Keywords
GPx1-antioxidant; GIT1; Hepatic, Ethanol-exposed
Ethanol (EtOH) exposure throughout gestation and breastfeeding leads to multiple adverse outcomes in the hepatic system. Under oxidative stress, alterations in the liver are related to the inhibition of induced nitric oxide synthase activity in sinusoidal cells as a consequence of low expression of G-protein-coupled receptor (GPCR)-kinase interacting (GIT1). Here, we hypothesized that both glutathione peroxidase 1 (GPx1) and GIT1 could be altered by EtOH exposure during the third trimester of human equivalent development.
We exposed rats during the third trimester equivalent [postnatal days (PD) 2-8] to moderate levels of maternal EtOH (20%). GPx1 and GIT1 expression was detected by western blotting, and the antioxidant activity of glutathione peroxidase GPx and the concentration of hepatic carbonyl groups (CG were determined by spectrophotometry. Serum biochemistry parameters such as alanine aminotransferase (ALT), glucose (gluc), cholesterol (chol), and triglycerides (TG) were also measured.
We found that ethanol decreased both GIT1 and GPx1 selenoprotein expression, affecting GPx antioxidant activity and increasing protein oxidation.
These results demonstrate for the first time that the GPx antioxidant system altered by EtOH exposure during the third trimester of development is related to a parallel decrease in GIT1 expression [1].
GPx1-antioxidant; GIT1; Hepatic, Ethanol-exposed
In this revised version of the article, we have addressed the referee's valuable suggestions to enhance clarity and improve the presentation of our findings. Specifically, we have updated Table 2, by including definitions of the acronyms directly in the table's description, making it more accessible and easier to interpret for readers. While these acronyms were already defined in the Methodology section of the original version, this addition ensures that essential information is readily available within the table itself.
No further modifications were made to the structure or content of the manuscript, as the original version was deemed scientifically robust and comprehensive. These changes were made solely to enhance the readability and user experience without altering the study's integrity or conclusions.
See the authors' detailed response to the review by Mauro Ceccanti
Prenatal ethanol (EtOH) exposure can result in significant negative consequence for offspring, such as impaired growth and range of congenital abnormalities, including Fetal Alcohol Spectrum Disorders (FASD).2–4 Numerous studies have demonstrated that sustained ethanol intake by mother disrupts the antioxidant homeostasis in their offspring.5–9 The detrimental teratogenic effects of this exposure are largely due to imbalance in redox processes, with the liver being particularly susceptible to ethanol-induced oxidative stress.10,11 Long-term alcohol consumption triggers the activation of Hepatic Stellate Cells (HSCs), which is linked to vascular changes and liver fibrosis resulting from increased deposition of Extracellular Matrix (ECM) proteins.11 In rodent models of cirrhosis induced by oxidative stress, sinusoidal capillarization occurs, involving the differentiation of Liver Sinusoidal Endothelial Cells (LSECs). This process is critical in HSC activation and fibrosis development.12,13
Ethanol exposure during gestation and breastfeeding impairs hepatic antioxidant glutathione peroxidase (GPx) activity and increases carbonyl groups in proteins.5,7 Consistent with this effect, pups of ethanol-exposed dams show some variation in the expression of selenium-dependent enzymes of the GPx family in liver tissue. Specifically, an increase in GPx4 expression levels has been reported at the expense of a decrease in GPx1 expression, likely due to GPx4’s physiological role in protecting phospholipids associated with several protein complexes of the mitochondrial inner membrane, such as cardiolipin. This protection helps inhibit cytochrome C release and maintain mitochondrial membrane potential and ATP generation.10,14,15
In the liver, acute and subacute oxidative stress results in the inhibition of induced nitric oxide synthase activity in sinusoidal cells through distinct mechanisms dependent on caveolin-1 inhibition, endothelin B receptor (ETBR) dissociation, and endothelial nitric oxide synthase (eNOS) phosphorylation.16 These alterations could influence the distribution and function of G-protein-coupled receptor kinase interacting protein-1 (GIT1), which is predominantly restricted to sinusoidal cells lining blood vessels and bile ducts.17 Furthermore, GIT1 is a multi-domain scaffold protein related to the regulation of cytoskeletal dynamics during cell spreading and migration, receptor internalization, and synapse formation.18
However, GIT1 also possesses intrinsic signaling capabilities in endothelial tissues. For instance, when fibroblasts adhere to the fibronectin extracellular matrix, GIT1 is phosphorylated by the tyrosine kinase Src.19,20 Furthermore, after endothelin 1 (ET-1) binds to the ETB receptor, eNOS interacts with GIT1, leading to eNOS activation.20 Interestingly, eNOS phosphorylation and activity are reduced in sinusoidal endothelial cells following liver injury,20–22 and oxidative stress has been associated with endothelial dysfunction alterations in both ET-1 and nitric oxide (NO) signaling pathways.23,24
Therefore, the third trimester of development is particularly vulnerable, and ethanol exposure induces multiple physiological alterations.25–32 Research has shown that ethanol consumption during pregnancy is relatively common.33 Given that exposure to ethanol during this developmental period in rodents has been used to model human exposure during the third trimester of pregnancy,4 we investigated whether ethanol-induced oxidative stress in the liver during this critical period results in alterations in the antioxidant proteins GPx1 and GIT1 in the liver tissues of offspring.
Unless otherwise indicated, all chemicals used in this research were obtained from PanReac AppliChem (Barcelona, Spain).
All animal procedures were approved by the University of Cartagena Animal Care and Use Committee and conformed to the NIH Guidelines, as well as the title V of Resolution 008430 of 1993 in Colombia. The study was conducted according to the ARRIVE guidelines 2.0, using the ARRIVE Essential 10 checklist for preclinical animal studies.34 Male and female Wistar rats (National Institute of Health, Colombia), weighing approximately 150–200 g, were randomized into two groups: control (C) and ethanol (EtOH) (E). The animals were maintained at an automatically controlled temperature (22–23°C) and a 12-h light–dark cycle (6:00–18:00).
All animal procedures were approved by the University of Cartagena Animal Care and Use Committee under Reference Number: 106 (approved on March 15th, 2018) and conformed to the NIH Guidelines, as well as the title V of Resolution 008430 of 1993 in Colombia. Animals were handled with care, provided with appropriate housing and management conditions, and anesthetics and analgesics were administered when necessary to alleviate any potential pain or discomfort.
Animals were exposed to ethanol starting on tap water that contained 5% v/v ethanol in the first and second weeks, and the ethanol concentration was increased to 10% in the third week, 15% in the fourth week, and 20% in the fifth week (induction period), which was maintained during the mating period. Pregnant rats were housed separately from male rats. To model third trimester-equivalent EtOH exposure, 20% ethanol in drinking water as the sole source of liquid with food ad libitum was restored to dams and their pups from postnatal days (PD) 2 to 8. During the paradigm, The gestational index calculated as (number of Successful Births (nSB), number of Pregnancy Rats (nPR)) was measured. In addition, morphometric parameters (Total Body Weight (BW) and craniocaudal length) were recorded at PD2 and PD8 using an electronic balance (Precisa XB220A, Gravimetrics AG) and a metric caliper (Digi-Max™ slide caliper Z503576, Sigma Aldrich). Additionally, Liver Weight (LW) and hepatic somatic index (HIS) calculated as (Liver weight (LW), Body weight (BW)) were registered at PD8. Finally, at the end of ethanol exposure, serum biochemical parameters, such as alanine aminotransferase (ALT), glucose (gluc), cholesterol (chol), and triglycerides (TG) were measured using an automated analyzer (Technicon RA-1000, Bayer Diagnostics).
At the end of the experimental period, rats were anesthetized with ketamine (250 mg/kg, i.p. Sigma-Aldrich, Cat. Number 1356009, St. Mo, USA). The abdomen was opened using a midline incision, and the entire liver was removed. Samples were immediately covered with liquid nitrogen and stored at -80°C prior to biochemical analysis. Blood was collected by heart puncture and centrifuged to obtain serum.
The hepatic tissue was sonicated in homogenization buffer (0.1 g tissue/1 mL of buffer) containing 25 mM HEPES (pH 7.4), 500 mM NaCl, 2 mM EDTA, 1 mM dithiothreitol, 0.1% Tween-2020 (Biorad, Cat. Number 170-6531. CA, USA), and 1 tablet in 10 mL of the Pierce Protease and Phosphatase Inhibitor Mini Tablets (Thermo Scientific, Cat. number A32961). Samples were centrifuged at 3000 rpm at 4 °C for 10 min to obtain supernatants rich in hepatic proteins and stored in 10 μL aliquots at −80°C. Protein concentration was determined by the Bradford Method (BioRad, Hercules, CA, USA) using bovine serum albumin as a standard and stored at −40 °C.35
Samples were mixed with sodium dodecyl sulfate polyacrylamide gel electrophoresis sample buffer (final concentration: 250 mM Tris–HCl (pH 6.8), 10% sodium dodecyl sulfate, 30% glycerol, 0.02% bromophenol blue and 5% β-mercaptoethanol (BioRad, Cat. number 161-0710)) and boiled at 95°C for 5 min. Samples were loaded at a concentration of 50 μg per lane. Control experiments demonstrated that this protein concentration was within the linear dynamic range for the western blot assay (not shown). Electrophoresis was performed on a 12% polyacrylamide gel at 150 V for 60 min at 4°C. Proteins were blotted onto polyvinylidene fluoride membranes (0.4 μm pore size. BioRad, Cat. Number 162-0177) at 100 V for 60 min at 4°C. Non-specific binding was blocked with 5% non-fat dry milk (BioRad, Cat. number 170-6404) in PBS for 1 h at room temperature and probed overnight at 4°C with either of the following specific primary antibodies: anti GPx1 antibody (Invitrogen, 1:5000 Cat. number PA5-30593) and anti GIT1 antibody (Invitrogen, 1:2,000, Cat. number PA5-78480; Invitrogen). Membranes were then incubated with a secondary antibody (goat anti-rabbit IgG (H+L) horseradish peroxidase conjugate, Invitrogen, Cat. number G21234;) at dilutions of 1:20,000 for GPx1 and GIT1. Monoclonal mouse anti-β-actin (Invitrogen, Cat. number MA1-140) was used to detect β-actin as a loading control, with a dilution of 1:20000, and a secondary antibody goat anti-mouse IgG (H+L), HRP (Thermo-Scientific, Cat. number 31430) was used at a dilution of 1:30000. Membranes were washed with PBS-milk-Tween 20, PBS-milk, and PBS for 5 min and visualized by chemiluminescence (Novex™ ECL Chemiluminescent Substrate Reagent Kit. Invitrogen, Cat. Number wp20005) for 3 min. The images were captured using a ChemiDoc ™ System (Bio-Rad, Hercules, CA, USA), and the intensity of the bands was analyzed using Image Lab 6.1 software (Biorad), For each sample, the protein expression was normalized to that of β-actin. The results were also used to calculate the GIT1/GPx1 ratio.
To measure the activity of GPx as well as the oxidation of protein, liver tissue samples were homogenized in a sucrose buffer (15 mM Tris/HCl, pH 7.4, 250 mM sucrose, 1 mM EDTA, and 1 mM DTT) in an ice bath, and the resulting supernatant was used for biochemical assays. The acquired data were also used to determine the ratio of GPx1 expression/GPx activity. Protein oxidation was measured according to a method based on spectrophotometric detection of the reaction of 2.4-dinitrophenylhydrazine (DNPH) with protein carbonyl (PC) to form protein hydrazones.36 The PC level was calculated at the maximum absorbance (366 nm), and the results were expressed as nmol/mg protein. The activity of the selenoprotein GPx was determined by NADPH-coupled assay according to the method of,36 which catalyzes the oxidation of glutathione by hydrogen peroxide. NADPH oxidation was monitored spectrophotometrically at 340 nm wavelength. Specific activity was expressed as mU/mg protein, where 1 mU is equal to nanomoles of NADPH oxidized/min.
All data were statistically analyzed using Prism 5 (GraphPad, San Diego, CA, USA) https://www.graphpad.com/. Initially, data were analyzed using the Pearson omnibus normality test. Only data that followed a normal distribution were analyzed using parametric tests. Statistical significance was set at p < 0.05.
To model EtOH exposure during the third trimester equivalent, dams and their offspring were exposed to EtOH (20%) ad libitum from PD2 to PD8. As shown ( Table 1), our model confirms that ethanol exposure leads to lower fertility and gestational index. To determine whether ethanol alters regular growth during this developmental stage, we recorded the morphometric parameters. In this context, the total BW at PD2 showed no alteration in the offspring exposed to ethanol compared to that in the control group (Control, n=29; EtOH, n=20; t=1.91, df= 45, p> 0.05, unpaired t-test). However, there were significant differences in pup weight between the control and ethanol group at the end of the exposure paradigm (PD8) (control, n=29; EtOH, n=20; t=2.28, df= 36, p<0.05, unpaired t-test). Similarly, the cranium-caudal length showed no difference in offspring from dams exposed to EtOH at PD2 (control, n=29; EtOH, n=20; t=1.93, df= 48, p> 0.05, unpaired t-test). In contrast, at the end of the ethanol exposure (PD8), alcohol-exposed pups registered a lower cranium-caudal length compared to the control (Control n=29; EtOH n=20; t=3.22, df= 48, p<0.01 by unpaired t-test). Despite the morphometric differences registered at the end of the ethanol exposure paradigm, the LW and HIS were similar in both groups.
| Parameters | Control | EtOH |
|---|---|---|
| Gestational index | 100 | 57.7 |
| WPD2(g) | 9.6±0.2 | 9.0±0.2 |
| WPD8(g) | 25.7±0.5* | 22.9±1.0 |
| LPD2(cm) | 8.6±0.1 | 8.2±0.2 |
| LPD8(cm) | 12.5±0.1** | 11.8±0.1 |
| LW-PD8(g) | 0.8±0.005 | 0.7±0.003 |
| HISPD8 | 3.11±0.06 | 3.05±0.06 |
Additionally, biochemical analysis of serum ( Table 2) revealed that EtOH-treated animals had a significant increase in alanine aminotransferase (ALT) enzyme activity (n=8; t=8.96, df= 14, p<0.001 by unpaired t-test), and in the products of lipid metabolism such as cholesterol (n=8; t=5.02, df= 14, p<0.001 by unpaired t-test), and triglycerides (n=8; t=2.19, df= 14, p<0.05, unpaired t-test). However, offspring of EtOH-exposed dams had significantly lower glucose concentrations (n=8; t=2.42, df= 14, p<0.05 by unpaired t-test).
To determine whether EtOH exposure during the third trimester alters the hepatic redox balance, we first evaluated GPx1 expression. Western blot analysis of total GPx1 within the liver tissue ( Figure 1A) showed a significant reduction in EtOH exposure when normalized to β-actin ( Figure 1B) (n=8; t=3.12, df= 14, p<0.01 by unpaired t-test). Knowing that hepatic oxidative stress alters parenchymal and non-parenchymal cells, similar to those found in sinusoidal ducts (34), and that GIT1 is restricted to cell lining blood vessels and bile ducts (16), we also evaluated whether liver ethanol-oxidative stress affected total GIT1 expression. Similarly, we found significant differences between the control and EtOH-exposed offspring when data were normalized to β-actin ( Figure 1A and C) (n=8; t=2.99, df= 14, p<0.01 by unpaired t-test). As a result of this altered expression, the GIT1/GPx1 expression ratio was also reduced by ethanol exposure ( Figure 1D) (n=8; t=2.19, df= 14, p<0.05 by unpaired t-test). Taken together, these data show that ethanol modulates the expression of GPx1 and GIT1, resulting in the depletion of both proteins during the third trimester equivalent of development.
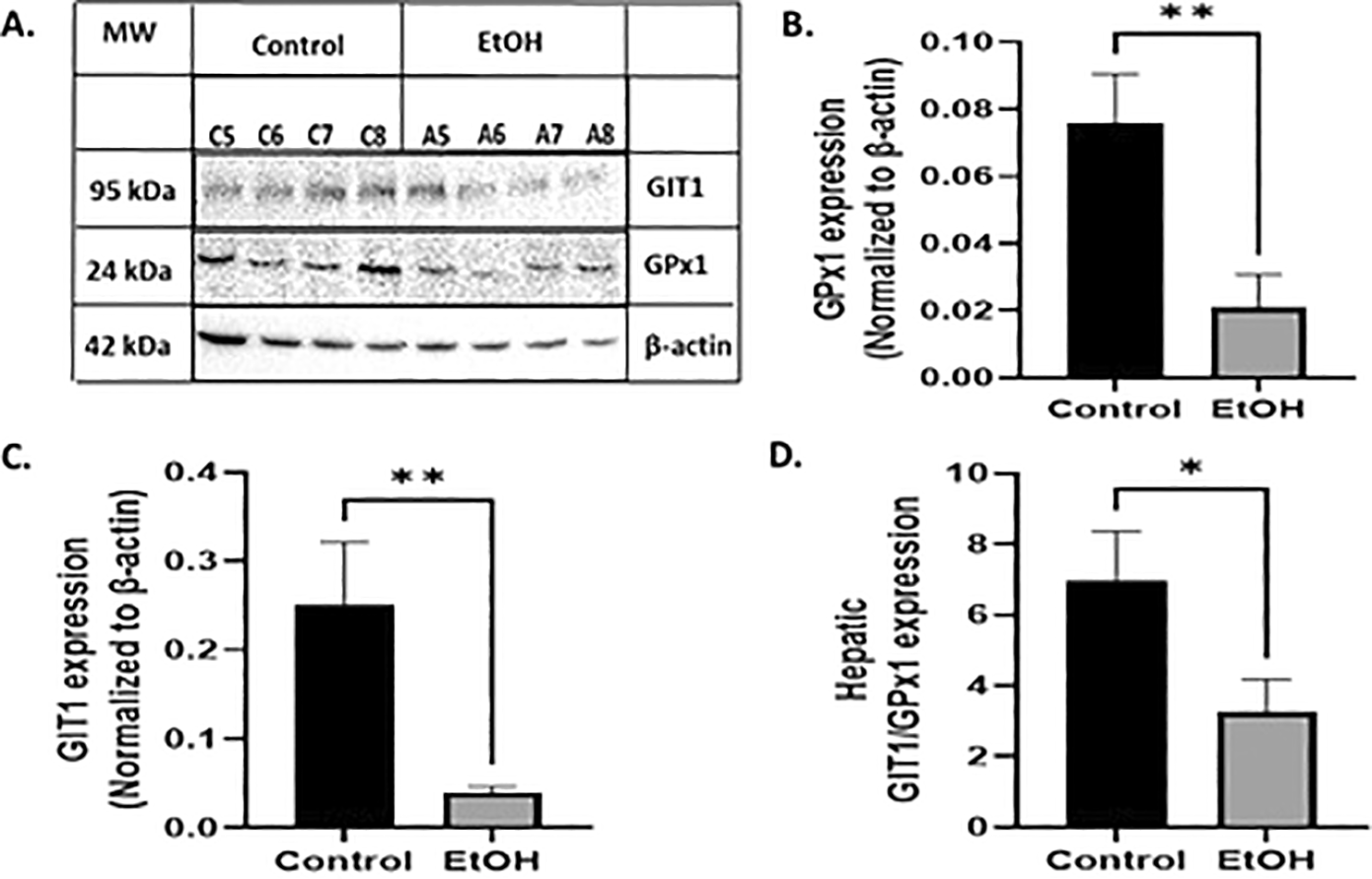
(A) Exemplar western blot illustrating levels of GIT1, GPx1 and β-actin from control and ethanol rats. (B) Graphic quantification of GPx1 expression normalized to β-actin. GPx1 was altered by ethanol exposure. (C) Graphic quantification of GIT1. GIT1 was also altered by ethanol exposure. (D) Ratio GIT1/GPx1 expression. Labels of C5 to 8 and A5 to -8 are representative samples of each experimental group. The results are expressed as mean ± SEM and analyzed by unpaired t-test. The number of animals in each group is 8. Statistic difference between groups was expressed as p value: C vs E: * p<0.05, ** p<0.01, *** p<0.001.
To confirm that the low expression of GPx1 selenoprotein is related to an altered hepatic redox balance, we determined GPx enzyme activity. Figure 2A shows a significant decrease in GPx activity in ethanol-exposed offspring at PD8 compared to that in the control (n=8; t=5.59, df= 14, p<0.001 by unpaired t-test). Furthermore, to evaluate the consequences of the altered antioxidant GPx system, we examined the levels of protein oxidation in the liver. Conversely, the data collected showed that pups from EtOH-treated dams had significantly higher concentrations of carbonyl groups (n=8; t=4.16, df= 14, p<0.001 by unpaired t-test) ( Figure 2B). Additionally, we found that when GPx1 expression was compared to GPx activity, the GPx1expression/GPx activity ratio decreased significantly in the liver after ethanol exposure ( Figure 2C) (n=8; t=5.66, df= 14, p<0.001 by unpaired t-test).
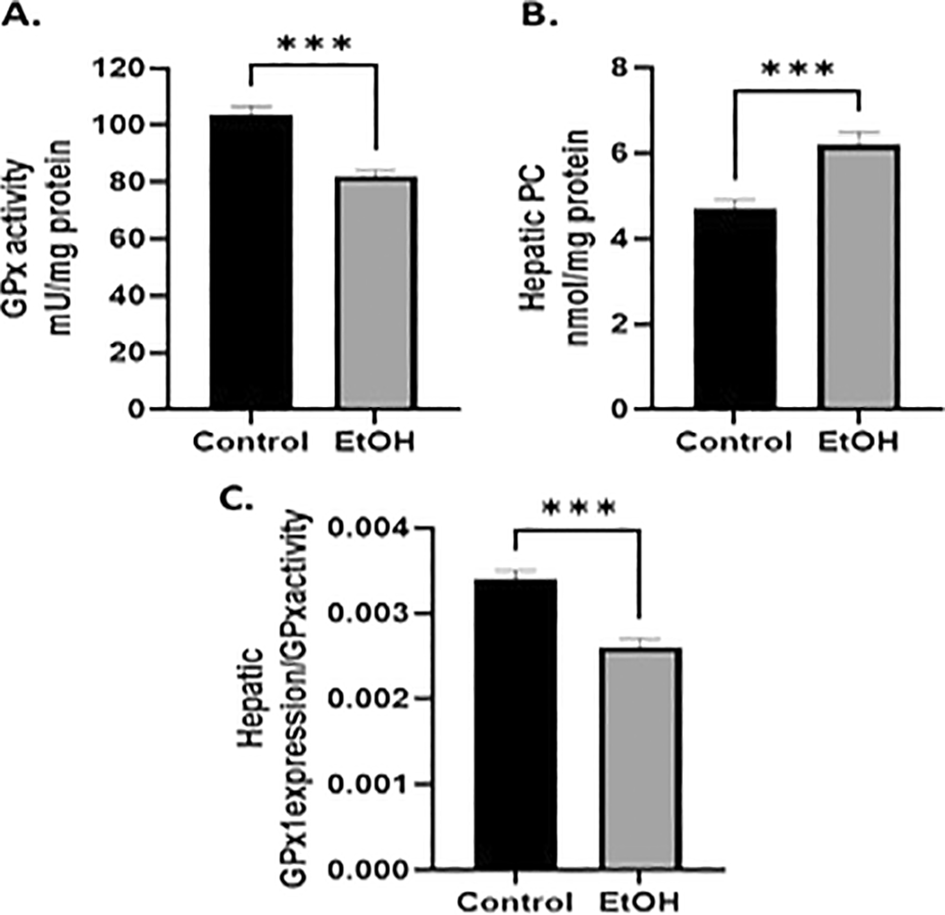
(A) Ethanol exposed-offspring showed a significantly reduced GPx activity. (B) Carbonyl groups were increased by ethanol exposure. (C) Ratio of GPx1expression/GPx activity. The results are expressed as mean± SEM and analyzed by unpaired t-test. The number of animals in each group is 8. Statistic difference between groups was expressed as p value: C vs E: *** p<0.001.
In this study, our data show that ethanol treatment during the initial period (PD2) does not cause any detrimental effects on the BW of pups or their cranium-caudal length. However, during the breastfeeding period (PD2-8), ethanol exposure induced a growth deficit at the end of the paradigm (PD-8). These results are consistent with several studies that also reported reduced growth rates at the end of lactation7,8 or even during the embryonic E19 stage,37 although those studies used models with more sustained ethanol exposure. It is likely that the findings reported here are related to the fact that ethanol consumption during lactation provokes a greater under-nutrition in dams, in part because in this period, the ethanol-exposed dams consumed more ethanol during lactation than during gestation, which affects the milk intake of the suckling pups; reduced in some cases until 30% of the amount consumed by offspring not exposed to ethanol.8 It is also likely that the detrimental growth registered in our data is correlated to a low quality of the milk consumed by pups, as previous works have reported that the low BW is reversed under selenium (Se) supplement treatment; Se (0.5ppm) and Se+Folic acid (FC) (0.5+0.8ppm),8,38 probably as a consequence that both diet supplements are able to increase the Se concentration in milk or in organs such as mammary glands.6 This trace element seems to play an essential role in offspring development, since it is necessary to synthesize deiodinase enzymes that enhance T3 tissue levels, specifically at the hypothalamus, where it produces an increase in the Neuropeptide Y inducing hyperphagia.39,40 Our model also demonstrated that ethanol exposure altered optimal metabolic conditions in the offspring. In this context, the results showed increased values of cholesterol and triglycerides and a low concentration of glucose, together with elevated levels of ALT, suggesting that metabolism in ethanol-exposed offspring is altered, leading to liver damage and altered cell membrane permeability.41 Taken together, these assumptions could explain, at least in part, the reduced BW and length of the offspring under EtOH exposure during the third trimester equivalent development.
Analysis of GPx1 expression and GPx activity in the liver on PD8 demonstrated that both were affected by ethanol exposure, indicating the existence of mechanisms affecting the synthesis and activation of this protein. This is consistent with previous studies demonstrating that chronic ethanol exposure during gestation and breastfeeding causes drastic changes in the hepatic GPx antioxidant system.5,36,42 In fact, it has been proven that reduced GPx activity is inversely related to the gluthatione reductase (GR) activity in liver of pups from ethanol exposed dams, which is associated with a depletion of reduced gluthatione (GSH). This mechanism seems to be activated as a possible adaptation response to oxidative stress to maintain regular GSH levels or to reduce NADPH levels associated with ethanol intoxication.5 Nevertheless, it is also likely that the ratio GPx1 expression/GPx activity imbalance is related to a decreased Se content in the liver, as previous work has reported a depletion of GPx activity dependent on the hepatic Se stored in embryos and pups, and subsequent administration of Se as supplement produces a moderate increase in GPx activity.5,7,36 In this context, we could assume that lower selenium-dependent GPx1 expression and its antioxidant activity could be linked to hepatic Se depletion induced by ethanol exposure. However, it is necessary to perform a more detailed analysis to confirm that Se depletion is the only cause of GPx reduction, as in the alcohol-damaged newborn model, more than one parameter has been used to describe the Se status and its biological function.35,43
To determine whether decreased GPx1 expression and GPx activity in ethanol-exposed offspring have repercussions on liver oxidative balance, we evaluated their effects on protein oxidation. In fact, oxidative stress in the offspring is supported by an increase in carbonyl groups in hepatic proteins (PC). This is consistent with studies performed under sustained ethanol exposure during gestation that promote high levels of PC and decrease GPx activity in the liver and kidney of suckling pups.36,44 Taken together, we suggest that liver tissue is trying to cope with ethanol-induced oxidative stress, but is incapable of doing so because it suffers peroxidation of its proteins. However, it is worth noting that the oxidative stress and enzymatic antioxidant system differ among tissues, and its activation also depends on the physiological quality of the tissue environment.44 It is not yet completely understood how the antioxidant GPx system functions under these conditions.
Ethanol exposure during the third trimester also decreased GIT1 expression in the liver. It is likely that such alterations could be related to the impairment of oxidative balance, which in turn leads to alterations in parenchymal and non-parenchymal cells. In this context, the GIT1/GPx1 decreased ratio found in ethanol-exposed offspring indicates the existence of mechanisms that possibly alter the synthesis of both proteins, perhaps as a result of the augmented hepatic oxidative stress produced by the increment of reactive oxygen species (ROS) that promote depletion of GIT1 and GPx1, as has been observed in cultured fibroblasts45 and in the liver,46 respectively. In addition, it is known that GIT1 is restricted to cells lining blood vessels and bile ducts.17 In portal-based fibrogenic response and portal hypertension in the liver, GIT1 level is reduced in injured cells, and subsequent decreased eNOS phosphorylation and activity with a markedly reduced GIT1/eNOS co-localization in the perinuclear region persists even after injury.47 Based on this, it is also likely that the decreased expression of GIT1 in our model is a response to an altered physiological condition induced by ethanol-oxidative stress, as in fibrosis, which is characterized by an excessive accumulation of extracellular matrix (ECM) proteins with very little degradation.37,48 Expression of Kupffer cell-derived tumor necrosis factor alpha (TNF-α),49 and interleukins (IL-1, IL-6, IL-8) have been reported in bile duct cells.50 This information could explain the possible mechanism related to EtOH-induced oxidative stress on GIT1 low expression and its role during the third trimester equivalent offspring. However, the effect of EtOH on GIT1 expression may be a more complex process involving several factors ( Figure 3). Significant evidence demonstrates that ethanol downregulates the endothelin B receptor (ETBR) and eNOS phosphorylation16 and likely also affects the G-protein-coupled receptor ligand endothelin-1 (ET-1). Oxidative stress is known to cause endothelial dysfunction and alterations in ET-1 signaling pathways.24 Together, these factors alter GIT1 expression and function. Additionally, ethanol can suppress Akt phosphorylation through cytochrome P4502E1 (CYP2E1)-induced oxidative stress in alcoholic fatty liver.51 This suppression may affect GIT1/eNOS association, as Akt phosphorylation activates eNOS (at Ser1177) and regulates the ability of Src to phosphorylate GIT1.20 Therefore, this is correlated with the fact that eNOS and NO production is reduced by sinusoidal endothelial cells as well as increased intrahepatic vascular resistance after liver injury.21,52 Furthermore, during preeclampsia, depletion of GIT1 impedes NO production and placental eNOS activity,53 and during the early neonatal period at different gestational ages, no correlation was found between ET-1 and the oxidative impairment product, MDA, and the tripeptide GSH.54 However, this could be explained by the effects of the transitional perinatal period, which reflect a mixed maternal and neonatal redox status.55
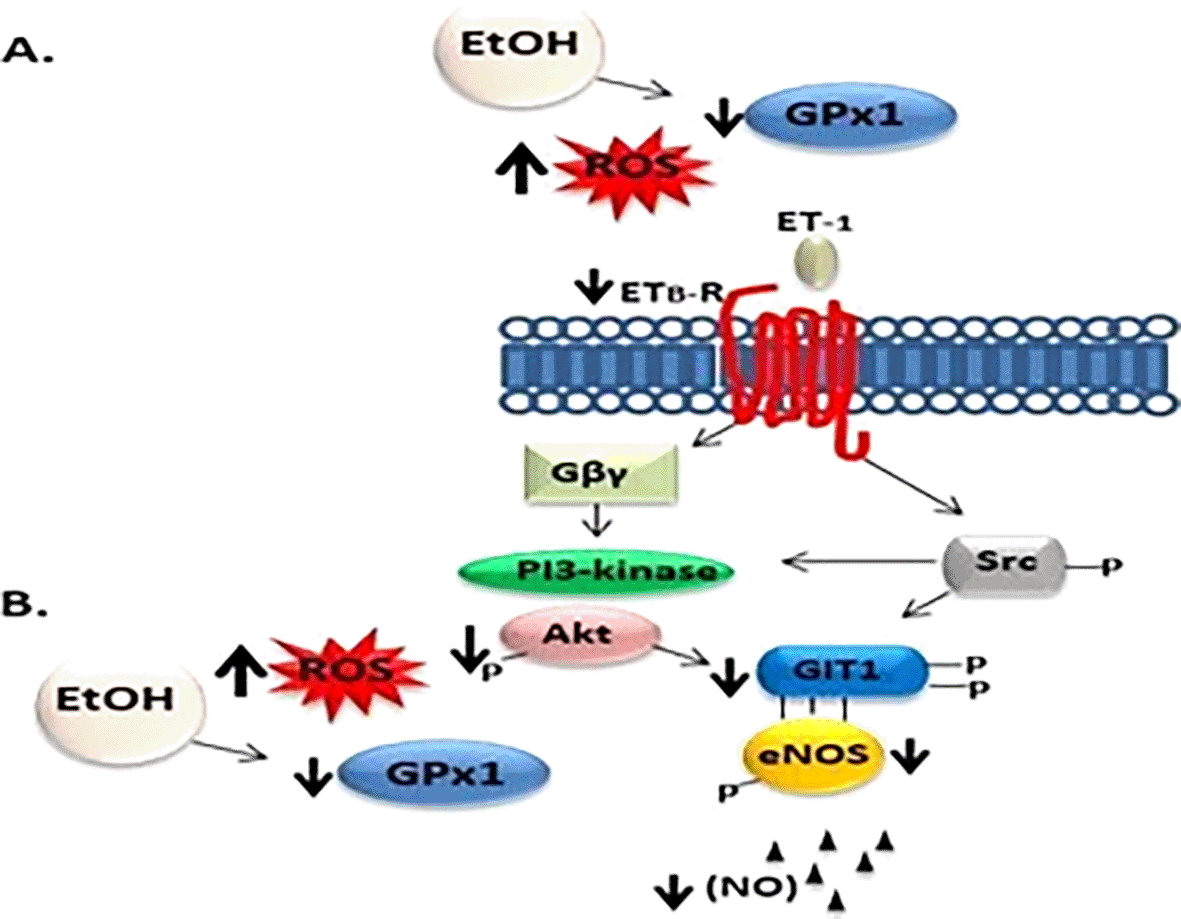
ET-1 activates its cognate ETB-R inducing Gα and Gβγ activation and dissociation. Akt and Src both are related to phosphorylation-enhanced GIT1/eNOS, eNOS activity and NO production. A) Ethanol exposure depletes GPx1 antioxidant system and increases ROS, which in turns, decreases endothelin B receptor, leading to a low co-expression and activation of GIT1/eNOS protein levels, and eNOS phosphorylation. B) Also, a decreased expression of GPx1 and increased ROS cytochrome P4502E1 (CYP2E1)-induced oxidative stress may be responsible for ethanol-induced suppression of Akt phosphorylation leading to downregulated of GIT1/eNOS expression. The consequence of altered GIT1 expression is reduced eNos activation and NO production. EtOH (Ethanol); ROS (Reactive Species Oxygen); GPx1 (Glutathione peroxidase 1); ET-1 (Endothelin-1); ETB-R (Endothelin b-Receptor); PI-3Kinase (Phosphatidylinositol (PI)-3 kinase); Akt (Protein kinase B); Src (Src kinase); (GIT1) (G-protein-coupled receptor (GPCR)-kinase interacting protein-1) eNOS (endothelial nitric oxide synthase); NO (Nitric Oxide).
Despite evidence that GIT1 functions are associated with hepatic endothelial physiology, it is worth noting that altered GIT1 expression due to ethanol exposure in hepatic cells could also affect focal cell migration and adhesion, as it was found that GIT1 in pathologies such as hepatocellular carcinoma (HCC) promotes the invasion, migration, and proliferation of HCC cells. Moreover, overexpression of GIT1 prompted epithelial mesenchymal transition (EMT) by activating the extracellular regulated kinase 1/2 (ERK1/2) pathway.56
Overall, it is clear that the third-trimester ethanol-exposed offspring have an impaired GPx1 antioxidant system in the liver. The altered antioxidant system seems to be related to the decreased expression of GIT1. It is possible that alterations in the transducing signal dependent on GIT1 underlie the mechanism of several harmful processes associated with ethanol exposure. Therefore, Future studies should focus on exploring the imbalance of the antioxidant system in the GIT1signaling pathway associated with hepatic LSEC injuries induced by ethanol exposure during this developmental period.
The assays confirmed that EtOH exposure during gestation and lactation significantly affects the morphological and biochemical development of offspring, critically affecting the hepatic antioxidant system. The reduction in GPx1 expression and activity, together with decreased levels of GIT1, suggests altered signaling pathways and functional integrity of the liver. These results underscore the importance of avoiding ethanol exposure during critical developmental stages because of its long-lasting adverse effects on liver health and overall offspring development.
All animal procedures were approved by the University of Cartagena Animal Care and Use Committee under Reference Number: 106 (approved on March 15th, 2018) and conformed to the NIH Guidelines, as well as the title V of Resolution 008430 of 1993 in Colombia. Animals were handled with care, provided with appropriate housing and management conditions, and anesthetics and analgesics were administered when necessary to alleviate any potential pain or discomfort.
To ensure transparency and reproducibility, we have deposited all the raw data underlying this study in https://figshare.com/articles/dataset/:Data of Hepatic GPx1 and GIT1 Expression Altered by Ethanol Exposure During Third Trimester-Equivalent Development. https://doi.org/10.6084/m9.figshare.27091666.57
Figshare: Data of Hepatic GPx1 and GIT1 Expression Altered by Ethanol Exposure During Third Trimester-Equivalent Development, https://doi.org/10.6084/m9.figshare.27091666.57
This project contains the following underlying files:
• Raw data Liver PC _ GPx activity_Jotty et aletal_2024/Analysis GPx and PC_ Alberto 2021.pzfx41.46 KB.xlsx Raw data Jotty et al_2024/Biochemestry parameters_Jotty et _2024.xlsx
• Raw data Jotty et al_2024/GPx1 and GIT1 Expression_Jotty et al_2024.xlsx
• Raw data Jotty et al_2024/Morphometric parameters_Jotty et al_2024.xlsx
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Figshare: Protein Quantification of ethanol exposed PD8 rat offspring for determining Hepatic GPx1 and GIT1 Expression. https://doi.org/10.6084/m9.figshare.27091804.58
This Project contains the following files:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Figshare: ARRIVE checklist Hepatic GPx1 and GIT1 Expression Altered by Ethanol Exposure During Third Trimester-Equivalent Development. https://doi.org/10.6084/m9.figshare.26863453.33
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The following free and open-source software can be used as equivalents to the software mentioned in this study:
PSPP: PSPP is a free and open-source alternative to Graph Pad for statistical analysis. It can be downloaded from the GNU Project at https://www.gnu.org/software/pspp/.
Image Lab: Image Lab is an open-source image processing program designed for scientific multidimensional images. It can be accessed and downloaded from https://www.bio-rad.com/. https://www.bio-rad.com/.
We would like to thank the following authors for their collaboration and commitment in the preparation of the preliminary work included in this project: Díaz Castillo A, Silgado Ortega JP, Jiménez Villalobos TP, Tuirán Ruiz M, Gómez Estrada H, Jotty Arroyo K.1
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Alcohol addiction, Gastroenterology, Alcohol Use Disorders.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Alcohol addiction, Gastroenterology, Alcohol Use Disorders.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 2 (revision) 06 Dec 24 |
read |
|
Version 1 29 Oct 24 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)