using Shapiro-Wilk and homogeneity data of COX-2, PGP 9.5, TNF-α and number of neutrophils using Levene’s test showed normal and homogeneous data (Table 1). Analysis using ANOVA showed that the expression of COX-2, PGP 9.5, TNF-α was significantly different (p<0.05) but the number of neutrophils was not significantly different (p>0.05). Data analysis was continued using Post hoc LSD (Figure 6). The results showed that COX-2 expression on all observation days 1st and 3rd was lower in the treatment group than in the control group (Figure 6). The results of immunohistochemical staining using anti-COX-2 antibodies showed a brown color in the extracellular matrix of the dental pulp with varying intensities, indicating positive COX-2 expression. The stronger the color intensity, the stronger is the COX-2 expression. Histological observations ( Figures 2 and 6)36 on the 1st day of both groups showed that there was COX-2 expression by the extracellular matrix in the area under the injury. The treatment group showed lighter color intensity than the control group. On the 3rd day, there was a significant decrease in COX-2 expression (Figure 6) in both groups, with the color intensity in the control group being stronger than that in the treatment group ( Figure 2). On the observation day, there was no significant difference in COX-2 expression between the control and treatment groups (Figure 6).36
(Magnification ×200).
(Magnification x200).
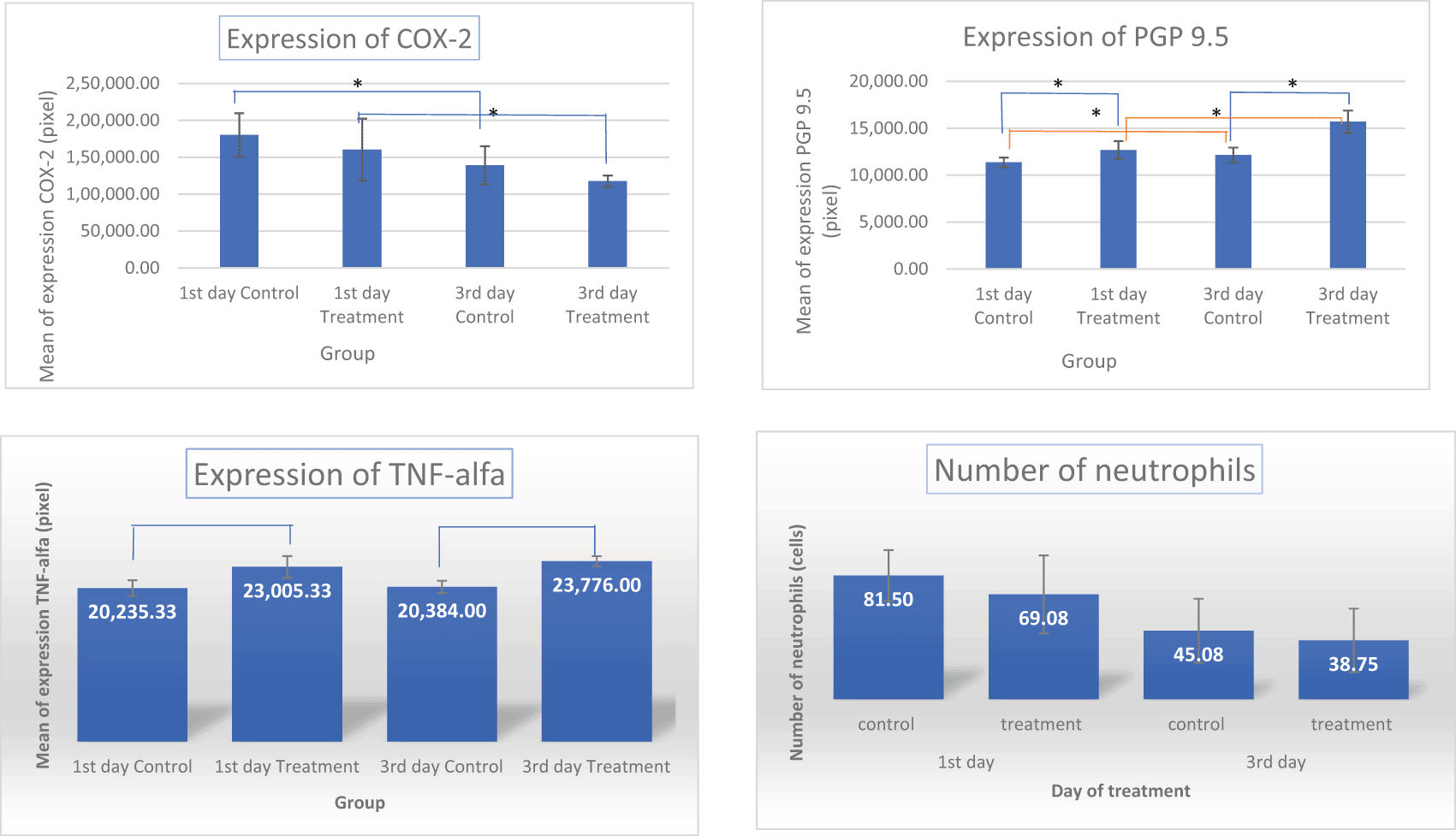
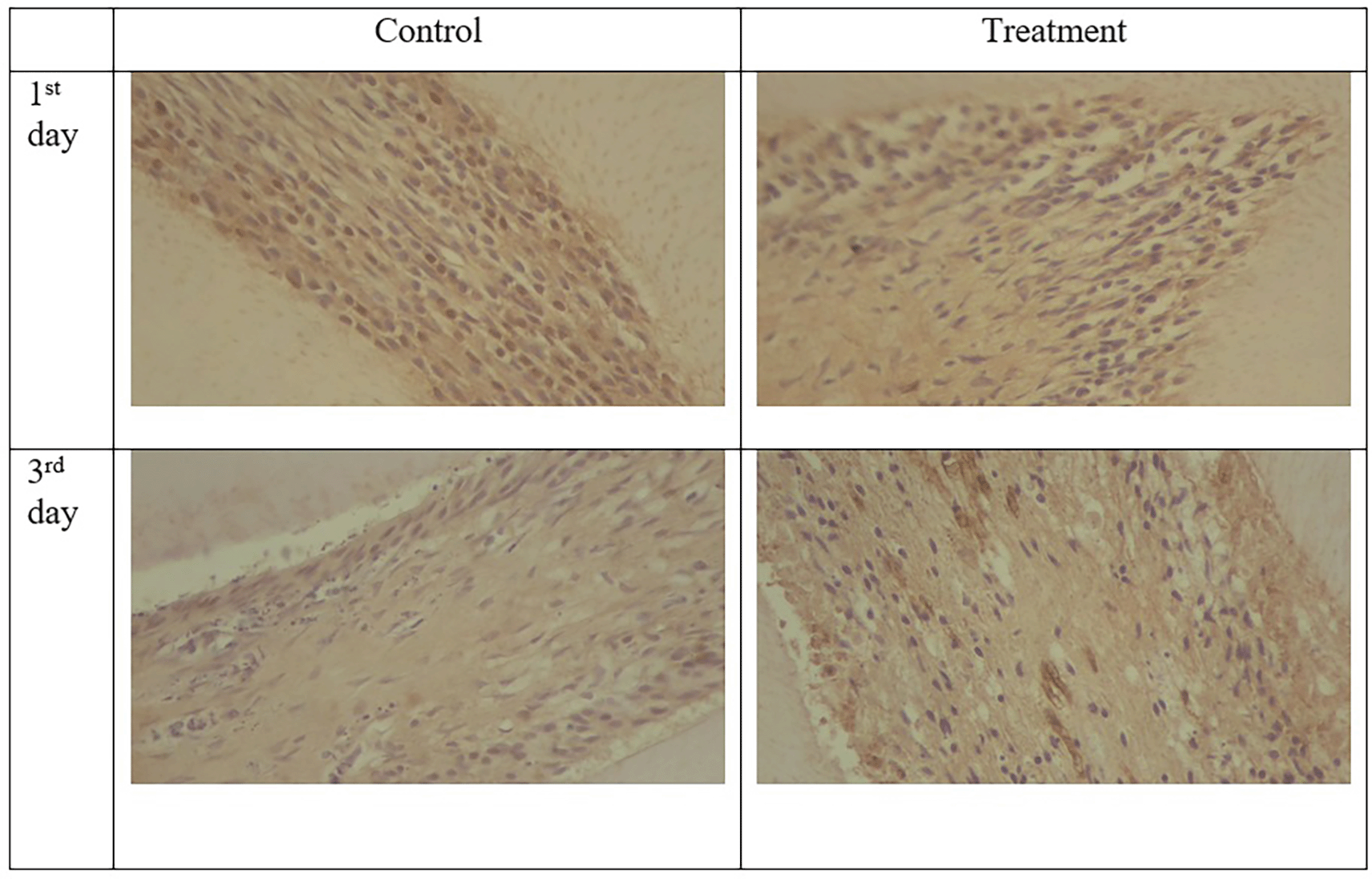
The results for PGP 9.5 showed brown nerve pulp after administration of gelatin-chitosan-TEOS-Ca (OH)2 composite compared to controls ( Figure 3),36 and the expression in the treatment group was significantly higher compared to controls on both observation days 1st and 3rd ( Figure 6). The highest expression of PGP 9.5 was observed in the treatment group on 3rd day of treatment ( Figure 6).
TNF-α expression appeared brown in the odontoblasts after administration of the gelatin-chitosan-TEOS-Ca (OH)2 composite and controls on the 1st and 3rd days ( Figure 4).36 The expression of TNF-α in the control group was weaker than that in the control group on 3rd ( Figure 6). On the 1st and 3rd days of observation, there was no significant difference between the control group; however, TNF-α expression increased significantly between the control and treatment groups based on the day of observation ( Figure 6). The highest TNF-α expression appeared on the 3rd day of treatment ( Figure 6).36
The results of histological observations ( Figures 5 and 6)36 showed that on 1st day in the group administered Ca(OH)2 (control), the number of neutrophils was higher than that in the treatment group. Results of the mean neutrophil number calculation. The mean number of neutrophils in both the groups decreased significantly on the 3rd days of observation ( Figure 6).
The results of this study showed that the use of a composite gelatin-chitosan-TEOS-Ca (OH)2 medicament as pulp capping leads to increased COX-2 expression on the 1st day then decreases significantly on the 3rd day ( Figures 2 and 6).36 These results were in line with the number of neutrophils which decreased in 3rd day of observation ( Figure 5). These findings suggest that the gelatin-chitosan-TEOS-Ca (OH)2 composite material induces a lower inflammatory response.
In this study, the cavity was prepared using a low-speed rotary instrument. Every rotary cutting instrument generates heat and vibrations that may damage the tooth pulp. If heat is delivered to the pulp, it may cause histopathological alterations can occur. Excessive heat transmission may also cause pulp inflammation and irritation.17,18
Inflammation of the dental pulp is a complicated process that is controlled by several molecular mediators, and the mechanism is the same as that of inflammation in other connective tissues. The primary role of these inflammatory mediators is to shield tissues from external irritants such as chemicals, mechanical forces, and germs.19–21 Injuries that result from the preparation of deep cavities can trigger the production of TNF-alpha and IL-1 by tissue-resident macrophages and fibroblasts, which can increase COX-2 expression in the injured area.22
The findings demonstrated that COX-2 expression was discernible in the extracellular matrix, neutrophils, macrophages, lymphocytes, and fibroblasts in the region of injury. Tissue damage, such as that caused by surgery or conditions such as pulpitis or periodontitis, causes the creation of COX-2, which in turn causes the synthesis of prostaglandins that sensitize pain fibers and encourage inflammation.23 PGE2 and prostacyclin are products of COX-2 activity and are implicated in various physiological and pathological processes, including inflammation and pain.24
The dental pulp can be impacted and harmed by exogenous irritants, such as mechanical, chemical, and bacterial agents, because they can activate the pulp’s innate and adaptive immune responses in addition to a variety of molecular mediators. Damage resulting from prepared deep holes has the potential to activate tissue-resident macrophages and fibroblasts, causing them to release TNF-α and IL-1, which in turn can elevate COX-2 expression in injured areas.25
There was a significant difference between the 1st and 3rd day of treatment as well as between the 1st and 3rd day of control, and the COX-2 color intensity in the treatment groups was lower on the first and third days compared to the control group. According to Jiang et al.,26 this outcome is assumed to be caused by the antibacterial and anti-inflammatory qualities of chitosan, which can reduce the bacterial load and reduce the initial inflammatory insult. The regulated release of Ca(OH)2 is facilitated by the matrix offered by gelatin. This minimizes the risk of excessive initial burst release, limits the entry of pathogens, and reduces the requirement for acute inflammatory cell infiltration. Both gelatin and Ca(OH)2 exhibit anti-inflammatory properties.27,28 The composite gelatin-chitosan-TEOS-Ca (OH)2 provides an environment that limits post-treatment inflammation in the pulp tissue, as seen by the lowered levels of these proinflammatory cytokines and mediators. (Extendd data).
The evaluation conducted on the first day post-treatment aided in determining the immediate reaction of COX-2 expression to the therapy, and we observed a non-significant decrease in COX-2 expression in the treatment group when compared to the control. The evaluation on the third day aided in tracking any short-term variations and possible trends in COX-2 expression after the first reaction, where the mean expression was much lower than on the first day.
After taking these factors into consideration, the composite gelatin-chitosan-TEOS-Ca (OH)2 material seems to help the pulp by reducing the initial inflammatory insult and, due to the synergistic effects of its bioactive components, fostering a more favorable healing environment. As the number of observation days increased, we saw a drop in the pattern of COX-2 expression. The overall pattern was reduced on the third day when compared to the first, with a significant decrease between the control and treatment groups ( Figure 6). On the 3rd day, a comparison of COX-2 expression and neutrophil counts between the treatment and control groups revealed no discernible differences. This suggests that the reduction in acute inflammation caused by the gelatin-chitosan-TEOS-Ca (OH)2 material is equivalent to that of Ca(OH)2. It is crucial to remember that further investigation and assessment are required to validate the long-term results and clinical effectiveness of this composite material. Specifically, the ratios of gelatin and chitosan within the material need to be changed to enhance their anti-inflammatory properties and maximize their efficacy.
The presence of chitosan may be the reason for the notable increase in PGP 9.5 expression on the first and third days after injection, which is consistent with other research showing the regeneration potential of chitosan-based composites. Chitosan, a natural copolymer of glucosamine and N-acetylglucosamine, is used in gene therapy, tissue engineering (TE), and other biomedical fields because of its biocompatibility, stability, sterilizability, biodegradability, antimicrobial activity, and immunostimulatory activity.29
Chitosan also offers a biomimetic microenvironment that promotes cell growth owing to its similarity to glycosaminoglycans (GAGs) found in naturally occurring extracellular matrix (ECM) materials. Furthermore, its osteoconductive properties can support osteogenic differentiation and biomineralization of stem/progenitor cells.29 The shortcomings of chitosan in terms of mechanical strength and early cell attachment can be effectively addressed by blending it with other biomaterials, such as gelatin.29 A protein fragment known as gelatin is produced when collagen fibers partially break down. Its numerous benefits, such as low antigenicity, biodegradability, hydrogel qualities, and affordability, have made it widely available in TE. Additionally, the Arg–Gly–Asp (RGD) integrin recognition motif supports the first cell attachment. Blends of chitosan and gelatin, or CS/Gel, have been suggested as scaffolding materials for bone regeneration and other tissues, including skin, cartilage, and peripheral nerves.29 These blends can potentially combine these features.
The incorporation of calcium hydroxide (Ca(OH)2) and tetraethyl orthosilicate (TEOS) into the composite may increase its effectiveness by promoting cellular adhesion, proliferation, and extracellular matrix formation. It is probable that the composite material triggered an early neurogenic response by triggering signalling pathways linked to cell differentiation and proliferation. Therefore, the persistent increase in PGP 9.5 expression may be a sign of continued axonal development and growth, which would ultimately aid in functional recovery.29 The study findings confirmed that the treatment group receiving gelatin, chitosan, TEOS, and Ca(OH)2 had the highest levels of PGP 9.5 expression.
Two distinct receptors attach to TNF-α and initiate signal transduction pathways. These pathways trigger numerous biological responses such as cell survival, differentiation, and proliferation.30 After administering the gelatin-chitosan-TEOS-Ca (OH)2 composite and Ca(OH)2 to the control group, odontoblasts were shown to express TNF-α, indicating that both materials triggered an inflammatory response. The brown coloration observed indicates an upregulation of TNF-α production, which is a key proinflammatory cytokine involved in various cellular processes, including tissue repair and inflammation. On the 1st and 3rd days, there was a similar expression of TNF-α in the treatment and control groups, indicating that the level of inflammatory response caused by both materials in tooth pulp was equivalent. This result is consistent with other research showing that Ca(OH)2 has strong immunomodulatory effects in dental pulp tissues, which upregulates TNF-α expression.30
On the third day, the control group showed lower TNF-α expression than the treatment group, which was remarkable. This disparity could point to variations in the TNF-α expression kinetics between the gelatin-chitosan-TEOS-Ca (OH)2 composite and pure Ca(OH)2. Compared with pure Ca(OH)2, the composite material might have produced a stronger inflammatory response or maintained TNF-α expression for a longer period of time. Further research is necessary to clarify the underlying mechanisms causing this variation in TNF-α expression between the two groups.
On the 1st day, infiltration of inflammatory cells, especially neutrophils, was observed in both groups. Neutrophils are leukocytes that first migrate to tissue/injury locations. These cells are dominant within 24-36 hours after injury and function to eliminate irritants and damaged tissue through phagocytosis.31,32 Changes in the endothelium surface caused by the activation of inflammatory mediators, such as histamine, cysteinyl-leukotrienes, and cytokines generated by tissue-resident leukocytes during damage can trigger the recruitment of neutrophils to the injured area.31 On the first and third days, the mean neutrophil count of the treatment group was lower than that of the control group. These results are in line with earlier research showing the inherent anti-inflammatory properties of the constituent parts of our composite, such as chitosan,33 gelatin,28 and Ca(OH)2.29 Post-treatment inflammation of the pulp tissue was inhibited by the composite gelatin-chitosan-TEOS-Ca (OH)2, as evidenced by the decreased levels of proinflammatory cytokines and mediators. This suggests that pulp-capping methods result in better pulp healing and regeneration rather than necrosis or infection, which could compromise the effectiveness of vital pulp therapies.34 Reductions in these indicators have been linked to improved clinical results in comparable studies. This suggests that pulp-capping treatments result in better pulp healing and regeneration rather than necrosis or infection, which compromises the effectiveness of vital pulp therapy procedures.35
The gelatin-chitosan-TEOS-Ca (OH)2 composite may facilitate a rapid resolution of inflammation and stabilization of the pulp capping wound site by restricting early neutrophil infiltration. Based on the results obtained, we conclude that the gelatin-chitosan-TEOS-Ca (OH)2 composite and Ca(OH)2 evoke similar biological responses in terms of neutrophil number, with no significant difference observed between the treatment group and the control group every day of observation. The results of the study also showed that gelatin-chitosan-TEOS-Ca(OH)2 composite can stimulate the pulp repair process and pulp vitality as indicated by the expression of TNF-alpha and PGP 9.5. TNF-α expression as a response to inflammation may also affect the formation of dentinal bridges by modulating cellular responses and extracellular matrices.
The results of this study support previous studies that the use of gelatin-chitosan-TEOS-Ca(OH)2 composite has the potential as a medicament for exposed pulp. This is evidenced by its ability to carry Ca(OH)2 more effectively by reducing the alkaline properties indicated by weak positive Nestin expression around the irritated pulp. This indicates the proliferation and differentiation of odontoblast cells and cells in the pulp towards tissue regeneration.16 To completely understand the therapeutic effectiveness and underlying mechanisms action of gelatin-chitosan-TEOS-Ca (OH)2 composite in endodontic applications, more research is required including its effects on odontoblast-like cells and TGF beta.
The gelatin-chitosan-TEOS-Ca (OH)2 composite material has potential as an anti-inflammatory agent in the exposed pulp by reducing COX-2 expression. These findings indicate a possible regenerative effect of the gelatin-chitosan-TEOS-Ca (OH)2 composite by increasing TNF-α expression and PGP 9.5, making it a promising candidate for further exploration in dental pulp therapy.
Figshare: Data final assignment recognition program (RTA)-2024. https://doi.org/10.6084/m9.figshare.2681988736
This project contains the following:
The data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Figshare: Data final assignment recognition program (RTA)-2024. https://doi.org/10.6084/m9.figshare.27021160.v137
This project contains the following:
The data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Figshare: Data final assignment recognition program (RTA)-2024. https://doi.org/10.6084/m9.figshare.27020713.v238
This project contains the following:
The data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pulp biology, in vitro and in vivo analysis of biomaterials, cytotoxicity of dental materials.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pulp biology, in vitro and in vivo analysis of biomaterials, cytotoxicity of dental materials.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Oral biology, biomedical, oral biochemistry, herbal medicine in dentistry
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Dental pulp biology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 20 Feb 25 |
read | ||
|
Version 2 (revision) 30 Jan 25 |
read | ||
|
Version 1 21 Oct 24 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)