Keywords
Glioblastoma multiforme, genetic mutation, MUC19, MUC17, MUC16, patient survival, siRNA, targeted therapy
This article is included in the Cell & Molecular Biology gateway.
Glioblastoma multiforme (GBM) is the most aggressive and lethal form of brain cancer, and is characterized by rapid progression and poor patient survival. Genetic mutations play a significant role in cancer development and recurrence. This study investigated the role of Mucin 19 (MUC19), a member of the mucin family that has been implicated in cancer progression. We aimed to assess whether MUC19 mutations are associated with a worse prognosis in patients with GBM and explore its potential as a therapeutic target.
Data from 16 independent GBM patient datasets were retrieved from the cBioPortal for Cancer Genomics, comprising over 5,600 patients. These patients were categorized into two groups based on their survival status: living and deceased. Clinical attributes, including mutation frequencies and survival outcomes, were analyzed to identify significant genetic alterations in the deceased group. MUC19 is one of the most prominent mutations. To functionally investigate the role of MUC19, we conducted RNA interference (RNAi) experiments using A172 glioblastoma cells. Small interfering RNA (siRNA) specific to MUC19 (siMUC19) was used to knock down MUC19 expression, whereas the negative control group was treated with non-targeting siRNA. The effects on cell viability, proliferation, and MUC19 expression were also assessed.
Our analysis identified 10 significantly mutated genes in deceased GBM patients, with MUC19 showing the most prominent association with poor outcome. siRNA-mediated knockdown of MUC19 resulted in a significant reduction in cell growth and viability compared to the control group, supporting its role in GBM progression.
MUC19 plays a significant role in GBM progression, and its suppression leads to reduced tumor cell growth. These findings suggest that MUC19 may be a promising therapeutic target for improving outcomes in patients with GBM. Further research is needed to explore its potential in clinical settings.
Glioblastoma multiforme, genetic mutation, MUC19, MUC17, MUC16, patient survival, siRNA, targeted therapy
Glioblastoma multiforme (GBM) is the most aggressive and common primary malignant brain tumor, accounting for approximately 45-50% of such cases.1 GBM typically originates from astrocytes, star-shaped cells that form part of the brain’s supportive tissue.2 It is more prevalent in older males than females. Despite aggressive treatments, such as surgical resection, radiotherapy, and chemotherapy, patient prognosis remains poor, with only minimal improvements in survival rates.3 GBM most commonly arises in the cerebral cortex, but can also occur in other areas, such as the spinal cord.4 Understanding the genetic alterations that occur in glioblastoma, particularly in deceased patients, is vital for unraveling tumor behavior, resistance mechanisms, and patient outcomes.5
Mutations in genes such as Phosphatase And Tensin Homolog (PTEN) and Epidermal Growth Factor Receptor (EGFR) contribute to aggressive tumor progression and poor survival rates.6 For example, EGFR mutations are linked to shorter survival times, whereas loss of PTEN is associated with treatment resistance.7 These genetic insights are critical for the development of personalized treatment strategies.8 However, limited research has comprehensively analyzed the relationship between specific genetic alterations and survival outcomes in glioblastoma, a gap this study seeks to fill.9 One such gene of interest is MUC19, a mucin family gene that plays a significant role in producing protective mucous layers in epithelial cells.10 While MUC19 is primarily linked to diseases such as inflammatory bowel disease, recent studies have suggested its involvement in cancer, making it a key target for understanding glioblastoma pathogenesis and potential therapeutic interventions.11
This study aimed to comprehensively analyze the genetic mutations in patients with glioblastoma, focusing on deceased patients. By organizing and assessing 16 significant studies, we aimed to investigate the influence of these genetic changes on survival outcomes. This analysis will provide insights into critical genes linked to disease progression and mortality, with the aim of identifying potential therapeutic targets and improving prognosis prediction in glioblastoma patients.
The cBioPortal for Cancer Genomics was used as the primary database to collect patient data and analyze survival outcomes.12 A total of 16 studies were organized, focusing on clinical data related to patient survival: Brain Lower Grade Glioma (TCGA, Firehose Legacy), Brain Lower Grade Glioma (TCGA, PanCancer Atlas), Diffuse Glioma (GLASS Consortium), Diffuse Glioma (GLASS Consortium, Nature 2019), Diffuse Glioma (MSK, Clin Cancer Res 2024), glioma (MSK, Clin Cancer Res 2019), glioma (MSK, Nature 2019), Low-Grade Gliomas (UCSF, Science 2014), Merged Cohort of LGG and GBM (TCGA, Cell 2016), Brain Tumor PDXs (Mayo Clinic, Clin Cancer Res 2020), glioblastoma (CPTAC, Cell 2021), glioblastoma (Columbia, Nat Med. 2019), glioblastoma (TCGA, Cell 2013), glioblastoma (TCGA, Nature 2008), Glioblastoma Multiforme (TCGA, Firehose Legacy), and Glioblastoma Multiforme (TCGA, PanCancer Atlas). The clinical tab was selected for analysis, and survival graphs were generated for the patient groups based on survival status (live and deceased groups). The p values associated with the survival curves were carefully evaluated to ensure the best accuracy. The survival data encompassed 2,444 patients in the living group and 3,226 patients in the deceased group.
A172 glioblastoma cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Cat. No. 11965092) supplemented with 10% fetal bovine serum (FBS; Gibco, Cat. No. 16000044) and 1% penicillin-streptomycin (Gibco, Cat. No. 15140122). The cells were maintained in a humidified incubator at 37°C and 5% CO2. The medium was replenished every 2–3 days, and cells were passaged upon reaching 80–90% confluence.
Transient knockdown of MUC19 was achieved using RNA (siRNA). The cells were seeded in 6-well plates (Corning, Cat. no. 3516) and grown to 70% confluence before transfection. MUC19-specific siRNA (Bioneer, Cat. No. 283463) and non-targeting control siRNA (NC) (Bioneer, Cat. no. SN-1001) were transfected into cells using Lipofectamine RNAimax (Invitrogen, Cat. No. 13778075) according to the manufacturer’s instructions. After a 6-hour incubation period, the transfection medium was replaced with fresh cell culture medium. Cells were harvested seven days post-transfection for subsequent assays.
Total RNA was extracted from cells using an AccuPrep® Universal RNA Extraction Kit (Bioneer, Cat. No. K-3141), according to the manufacturer’s instructions. RNA was quantified using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Cat. No. ND-2000), and 1 μg RNA was reverse transcribed into complementary DNA (cDNA) using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Cat. No. 4368814). The resulting cDNA was used as a template for PCR amplification, using specific primers for the gene of interest. PCR amplification was performed using Taq polymerase (Takara, Cat. No. R001A) under the following cycling conditions: initial denaturation at 95°C for 5 min, followed by 35 cycles at 95°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. The amplified products were separated by electrophoresis on a 1.5% agarose gel containing ethidium bromide, and visualized under UV light using a gel documentation system (Biorad, Cat. No. 170-8185). DNA ladder markers (New England Biolabs, Cat. No. N3232S) were used to determine the molecular weights of the PCR products, and band intensities were quantified using ImageJ software (NIH, version 1.53k).
Cell viability was assessed using a Luna-FL Automated Cell Counter (Cat. No. L20001). Cells were harvested, resuspended in phosphate-buffered saline (PBS; Thermo Fisher Scientific, Cat. No. 10010023) and stained with acridine orange/propidium iodide (AO/PI) staining solution (Logos Biosystems, Cat. No. F23001). Viable and non-viable cells were counted using a Luna-FL device, which provides accurate quantification based on fluorescence and bright-field imaging. Data from triplicate experiments were averaged and statistical analyses were performed to assess the impact of MUC19 knockdown on cell viability.
Statistical analyses were performed to evaluate the significance of genetic alterations and their association with survival outcomes in patients with glioblastoma multiforme (GBM) patients. Survival curves were generated using the Kaplan-Meier method, and differences between groups (living and deceased) were assessed using the log-rank test. The mutation frequencies for the top genes were compared between groups using Fisher’s exact test. P-values were adjusted for multiple comparisons using the Benjamini-Hochberg false discovery rate (FDR) correction to obtain q-values. A threshold of p < 0.05 and q < 0.05 was considered statistically significant. All analyses were performed using R software (version 4.1.2), and the results were visualized using appropriate plotting functions. For siRNA experiments, statistical significance between control and siMUC19-treated groups was determined using Student’s t-test, with a p-value of < 0.05 considered significant.
The top ten mutated genes were significantly enriched in the deceased group of patients with GBM ( Table 1). These genes, including PTEN, Aquaporin 7 Pseudogene 1 (AQP7P1), EGFR, MUC19, FSHD Region Gene 2 Family Member B (FRG2B), Mucin 17 (MUC17), Transmembrane Protein 191A (TMEM191A), Mucin 16 (MUC16), Rho Guanine Nucleotide Exchange Factor 33 (ARHGEF33), and NACHT And WD Repeat Domain Containing 2 (NWD2), had notably higher mutation rates in the deceased group than in the living group. The analysis highlights the cytoband locations, mutation rates, and statistical significance of the differences, measured through p-values and q-values. Three members of the mucin (MUC) gene family (MUC19, MUC17, and MUC16) were prominent, with MUC19 being the most significant gene in the group.
The patients were divided into a living group (n = 2,444) and a deceased group (n = 3226). This table identifies ten of the most significant mutated genes enriched in the deceased groups. The table also displays the gene’s cytobands, their rates in both deceased and living groups, their log2 ratio, their p-value, and their q-value. Phosphatase and tensin homolog (PTEN), Aquaporin 7 Pseudogene 1 (AQP7P1), Epidermal growth factor receptor (EGFR), Mucin 19 (MUC19), FSHD Region Gene 2 Family Member B (FRG2B), Mucin 17 (MUC17), Transmembrane Protein 191A (Pseudogene) (TMEM191A), Mucin 16 (MUC16), Rho Guanine Nucleotide Exchange Factor 33 (ARHGEF33), NACHT And WD Repeat Domain Containing 2 (NWD2) genes were identified to be enriched in the deceased groups. This table has been reproduced with permission from Ref. 22.
The effect of siRNA targeting MUC19 (siMUC19) on MUC19 expression levels and cell viability in A172 glioblastoma cells was analyzed ( Figure 1). The gel electrophoresis results showed two bands corresponding to MUC19 and GAPDH in the negative control (NC) and siMUC19-treated cells ( Figure 1A). After siRNA treatment, MUC19 expression in the siMUC19 group was significantly reduced compared with that in the control group. Quantitative analysis revealed that control cells showed significantly decreased cell viability ( Figure 1A).
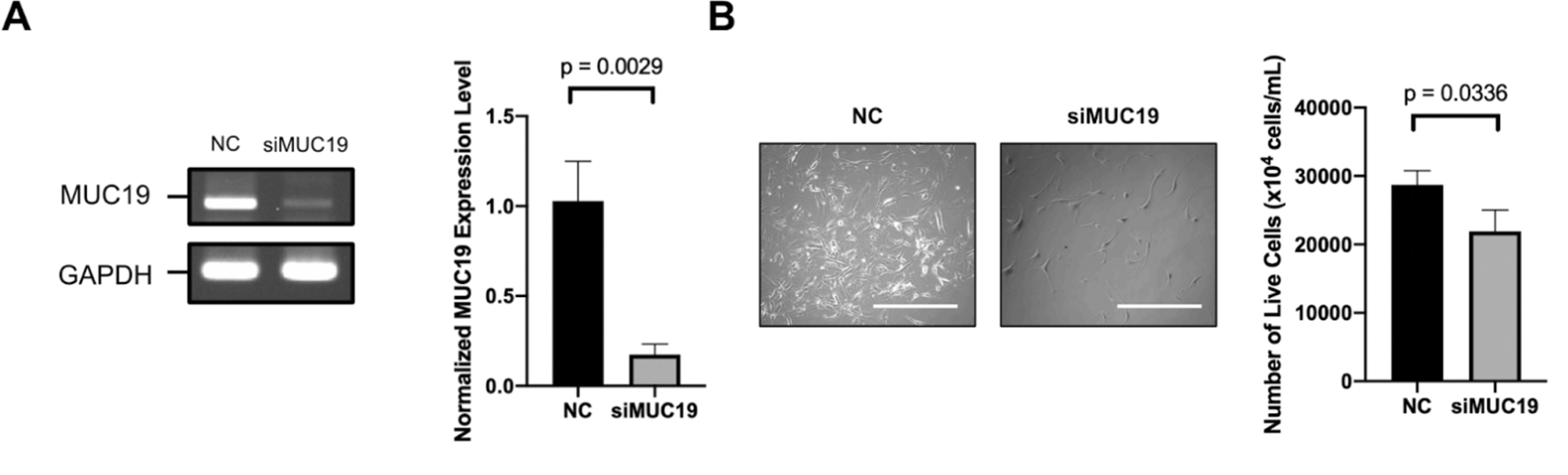
(A) Gel electrophoresis results showing MUC19 and GAPDH (control) expression in negative control (NC) and siMUC19-treated A172 cells seven days post-transfection. The siMUC19 group exhibits significantly reduced MUC19 expression compared to the control (p = 0.0029) (N = 3). (B) Quantitative analysis of cell viability after siMUC19 transfection. Representative microscopy images demonstrating decreased cell density in siMUC19-treated cells compared to the control group. The bar graph shows significantly lower MUC19 expression in the siMUC19 group compared to the control (p = 0.0336) (N = 3), Scale bar = 200 μm.
The normalized MUC19 expression levels in the siMUC19 group were significantly lower than those in the control group, and microscopic images further confirmed a decrease in cell density ( Figure 1B). siMUC19-treated cells exhibited visibly fewer cells than the control group, supporting the observation of reduced cell growth. Statistical analysis revealed a significant difference between the two groups, with a p-value of 0.0336 ( Figure 1B). These findings suggest that MUC19 plays a key role in glioblastoma cell growth, supporting the hypothesis that targeting MUC19 could be a potential therapeutic strategy.
This study provides significant insights into the genetic alterations associated with glioblastoma multiforme (GBM) mortality and highlights potential therapeutic targets, mainly focusing on mucin genes. Our findings revealed important differences in mutation frequencies between deceased and living GBM patients, offering new perspectives on disease progression and potential treatment strategies.
Our analysis identified ten key mutated genes that were significantly enriched in deceased GBM patients, suggesting their potential role in disease severity and mortality. These genes, including PTEN, EGFR, AQP7P1, and FRG2B, as well as three mucin genes (MUC19, MUC17, and MUC16), were selected based on their low q-values and p-values, indicating strong statistical significance. The diverse chromosomal locations of these genes and their higher presence in deceased individuals emphasize their possible involvement in critical biological processes that impact patient outcomes.
The identification of well-known cancer-associated genes, such as PTEN and EGFR, in our analysis, aligns with previous studies, reinforcing their importance in GBM pathogenesis.13 PTEN loss and EGFR amplification have been associated with poorer survival outcomes in patients with GBM, suggesting that personalized treatment strategies targeting these alterations could be more effective.14 PTEN, which is located on chromosome 10q23.31, regulates cell growth and division.15 Its mutation rate of 26.37% in deceased patients compared to 12.16% in living patients underscores its significance in GBM progression. EGFR, found on chromosome 7p11.2, is involved in the cell growth and survival pathways. Its amplification, observed in 20.41% of deceased patients versus 8.75% of living patients, further emphasizes its role in aggressive GBM phenotypes. AQP7P1, a gene encoding an integral membrane protein involved in water transport, showed a striking difference, with a 23.16% mutation rate in deceased patients compared to 0% in living patients.16 This finding suggests a potential novel role of this gene in GBM pathogenesis, which warrants further investigation.
A novel aspect of our findings is the identification of three mucin genes (MUC19, MUC17, and MUC16) among the top mutated genes in deceased patients with GBM. Although these genes are traditionally associated with the formation of protective mucous barriers in various bodily systems, their involvement in cancer pathogenesis, particularly in GBM, is less understood.17 MUC19 emerged was the most significant mucin gene in our analysis, with a mutation rate of 4.55% in deceased patients compared with 0% in living patients. Our siRNA experiments targeting MUC19 demonstrated a significant reduction in cell viability in A172 glioblastoma cells, suggesting its potential role in tumor cell survival and proliferation. These results indicate that MUC19 may be a viable therapeutic target, with the potential for the future development of targeted therapies aimed at silencing MUC19 expression. MUC17, located on chromosome 7q22.1, had a mutation rate of 9.93% in deceased patients versus 2.68% in living patients. This gene is known to play a role in creating a mucous barrier that protects the digestive system lining.18 Its increased mutation rate in deceased GBM patients suggests a potential role in tumor progression, possibly through cell adhesion or signaling pathway alterations. MUC16, found on chromosome 19p13.2, exhibited a mutation rate of 17.19% in deceased patients compared with 8.14% in living patients. This gene forms protective barriers on epithelial surfaces and is involved in cellular adhesion and immune responses. The higher mutation rate in deceased GBM patients may indicate a role in immune evasion or altered cell-cell interactions in more aggressive tumors.19 The roles of MUC17 and MUC16 in GBM progression require further investigation. Their potential contributions to GBM pathogenesis may involve cell adhesion, signaling, and immune modulation mechanisms that are yet to be fully elucidated in the context of brain tumors.20
Although our study provides valuable insights, it has several limitations that should be addressed in future research. Our reliance on data from deceased GBM patients may introduce bias as these patients likely have more aggressive tumor phenotypes. Future studies should include a more balanced cohort of patients at various disease stages to provide a more comprehensive understanding of the genetic alterations throughout GBM progression.21 Focusing on mutations in specific genes may overlook other potential factors such as epigenetic changes or microenvironmental influences, which could also significantly affect patient outcomes. A more comprehensive approach that incorporates these factors would provide a fuller picture of GBM progression and potentially reveal additional therapeutic targets. While our siRNA experiments provided evidence for the role of MUC19 in glioblastoma cell viability, further studies are needed to explore the exact mechanisms by which MUC19, MUC17, and MUC16 contribute to tumor progression.20
This study highlights the importance of genetic alterations, particularly in mucin genes, in GBM progression and mortality. These findings underscore the potential for developing personalized treatment strategies targeting MUC19, MUC17, and MUC16 in future therapeutic approaches. Although our results provide a promising foundation, further research is essential to validate these findings and explore new avenues for GBM treatment.
This study used publicly available clinical data obtained from the cBioPortal for Cancer Genomics, which was anonymized and de-identified to protect patient confidentiality. No direct involvement of human or animal participants was observed in this study. All experimental procedures involving cell cultures adhered to institutional guidelines and regulations regarding ethical conduct in the research. Specifically, A172 glioblastoma cell lines were approved under standard laboratory protocols, ensuring the proper handling and disposal of biological materials to minimize environmental or safety risks.
Figshare: Data_A comparative analysis of mutated genes that are enriched in deceased patients from cBioPortal analysis.xlsx, https://doi.org/10.6084/m9.figshare.27190986.v1.
This project contains the following underlying data:
• Data_A comparative analysis of mutated genes enriched in deceased patients from cBioPortal analysis.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Bioinformatics, Gene ontology, Cancer, genetic diseases
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 03 Dec 24 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)