Keywords
Tuberculosis, Drug-induced Liver Injury, Autoimmune Hepatitis, Primary Biliary Cholangitis, Overlap Syndrome
This article presents two patients who were diagnosed with autoimmune liver disease (autoimmune hepatitis and primary biliary cholangitis overlap syndrome) during anti-tuberculosis therapy, which is a rarely reported occurrence. It highlights the challenges in distinguishing drug-induced liver injury from authentic autoimmune liver disease. It also points out the importance of considering autoimmune liver disease as a potential diagnosis revealed by the setting of drug-induced liver injury.
Tuberculosis, Drug-induced Liver Injury, Autoimmune Hepatitis, Primary Biliary Cholangitis, Overlap Syndrome
Tuberculosis (TB) remains a significant public health challenge in numerous countries worldwide, raising concerns about the potential risks associated with anti-TB drugs. Specifically, these drugs have been implicated in drug-induced liver injury (DILI) with a reported incidence ranging from 2% to 28%.1 DILI can manifest as mild liver enzyme elevation to life-threatening liver failure, commonly resulting from an idiosyncratic reaction involving either metabolic or immune-mediated mechanisms.2
Moreover, DILI with autoimmune features often presents diagnostic complexities, particularly when distinguishing it from autoimmune liver diseases (ALD) such as autoimmune hepatitis (AIH) and primary biliary cholangitis (PBC). In this article, we present two compelling instances of ALD, specifically the AIH-PBC overlap syndrome, which were unveiled during TB therapy. In addition, we conducted a review of the literature, seeking insights into the intricate relationship between TB treatment and ALD.
A 49-year-old Caucasian woman, with atopic background, was diagnosed with abdominal lymph node and peritoneal tuberculosis (TB). She was started on first-line anti-TB therapy with Isoniazid (INH), Rifampicin (RIF), Pyrazinamide (PZA) and Ethambutol (EMB) following normal pre-treatment liver function tests (LFTs).
A month after starting the treatment, the patient presented with Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) syndrome attributed to EMB. DRESS syndrome was diagnosed based on a pruritic macular-papular skin rash, fever, eosinophilia (3100/mm3), immunoallergic leukocytoclastic skin vasculitis, and significantly elevated liver enzymes including Aspartate Aminotransferase (AST) at 7 to 9 times the upper limit of normal (ULN), Alanine Aminotransferase (ALT) at 3 to 4 xULN, Alkaline Phosphatase (ALP) at 2.6 xULN, and Gamma Glutamyl Transferase (GGT) at 7 xULN, with normal bilirubin levels (BIL). Anti-TB drugs were suspended, and DRESS syndrome resolved within a month, allowing for the restart of a modified treatment regimen, replacing EMB with a fluoroquinolone: Levofloxacin (LFX) with INH-RIF-PZA during 2 months followed by 6 months of INH-RIF.
However, by the 8th month of anti-TB treatment (INH-RIF), the patient exhibited clinical signs of acute hepatocellular injury, presenting with jaundice and significantly elevated levels of AST (18xULN), ALT (10xULN), ALP (1.3xULN), GGT (3xULN), and BIL (12 mg/dl) predominantly conjugated bilirubin (CBIL: 9 mg/dl), with normal factor V levels (84%). There were no clinical or biological signs of hypersensitivity, and abdominal ultrasound (US) showed no abnormalities.
Aetiological investigation for liver injury ruled out a viral hepatitis A, B and C, as well as HIV infection (negative anti-HAV IgM, negative HBsAg, positive anti-HBc, negative DNA-HBV, negative anti-HVC and negative HIV serology). Meanwhile, immunological assessment revealed positivity for anti-nuclear antibody (ANA) with a titre of 1/400, anti-Smooth Muscle Antibody (ASMA), anti-Mitochondrial M2 antibody (AMA-M2) and anti-gp210 antibody. The rest of the Liverdot was negative (anti-sp100, anti-LKM1, anti-LC1, anti-SLA/LP). Additionally, the patient had mild hypergammaglobulinaemia (1750 mg/dl) with normal levels of IgA, IgM, and IgG.
Based on these findings and the pharmacovigilance survey, the diagnosis of drug-induced liver injury (DILI) with auto-immune features was considered, leading to the indefinite discontinuation of anti-TB drugs, especially considering that TB was declared cured.
At follow-up, LFTs returned to normal levels within 2 months. However, the patient complained of persistent right upper quadrant abdominal discomfort without pruritus or fatigue. Immunological assessment at 3 months showed the same previous findings with an increased ANA titre of 1/800. Abdominal ultrasound at 6 months revealed a dysmorphic liver with surface irregularity, right lobe hypotrophy, and left lobe hypertrophy, but no signs of portal hypertension or impaired liver function. Transient elastography using Fibroscan® revealed a median liver elasticity of 12.2 kPa. Subsequently, a liver biopsy was performed for diagnostic and prognostic purposes, which demonstrated septal fibrosis, interface hepatitis (Figure 1), hepatic rosette formation, and non-suppurative destructive lymphocytic cholangitis (florid duct lesions).
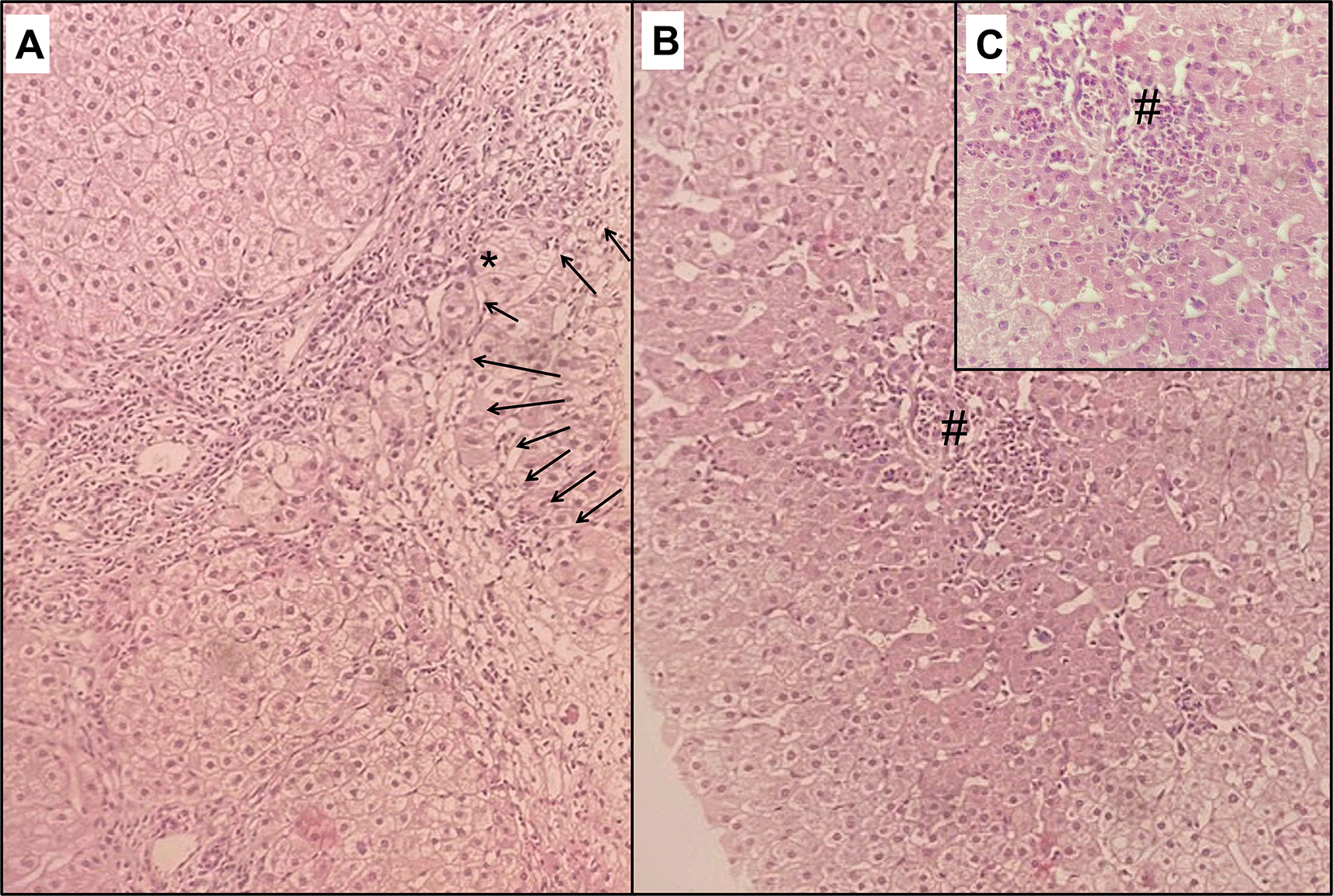
Hematoxylin and eosin staining showing in (A), on x100 magnification, a portal tract enlarged with fibrosis along with fibrous bands separating clusters of hepatocytes with an annular tendency. This fibrosis contains lymphoplasmacytic and polymorphic inflammatory infiltrate disrupting the limiting plate and extending to periportal hepatocytes, resulting in interface hepatitis (arrows). Ballooned hepatocytes are also visible (*). Lobular inflammatory infiltrate with necrosis (#) is also noted in (B) and in x200 magnification (C).
These histological features aligned with an overlap syndrome involving AIH and PBC. Accordingly, the patient was started on ursodeoxycholic acid (UDCA) 15 mg/kg/day and azathioprine 1.2 mg/kg/day with good treatment adherence and tolerability. Given that LFTs were normal, no corticosteroids were prescribed, and LFTs remained normal during a 7-month follow-up period.
A 64-year-old Caucasian man with no prior medical history was diagnosed with pulmonary TB following a positive sputum polymerase chain reaction result. Pre-treatment LFTs showed AST levels at 1.5 xULN with normal levels of ALT, ALP, GGT, and BIL. He received initial anti-TB therapy (INH, RIF, PZA, and EMB) for two months. At the end of this period, the patient developed jaundice due to acute hepatitis, with levels of AST at 15 xULN, ALT at 4 xULN, ALP at 1.6 xULN, GGT at 5 xULN, BIL at 2 mg/dl, and prothrombin time (PT) at 51%. The treatment was consequently discontinued. However, an acute liver injury (ALI) was settled, as indicated by the decline in PT to 46% alongside hypertransaminasemia worsening (AST at 22 xULN, ALT at 8 xULN) and a rise in BIL levels to 3 mg/dl.
An aetiological investigation revealed active hepatitis B (positive AgHBs, negative AgHBe, and a viral load of 8,000,000 IU/ml). Alcohol intake and other viral hepatitis were excluded (negative IgM HAV, negative IgM HEV, negative anti-HCV, negative HIV serology, and previous immunity to EBV, CMV, and HSV 1 and 2). The patient was started on entecavir 0.5 mg/d. Disease assessment showed a dysmorphic liver on ultrasound with irregular contours and the presence of oesophageal varices on endoscopy.
Within one week of initiating entecavir, LFTs continued to worsen, with AST reaching 26 xULN, ALT reaching 9 xULN, BIL increasing to 3.5 mg/dl, and PT dropping to 30%. Further immunological assays showed positive ANA at 1/100 and ASMA at 1/100. Other liver-related autoantibodies were negative. The diagnosis of drug-induced autoimmune liver disease was suspected, and the patient was started on oral corticosteroids (prednisolone 30 mg/d) following negative sputum bacilloscopy tests and an expert opinion.
In 3 weeks, LFTs significantly improved (AST at 5 xULN, ALT at 2 xULN, BIL at 2 mg/dl, and PT at 48%), with persistent cholestasis (ALP at 1.6 xULN, GGT at 5 xULN). A liver biopsy revealed portal and periportal extensive fibrosis with regenerative nodules, lymphoplasmocytic infiltrate in the portal tract extending into the lobule with hepatic rosette formation, and a marked interface hepatitis. Florid bile duct lesions with evident ductopenia were also observed. Several ground glass hepatocytes and central ballooning degeneration were found, attesting to active hepatitis B infection.
In summary, coupled with other findings, pathology results provided evidence of chronic liver disease at cirrhosis stage with severe activity (A3-F4 according to Metavir score) due to an AIH-PBC overlap syndrome, along with viral hepatitis B. Accordingly, the patient was kept on entecavir and corticosteroids, and UDCA was started at a 15 mg/kg/d.
LFTs continuously improved within two months. Nevertheless, expert opinion recommended no anti-TB restart until the total normalisation of liver enzymes, given that TB symptoms and radiographic lesions had considerably recovered. Immunosuppressive therapy was allowed at that point.
Therefore, the patient was started on azathioprine (50 mg/d, then 100 mg/d) alongside tapering corticosteroids (5 mg/3 weeks), with good compliance and tolerability to the treatment. After six months, biological findings showed complete normalisation of LFTs, and the viral load of HBV was 40 IU/ml.
Though the manifestation of AIH-PBC overlap syndrome during anti-TB treatment is uncommon, drug-induced autoimmune liver disease (DIALD), which encompasses various combinations of AIH and DILI, has been increasingly studied. DIALD can be classified into three main types3 (Table 1). First, AIH with DILI includes patients already diagnosed with AIH, who reactivate their quiescent disease upon the introduction of a new drug, often with advanced fibrosis. Second, drug-induced AIH (DI-AIH) involves patients with undiagnosed low-grade disease or genetically predisposed to develop AIH, where the drug generates an immune reaction sustaining AIH, leading to chronic inflammation and requiring continuous immunosuppressive therapy. DI-AIH patients usually carry typical HLA-DR haplotypes associated with AIH. The third type is immune-mediated DILI (IM-DILI), where the drug triggers an adverse immune reaction with hypersensitivity. The liver injury in IM-DILI can be acute or chronic, and it may resolve or remain quiescent after drug cessation. IM-DILI is the most common DIALD type and is also referred to as drug-induced AIH-like injury. It often resembles idiopathic AIH, showing a complete response to corticosteroid treatment, yet with no relapse.4–6
The immunopathogenesis of DILI with autoimmune features is not fully understood. It has been hypothesised that these idiosyncratic adverse events involve reactive drug metabolites, primarily produced by CYP450, which bind covalently to this enzyme, forming neoantigens that activate humoral and cellular immune reactions, leading to liver injury.2,4
Distinguishing classical AIH from DIALD is challenging as no specific feature is exclusive to either entity. Certain characteristics favour drug-induced AIH-like liver injury, including the presence of hypersensitivity features (in up to 30% of cases), absence of advanced fibrosis or cirrhosis at presentation, and no relapse after steroids discontinuation.4–6
As both conditions can share similar clinical, biochemical, and serological patterns, histological findings may not always be helpful in distinguishing between the two diagnoses. Nevertheless, Suzuki et al.7 found that certain features such as portal and intra-acinar plasma cells, rosette formation, and emperipolesis, along with more severe histological inflammation scores (Ishak score), supported the diagnosis of AIH. On the other hand, portal neutrophils and intracellular cholestasis were more prevalent in DILI. However, interface hepatitis, emperipolesis, and rosette formation were also present in 89%, 34%, and 40% of DILI cases, respectively.
Recently, Lammert et al.8 suggested that autoantibody profiling could be a promising and useful tool to distinguish idiopathic AIH and DILI with AIH-like phenotype. Among 65 patients with autoimmune phenotype DILI and 17 patients with de novo AIH, they found that the former group had elevated IgM (55%) but not IgG autoantibodies, whereas the latter group had elevated IgG (46%) and IgM (70%) autoantibodies, theorising the unique IgG autoantibodies signature in authentic AIH. Based on this, they developed a model incorporating IgG autoantibodies directed against centromere protein B, myosin, mitochondrial antigen, and nucleosome antigen, which accurately predicted de novo AIH, distinguishing it from autoimmune DILI, with an area under the receiver operating characteristic (ROC) curve of 0.88.
A study by Björnsson et al.9 included 216 patients with AIH, of which 9.2% were drug-induced, and both groups showed similar clinical and histological characteristics. Notably, overlap syndromes with PBC were excluded from this study. They found that nitrofurantoin and minocycline accounted for more than 90% of the drug-induced AIH cases.
Anti-TB drugs are the leading cause of DILI in India and China, given the prevalence of TB in these countries.10,11 The reported incidence of DILI due to anti-TB drugs varies between 2% and 28%.1 Isoniazid, pyrazinamide, and rifampicin are recognised as potentially hepatotoxic drugs. Isoniazid, in particular, is notorious for causing hepatocellular injury and has been incriminated in some cases of idiosyncratic DILI. It ranks second among antimicrobials responsible for DILI (11.7%) in the United States DILI Network registry12 and is considered to have a probable association with liver injuries resembling AIH.5 Previous literature has reported cases of DILI with autoimmune features due to anti-TB treatment.13,14
Certain risk factors have been suggested to predict DILI, including age, female gender, malnutrition, alcohol intake, and pre-existing liver disease.12 Slow acetylator status and specific genetic polymorphisms have also been associated with DILI occurrence due to isoniazid.2,15,16 Additionally, polymorphisms of drug-metabolising enzymes, mainly CYP, and major histocompatibility complex (MHC) are plausible genetic factors for autoimmune DILI susceptibility.2,4
An interesting study conducted by Lucena et al.17 reported that 9 out of 742 patients experienced a second episode of DILI caused by a different offending agent than the first one. Among these 9 patients, four had a presentation of AIH. The authors concluded that recurrent DILI is likely to be associated with autoimmune features. However, the mystery remains unresolved whether the episode was a DILI unmasking an authentic AIH or an IM-DILI.
Immune-mediated hepatotoxicity can also present with a cholestatic phenotype, characterised by small bile duct injury. This type of DILI is more common in elderly patients and typically exhibits delayed recovery and a tendency to become chronic even after drug withdrawal.4,12,15 In some cases, it may progress to ductopenia, known as vanishing bile duct syndrome (VBDS), and biliary cirrhosis. This DILI phenotype is well established with amoxicillin-clavulanic acid association.4,12,15 Notably, chronic cholestatic hepatitis, or VBDS, has not been linked to anti-TB drugs (INH, RIF, PZA, EMB) according to LiverTox database.
Ultimately, both of our patients were diagnosed with overlap syndrome AIH-PBC, based on the Paris criteria, as recommended by guidelines.5,6 An undiagnosed quiescent AIH, unmasked by anti-TB treatment, appeared to be a more plausible explanation than a de novo IM-DILI. The presence of advanced fibrosis, characteristic histology findings, and the association with PBC support this conclusion. Furthermore, PBC is known for its progressive nature and slow onset. Additionally, the positivity of AMA-M2 and anti-gp210, as well as the presence of florid bile duct lesions, strongly suggest PBC and argue against a cholestatic DILI.
In cases of AIH-like DILI, the backbone of the treatment is cessation of the causative drugs and close monitoring of liver enzymes until recovery, which typically occurs within a month.5 Corticosteroid therapy should be initiated for severe hepatitis or if lab tests do not improve or worsen after drug withdrawal.5,6 Corticosteroids may also be prescribed for severe hypersensitivity reactions, DRESS syndrome, and when autoimmune features are observed on liver biopsy.15 Typically, glucocorticoids lead to a response with no relapse after discontinuation. Unlike authentic AIH, long-term immunosuppression is not required. Therefore, follow-up can help differentiate AIH and DILI.5,6 UDCA might be beneficial in cases of DILI with prolonged cholestasis.4,15
In the cases of our patients, both of them required immunosuppressive therapy, along with UDCA, given that they were diagnosed with AIH-PBC overlap syndrome. In the first case, discontinuation of anti-TB drugs led to resolution of liver injury without the need for corticosteroids. Instead, the decision to prescribe immunosuppressants was based on histological disease activity and advanced fibrosis. As for the second patient, the withdrawal of anti-TB drugs and the use of entecavir were not sufficient to induce remission of ALI until steroids were introduced. Subsequently, normalisation of cholestasis was slowly achieved with UDCA treatment.
In conclusion, our article emphasises the significance of monitoring for DILI during TB therapy. It also highlights the importance of considering ALD, despite overlapping features, which can make accurate diagnosis challenging. A comprehensive understanding of these entities, aided by histological findings, is essential for initiating appropriate treatment strategies.
Written informed consent for publication of their clinical details and/or clinical images was obtained from the patients.
No data are associated with this article.
Zenodo Repository: CARE checklist for ‘Autoimmune Liver Disease Revealed by Tuberculosis Treatment: Report of Two Cases and Literature Review’. DOI: https://doi.org/10.5281/zenodo.13934956 . 18
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the cases’ history and progression described in sufficient detail?
No
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
No
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
No
Is the conclusion balanced and justified on the basis of the findings?
Partly
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 3 (revision) 31 Mar 25 |
|
|
Version 2 (revision) 17 Jan 25 |
read |
|
Version 1 24 Oct 24 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)