Keywords
brain death; hematologic analysis; Wistar rats
This study aimed to evaluate hematologic, biochemical, and gasometric parameters in Wistar rats to better understand brain death parameters and reduce early misdiagnoses.
Fifteen adult male Wistar rats (Rattus norvergicus; HanUnib: WH) were randomly distributed into three groups of five animals: the control group (G0) with evaluation performed before brain death, and two groups (G1 and G2) with brain death induced at different times: immediately after induction (G1) and one hour after induction (G2). Venous and arterial blood samples were taken to perform complete blood count, biochemical, and blood gas assays. Samples were taken at specific times based on the group each rat belonged to.
Statistically significant mean values were observed (P<0,05) for segmented cells (G1>G2 and G0>G2), monocytes (G2>G1 and G0>G1), creatinine (G2>G0), aspartate aminotransferase (G1>G0), potassium (G2>G0), and bicarbonate (G0>G1).
Furthermore, brain death showed a unique response in each organism, complicating its precise determination even more.
brain death; hematologic analysis; Wistar rats
This study aimed to evaluate hematologic, biochemical, and gasometric parameters in Wistar rats to better understand brain death parameters and reduce early misdiagnoses. Brain death (BD), the complete and irreversible loss of brain function, is a complex process characterized by an inflammatory state leading to cellular and molecular disturbances that alter the physiology and biochemistry of the organic system. Wistar rats were randomly distributed into groups, and the time criteria used in the research followed the main goal of this research, as we aim to evaluate the brain death in different levels of death induction. We were able to evaluate the progression of the parameters at different times and understand how the brain death progresses, since the acute changes manifest immediately after injury and can last for a few days. The laboratory parameters play a fundamental role in understanding the progression of brain death, as death results in leakage of cytokines, proteins, and other components, mainly in the acute phase. Also, several changes in brain death may not be detectable in macroscopical examination, so the study primarily focused on laboratory parameters, which could provide early indications of physiological changes. Our results, even in a short interval time evaluated and with a short number of animal models, demonstrate the tendency of those changes. This type of study, in which brain death is evaluated by animal laboratory changes, is not well studied and explored. We hope our study stimulates further research. We wish we could have explored these points more in our response and paper. In closing, we sincerely thank the reviewers for providing an opportunity to clarify our perspective and initiating a professional, productive, and interesting discussion.
See the authors' detailed response to the review by Mario Abbud-Filho and Ludmila Marzochi
See the authors' detailed response to the review by Chiara Lazzeri
Brain death (BD), the complete and irreversible loss of brain function, is a complex process characterized by an inflammatory state leading to cellular and molecular disturbances that alter the physiology and biochemistry of the organic system.1 However, criteria for determining BD vary among countries, each having national laws or guidelines, resulting in a lack of standardization. In 50% of countries, clinical examination is sufficient to determine BD, whereas the other 50% require complementary tests.2
Currently, the American Association of Neurology (AAN) defines BD with three cardinal signs: interruption of brain functions, including the brainstem, coma or unresponsiveness, and apnea.3 In Brazil, social changes and advancements in medicine led the Federal Council of Medicine (CFM) to update the criteria for diagnosing BD in 2017, with Resolution No. 2,173 of November 23, replacing No. 1,408/97. The changes included specific physiological prerequisites for patients, requirements for doctors to provide care before diagnosing BD, the necessity of complementary tests, and specific training for doctors making this diagnosis.4,5
Determining the moment of BD is complicated by significant complex pathophysiological changes involving excitation of the sympathetic nerves, hormonal imbalance, hemodynamic instability, and metabolic disorders with the release of cytokines,6 which peak at alternating times due to the type of reaction and injury severity.7,8 During BD progression, a systemic inflammatory response can worsen, leading to disseminated intravascular coagulation mediated by inflammatory mediators from the ischemic brain, ischemic reperfusion injury, metabolic changes during the catecholamine storm, and an inadequately restored cardiovascular state. This also produces sudden changes in blood pressure, hypoxemia, hypothermia, coagulopathy, and electrolyte and hormonal disorders.9,10
More studies are needed to accurately determine the moment of BD, especially due to the scarcity of data. The literature on BD in animals is even more limited. Given the social importance of animals and the growing demand for scientific data to determine and establish death in animals, this has become an important issue for veterinarians. This importance is underscored by the increase in the animal market (farming, pet market, etc.) and the evolution of veterinary medicine (freelancers, clinics, hospitals, and research centers). Therefore, this study aimed to evaluate the hematological, biochemical, and gasometric parameters in rats, as these can be determinants of BD and help elucidate its concept, reducing errors in early diagnosis.
Fifteen Wistar adult male rats (Rattus norvegicus) of the HanUnib: WH line were acquired from the Multidisciplinary Center for Biological Research at the State University of Campinas (CEMIB - UNICAMP). They were housed in the Central Experimental Vivarium of the Experimental Research Unit (UNIPEX) at the Medical School (FMB) of São Paulo State University (UNESP). The rats were kept in polypropylene cages with metal grid covers in a room maintained at 22 °C and 55% humidity under a light-dark cycle. The protocols used in this study are in accordance with the Ethical Principles in Animal Research adopted by the Brazilian College of Animal Experimentation (COBEA) and were approved by the Ethics Committee on the Use of Animals (CEUA) of the Faculty of Medicine (FMB) of UNESP, Botucatu Campus, protocol No. 0259/2018 on 16th January of 2019.
Food and water were provided ad libitum, and environmental enrichment was performed using polyvinyl chloride (PVC) pipes and paper balls during the experimental period. Individual body weights were recorded weekly to monitor hygiene, adjust the number of animals per cage, and ensure their welfare. After an acclimatization period of 2 weeks, the animals were randomly divided into three groups, each with five animals: control group (G0) (n=5), evaluated before BD, and two groups with BD induction, immediately after induction (G1) (n=5) and 1 h after induction (G2) (n=5).
Before the induction of BD, the animals were anesthetized, initially in an induction box with isoflurane (9–5%) and then with an isoflurane mask (5–2.5%). Intubation was performed using a 14G or 16G catheter, and rats were maintained in the inhalation anesthesia circuit with isoflurane (3–1.5%). Trichotomy and asepsis were performed using chlorhexidine degermante and 70% alcohol in the area of access to the aorta and femoral vein. A 24G catheter was used to access the aorta and femoral vein, and a three-way tap circuit was set up to monitor blood pressure and collect blood for blood gas analysis, along with venous blood collection for blood count and biochemical tests. Animals with mean arterial pressure (MAP) >70 mmHg were used for hematological, biochemical, and hemogasometric parameters. Those without stable MAP were excluded from the experiment.
Blood samples were collected from each animal according to their respective group timings: G0 at time 0 (M0, before BD), G1 at time 1 (M1, shortly after BD induction), and G2 at time 2 (M2, 1 h after BD induction). The time criteria used in the research follow the main goal of this research as we aim to evaluate the brain death in different levels of death induction. Using these criteria, we were able to evaluate de progression of the parameters in different times and understand how the brain death progress, from immediately after death, hour after death compared with our control group. According to Hannoodee and Nasuruddin the acute changes manifest immediately after injury and can last for a few days.11 Venous blood samples were drawn from the femoral vein into 0.5 ml ethylenediaminetetraacetic acid (EDTA) tubes and refrigerated (6–10 °C) for subsequent erythrogram examination (including red blood cells [RBC], hemoglobin [Hb], hematocrit [HCT], mean corpuscular volume [MCV], mean corpuscular hemoglobin concentration [MCHC], mean corpuscular hemoglobin [MCH], total plasma protein [TPT], red cell distribution width [RDW], and platelets) and leukogram (covering total leukocytes, neutrophils, segmented, lymphocytes, eosinophils, and monocytes).
Processing was performed using a Hemacounter 60-RT 7600 hematological analyzer (Hemogram, China). Hematocrit was determined by centrifuging microhematocrit samples at 12,000 rpm for 5 min. Platelet counts were performed manually by diluting 20 μL of blood in 2 mL of Brecher solution and counting in a Neubauer chamber. The differential leukocyte count was executed on panotype-stained blood smears (Laborclin) under an optical microscope with immersion (1,000× magnification).
The biochemical examination was conducted using the BS200E Analyzer (Mindray, Brazil). Venous blood samples from the femoral vein were collected in dry tubes, refrigerated (6–10 °C), and centrifuged at 2,500 rpm for 5 min. This process was followed by analyzing urea, creatinine (Cr), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), serum total protein (TP), albumin, globulin, creatine kinase (CK), and CK-MB.
Blood gas analysis was performed using the ABL80 Flex Analyzer - BASIC Version (RADIOMETER). Arterial blood samples were collected from the aortic artery in 1 mL heparinized syringes (volume collected 0.7 mL), immediately packed in a styrofoam container with crushed ice, and analyzed for blood gases (pH, pCO2, and pO2), electrolytes, and metabolites (Na+, K+, Ca2+, Cl-, Lac), and derived values (HCO3 and sO2).
BD induction followed the protocol described by Esmaeilzadeh et al.10 A Fogarty 14G catheter (Baxter Health Corp., CA, USA) was inserted via frontolateral trepanation into the skull using a surgical drill. Intracranial pressure (ICP) was increased by slowly inflating with 400–700 μL of saline. The catheter balloon inflation began with 100 μL of saline solution, and after 1 min, an additional 100 μL of solution was added. This procedure was repeated until the absence of corneal reflexes confirmed the BD, maximally dilated fixed pupils and 60 s apnea.
Following BD confirmation, all animals had their body temperature maintained using a thermal mattress, and blood pressure (BP) and heart rate (HR) were continuously monitored with a multiparameter monitor, adjusted according to the respective group. Animals in the BD-induced groups were supported with mechanical ventilation after anesthesia withdrawal. After BD, the animals received a heated infusion of sodium chloride 0.9% supplemented with potassium chloride at a rate of 3–5 mL/kg/h, along with dopamine infusion (5–15 mcg/kg/min), with bolus administration as needed, to conclude animals’ euthanasia.
In the comparison among G0, G1, and G2, no statistically significant differences (P<0.05) were observed in the mean values of RBC, Hb, HCT, MCV, MCHC, MCH, TPP, RDW, and platelets among the experimental animals ( Table 1, Figures 1 and 2).
Differences in group least squares means, adjusted for multiple comparisons - Tukey-Kramer of the variables.
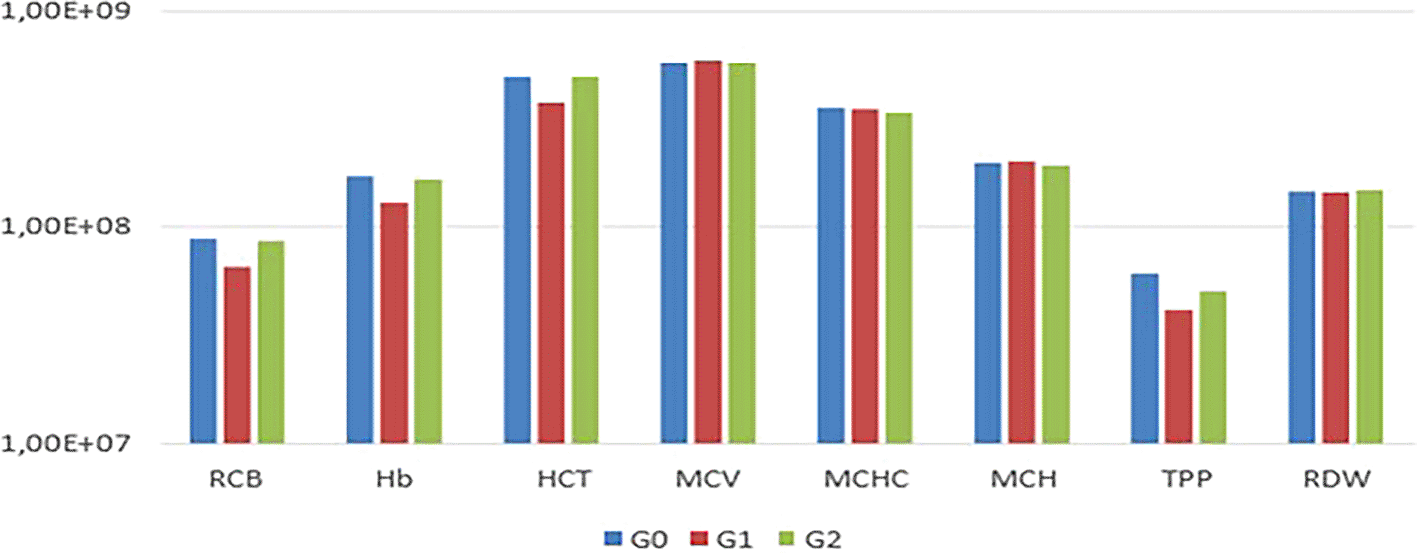
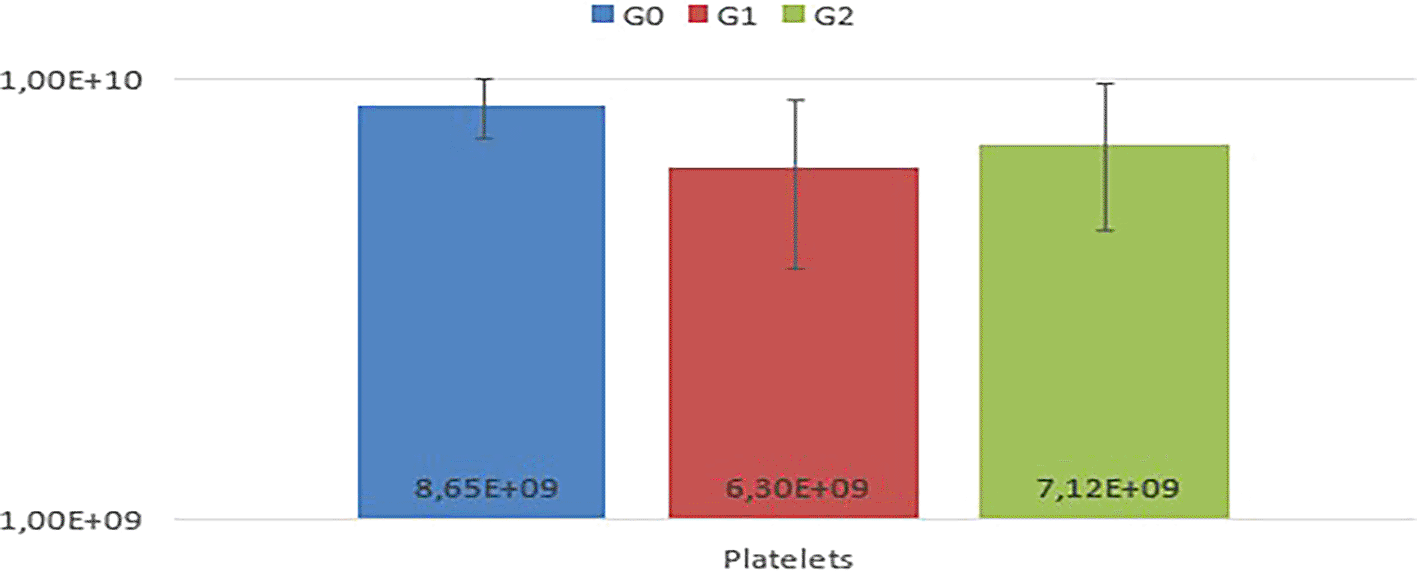
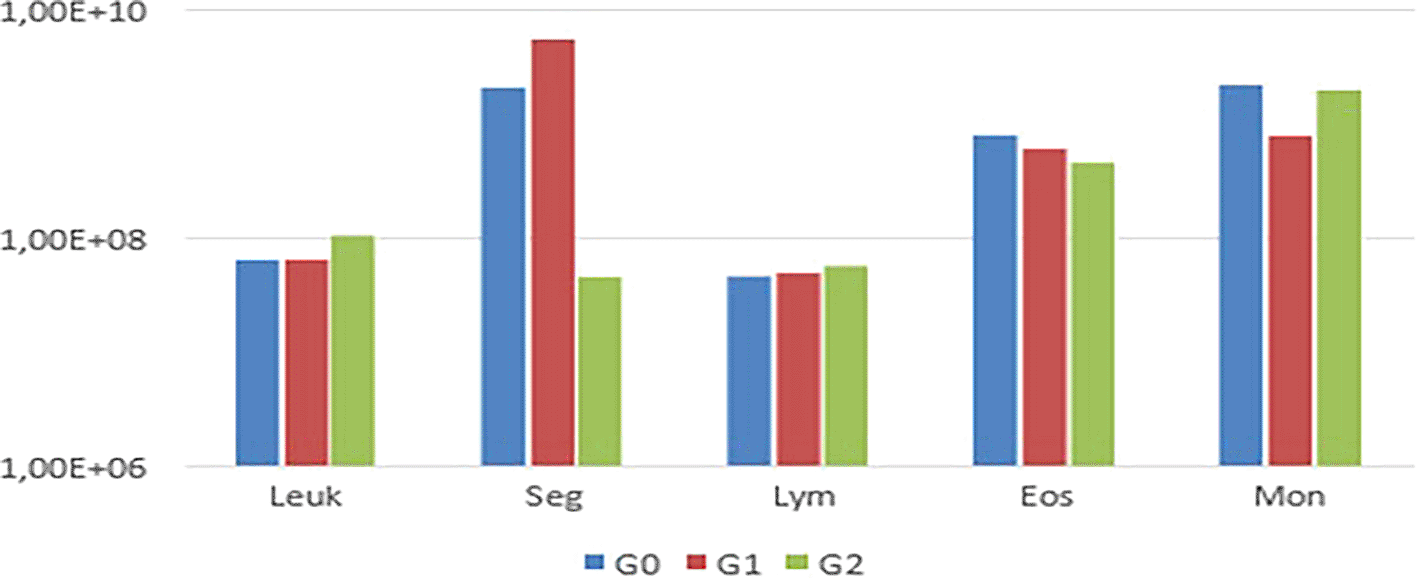
Notably, animals in G1 exhibited thrombocytopenia. In the leukogram ( Table 2), comparisons among G0, G1, and G2 regarding total leukocytes, segmented leukocytes, lymphocytes, eosinophils, and monocytes from the experimental animals revealed variations in segmented leukocytes and monocytes (p<0.05) (Figure 3).
Differences in group least squares means, adjusted for multiple comparisons - Tukey-Kramer of the variables.
| Variable | Group | _Group | Estimate | Standart Error | Value z | Pr˃│z│ | Adj p |
|---|---|---|---|---|---|---|---|
| Leukocytes | 1 | 2 | -0.4788 | 0.3086 | -1.55 | 0.1208 | 0.2670 |
| Leukocytes | 1 | 0 | 0.02027 | 0.3273 | 0.06 | 0.9506 | 0.9979 |
| Leukocytes | 2 | 0 | 0.4991 | 0.3273 | 1.52 | 0.1273 | 0.2793 |
| Segmented | 1 | 2 | 4.8179 | 0.8869 | 5.43 | <.0001 | <.0001* |
| Segmented | 1 | 0 | 0.9925 | 0.9407 | 1.06 | 0.2913 | 0.5420 |
| Segmented | 2 | 0 | -3.8245 | 0.9407 | -4.07 | <.0001 | 0.0001* |
| Lymphocytes | 1 | 2 | -0.1416 | 0.3453 | -0.41 | 0.6816 | 0.9114 |
| Lymphocytes | 1 | 0 | 0.06694 | 0.3662 | 0.18 | 0.8550 | 0.9817 |
| Lymphocytes | 2 | 0 | 0.2086 | 0.3662 | 0.57 | 0.5690 | 0.8363 |
| Eosinophils | 1 | 2 | 0.2800 | 0.3098 | 0.90 | 0.3660 | 0.6378 |
| Eosinophils | 1 | 0 | -0.4601 | 0.3463 | -1.33 | 0.1841 | 0.3794 |
| Eosinophils | 2 | 0 | -0.7401 | 0.3463 | -2.14 | 0.0326 | 0.0825 |
| Monocytes | 1 | 2 | -0.9122 | 0.3619 | -2.52 | 0.0117 | 0.0314* |
| Monocytes | 1 | 0 | -1.0284 | 0.3838 | -2.68 | 0.0074 | 0.0202* |
| Monocytes | 2 | 0 | -0.1162 | 0.3838 | -0.30 | 0.7621 | 0.9607 |
The biochemical tests ( Table 3) compared G0, G1, and G2 regarding urea, Cr, ALT, AST, ALP, GGT, serum TP, albumin, globulin, CK, and CK-MB from the experimental animals (Figure 4). Only Cr and AST exhibited variations in their values.
Differences in group least squares means, adjusted for multiple comparisons - Tukey-Kramer of the variables.
| Variable | Group | _Group | Estimate | Standart Error | Value z | Pr˃│z│ | Adj p |
|---|---|---|---|---|---|---|---|
| Urea | 1 | 2 | -0.05071 | 0.1513 | -0.34 | 0.7375 | 0.9400 |
| Urea | 1 | 0 | 0.07189 | 0.1513 | 0.48 | 0.6348 | 0.8831 |
| Urea | 2 | 0 | 0.1226 | 0.1513 | 0.81 | 0.4179 | 0.6967 |
| Creatinine | 1 | 2 | -0.1210 | 0.1460 | -0.83 | 0.4071 | 0.6850 |
| Creatinine | 1 | 0 | 0.2935 | 0.1460 | 2.01 | 0.0444 | 0.1096 |
| Creatinine | 2 | 0 | 0.4146 | 0.1460 | 2.84 | 0.0045 | 0.0126* |
| ALT | 1 | 2 | -0.3916 | 0.2849 | -1.37 | 0.1692 | 0.3542 |
| ALT | 1 | 0 | 0.2354 | 0.2849 | 0.83 | 0.4087 | 0.6867 |
| ALT | 2 | 0 | 0.6270 | 0.2849 | 2.20 | 0.0277 | 0.0710 |
| AST | 1 | 2 | 0.7952 | 0.3469 | 2.29 | 0.0219 | 0.0568 |
| AST | 1 | 0 | 0.9538 | 0.3469 | 2.75 | 0.0060 | 0.0164* |
| AST | 2 | 0 | 0.1586 | 0.3469 | 0.46 | 0.6474 | 0.8911 |
| AF | 1 | 2 | -0.5406 | 0.2725 | -1.98 | 0.0472 | 0.1161 |
| AF | 1 | 0 | 0.08733 | 0.2725 | 0.32 | 0.7486 | 0.9449 |
| AF | 2 | 0 | 0.6280 | 0.2725 | 2.30 | 0.0212 | 0.0551 |
| GGT | 1 | 2 | -0.06188 | 0.05020 | -1.23 | 0.2177 | 0.4339 |
| GGT | 1 | 0 | -0.06188 | 0.05020 | -1.23 | 0.2177 | 0.4339 |
| GGT | 2 | 0 | 1.03E-17 | 0.05020 | 0.00 | 1.0000 | 1.0000 |
| TSP | 1 | 2 | -0.06744 | 0.1127 | -0.60 | 0.5497 | 0.8210 |
| TSP | 1 | 0 | -0.1588 | 0.1127 | -1.41 | 0.1589 | 0.3364 |
| TSP | 2 | 0 | -0.09135 | 0.1127 | -0.81 | 0.4177 | 0.6966 |
| Albumin | 1 | 2 | -0.01026 | 0.1774 | -0.06 | 0.9539 | 0.9982 |
| Albumin | 1 | 0 | -0.2044 | 0.1774 | -1.15 | 0.2493 | 0.4821 |
| Albumin | 2 | 0 | -0.1942 | 0.1774 | -1.09 | 0.2739 | 0.5177 |
| Globulin | 1 | 2 | -0.1121 | 0.08407 | -1.33 | 0.1824 | 0.3765 |
| Globulin | 1 | 0 | -0.1197 | 0.08407 | -1.42 | 0.1546 | 0.3288 |
| Globulin | 2 | 0 | -0.00755 | 0.08407 | -0.09 | 0.9285 | 0.9956 |
| CK | 1 | 2 | 0.8398 | 0.5325 | 1.58 | 0.1148 | 0.2556 |
| CK | 1 | 0 | 0.5379 | 0.5325 | 1.01 | 0.3125 | 0.5705 |
| CK | 2 | 0 | -0.3019 | 0.5325 | -0.57 | 0.5707 | 0.8377 |
| CK-MB | 1 | 2 | 0.2818 | 0.5311 | 0.53 | 0.5956 | 0.8563 |
| CK-MB | 1 | 0 | -0.1141 | 0.5311 | -0.21 | 0.8299 | 0.9749 |
| CK-MB | 2 | 0 | -0.3959 | 0.5311 | -0.75 | 0.4559 | 0.7363 |
Overall, blood gas analysis ( Table 4) showed that most animals in all groups had low hydrogen potential (pH) levels, increased oxygen partial pressures (PaO2), and carbon dioxide partial pressures (PaCO2).
In some animals, bicarbonate (HCO3) levels were decreased, and the oxygen saturation index (sO2) and lactate values tended to increase. Moreover, sodium levels showed a tendency to increase in most animals, potassium increased in only one, calcium decreased uniformly across all, and chloride tended to rise in the majority.
In the bivariate analysis, the initial analysis of the nine erythrogram variables in group 0 using the Spearman test showed positive correlations with hemoglobin (Hb), platelets, mean corpuscular volum (MCV), and mean corpuscular hemoglobin (MCH) ( Tables 5 and 6).
| Spearman's Correlation Coefficients (N = 4) Prob >|r|sob H0: Rho=0 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| RBC | Hb | HCT | MCV | MCHC | MCH | TPP | RDW | Platelets | |
| RBC | 1.00000 | 0.40000 | 0.80000 | -0.80000 | -0.80000 | -0.80000 | 0.25820 | 0.20000 | 0.40000 |
| 0.6000 | 0.2000 | 0.2000 | 0.2000 | 0.2000 | 0.7418 | 0.8000 | 0.6000 | ||
| Hb | 0.40000 | 1.00000 | 0.80000 | 0.00000 | -0.20000 | 0.00000 | 0.77460 | -0.80000 | 1.00000 |
| 0.6000 | 0.2000 | 1.0000 | 0.8000 | 1.0000 | 0.2254 | 0.2000 | <.0001* | ||
| HCT | 0.80000 | 0.80000 | 1.00000 | -0.60000 | -0.40000 | -0.60000 | 0.77460 | -0.40000 | 0.80000 |
| 0.2000 | 0.2000 | 0.4000 | 0.6000 | 0.4000 | 0.2254 | 0.6000 | 0.2000 | ||
| MCV | -0.80000 | 0.00000 | -0.60000 | 1.00000 | 0.40000 | 1.00000 | -0.25820 | -0.40000 | 0.00000 |
| 0.2000 | 1.0000 | 0.4000 | 0.6000 | <.0001* | 0.7418 | 0.6000 | 1.0000 | ||
| MCHC | -0.80000 | -0.20000 | -0.40000 | 0.40000 | 1.00000 | 0.40000 | 0.25820 | -0.40000 | -0.20000 |
| 0.2000 | 0.8000 | 0.6000 | 0.6000 | 0.6000 | 0.7418 | 0.6000 | 0.8000 | ||
| MCH | -0.80000 | 0.00000 | -0.60000 | 1.00000 | 0.40000 | 1.00000 | -0.25820 | -0.40000 | 0.00000 |
| 0.2000 | 1.0000 | 0.4000 | <.0001* | 0.6000 | 0.7418 | 0.6000 | 1.0000 | ||
| TPP | 0.25820 | 0.77460 | 0.77460 | -0.25820 | 0.25820 | -0.25820 | 1.00000 | -0.77460 | 0.77460 |
| 0.7418 | 0.2254 | 0.2254 | 0.7418 | 0.7418 | 0.7418 | 0.2254 | 0.2254 | ||
| RDW | 0.20000 | -0.80000 | -0.40000 | -0.40000 | -0.40000 | -0.40000 | -0.77460 | 1.00000 | -0.80000 |
| 0.8000 | 0.2000 | 0.6000 | 0.6000 | 0.6000 | 0.6000 | 0.2254 | 0.2000 | ||
| Platelets | 0.40000 | 1.00000 | 0.80000 | 0.00000 | -0.20000 | 0.00000 | 0.77460 | -0.80000 | 1.00000 |
| 0.6000 | <.0001* | 0.2000 | 1.0000 | 0.8000 | 1.0000 | 0.2254 | 0.2000 | ||
The meticulous control and interpretation of laboratory test changes are intrinsic to diagnosing BD.12,13 Individual examination within each group revealed a tendency towards polycythemia in most animals, particularly notable in G2, consistent with findings from Fiocruz.14 This can be explained by hypoxemia resulting from BD, leading to secondary absolute polycythemia. One subject in G1 showed anemia and hypoproteinemia. One study found hemoglobin and hematocrit concentrations also decreased after BD induction.7 There were also animals in this group that showed thrombocytopenia. Another research revealed 38.5% of organ donors exhibited anemia and 30.8% had thrombocytopenia.29 This phenomenon is attributed to common coagulation disorders following BD, driven by factors such as thromboplastin release, damaged brain tissue fibrinogen release, disseminated intravascular coagulation (DIC), and platelet and coagulation factor consumption due to fluid resuscitation volumes.13
Regarding the analysis of variables in the leukogram examination, the reduction in segmented neutrophils in G2 may be related to increased bone marrow immunosuppression 1 h after BD. An increase in the number of circulating hematopoietic precursor cells, followed by bone marrow dysfunction, has been observed in patients with severe trauma, hemorrhagic shock, or burns. Stroke induces systemic inflammatory response and subsequent immunosuppression.15,16
Evaluating the animals in each group individually, two animals (A6 and A11) in G1 and G2, respectively, presented leukocytosis. This change is justified by the inflammatory state resulting from the BD process, which activates inflammatory mediators like thromboxanes and leukocyte factors. Among the potential human donors, 66.2% had leukocytosis.17,18 Conversely, Menegat and Sannomiya observed BD-induced leukopenia in rats, resulting in a reduction in lymphocytes, monocytes, and granulocytes.19
The difference in Cr values between G0 and G2 in this study may be associated with renal function failure due to hemodynamic instability and hypotension, leading to decreased perfusion.20 Another factor that can affect the kidneys and cause a sudden elevation of plasma Cr, which is widely used as an indicator of acute renal failure and changes in diuresis volume, is rhabdomyolysis.21 In this study, most of the gas tests presented an acid-base imbalance. Most animals tended towards respiratory acidosis, and some presented metabolic acidosis. These disorders are associated with an increased risk of organ and system dysfunction, and metabolic acidosis is an indicator of unfavorable clinical outcomes.22,23
In BD, gasometry values vary considerably, as the lungs are among the first organs to suffer changes in the body. The lungs seek to compensate and preserve a physiologically acceptable pH when the renal ability to maintain homeostasis is lost.24 However, the lung tissue can be affected by pathophysiological and endocrine changes and the inflammatory reactions caused by BD.25 Additionally, perfusion-ventilation imbalance and hypoxemia are the main manifestations of pulmonary changes due to intense adrenergic discharge, leading to increased venous return to the right ventricle consequently increased pulmonary flow. Along with elevated left atrial pressure from intense peripheral vasoconstriction, this results in increased capillary hydrostatic pressure, promoting capillary rupture, interstitial edema, and alveolar hemorrhage.26
Abnormal partial oxygen pressure promotes the release of tumor necrosis factor-alpha and IL-1 beta, which are inflammatory cytokines that mediate lung lesions.21 According to Mascia et al.19 and Botha et al.,6 30–45% of potential donors develop lung injury, with acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) being the most frequent. Nogueira and Pereira25 observed that 69.2% of patients had hyperoxia due to oxidative stress in the clinical picture of BD. In this study, lactate levels tended to increase. This is mainly due to altered energy metabolism, where excessive prolongation of anaerobic metabolism overcomes the body’s ability to remove lactate, leading to metabolic acidosis, which can become severe and cause fatigue, even when buffering mechanisms are well-developed.27,28 According to Hodgson and Rose, an anaerobic threshold is commonly reached when lactate.29 concentration is between 2–4 mmol/L. In our study, higher lactate levels were observed.
In most animals, hypernatremia was observed, and an increase in potassium was seen in only one animal, contradicting Westphal et al.,20 who observed hyperkalemia and hypomagnesemia as common disorders associated with BD, potentially leading to arrhythmia. Additionally, calcium decreased in all animals, whereas chlorine levels tended to increase in most cases. Hypernatremia was observed in most animals, and an increase in potassium was seen in only one animal. This contradicts Westphal et al.,20 be related to progressive dysfunction of the hypothalamic-pituitary axis after BD and, in 80% of cases, the development of diabetes insipidus, which results in a decline in circulating antidiuretic hormone.30 The polyuria resulting from this endocrine/metabolic change can lead to serious electrolyte disorders, such as hypernatremia, hypokalemia, hypocalcemia, hypophosphatemia, and hypomagnesemia.14 In a study by Vasconcelos et al. with possible human donors, 47.7% had hypernatremia, similar to the values obtained in this study.31
Therefore, this study recognizes and highlights the deleterious effects caused by BD, noting that these effects are multiple and cause various complications in the body, so an evaluation using different combined analyses is important, as shown in this study, to also diagnose different critical diseases prior to the brain death itself. Early detection is important to avoid progressive somatic deterioration and ensure better organ function.31,32 The experimental model used in this study, which is interesting for evaluating a short period of time, did not produce more significant results for many variables. The speed of diagnosing BD may not lead to major changes in tests within 1 h after BD. Additionally, the number of animals (n) used was a limiting factor. In conclusion, BD showed a unique response in each organism, complicating its precise determination even more.
However, the time criteria used in the research follow the main goal of this research as we aim to evaluate the brain death in different levels of death induction. Using these criteria, we were able to evaluate the progression of the parameters at different times and understand how the brain death progresses, from immediately after death, hour after death, compared with our control group. And it is settled that the acute changes manifest immediately after injury and can last for a few days.11 So maybe the results we have had reflect the reality of the changes that we can and cannot observe in brain death.
The small sample size of our study was a limitation for establishing parameters to settle brain death characteristics. However, we have calculated the minimum number of animals to use and validate the data. The main problem was the inconsistency of the values found, which supports our belief that the brain death parameters have large intervals, so future research could be elaborated with a more significant number of animals to see if it would impact the results. We indeed believe that BD shows a unique response in each organism, so it is a challenge to determine its precise time. But our results support the hypothetical theories we found during the literature review with a pilot study conducted in laboratory animals.
We believe, within the results we found in this research that the laboratory parameters play a fundamental role in understanding the progression of brain death, as death results in leakage of cytokines, proteins, and other components, mainly in the acute phase. Also, several changes in brain death may not be detectable in macroscopical examination, which is why this study primarily focused on laboratory parameters, which could provide early indications of physiological changes. the primary objective of this paper was to evaluate the hematologic, biochemical, and gasometrical parameters in brain death to understand the modifications and reduce misdiagnosis by using these parameters as an indication in early stages.
Our research is experimental, and we performed an individual examination of the specific groups used in the research. The experimental nature of this paper should be considered, as well as the small number of animals used in this research. Our results, even in a short interval time evaluated and with a short number of animal models, demonstrate the tendency of those changes. We hope our study stimulates further research, which could provide a better discussion of the results. Further studies with more animals and extended observation periods are necessary to elaborate a more comprehensive discussion of the mechanisms involved.
In conclusion we believe that this study highlights the complex physiological changes associated with brain death (BD) in Wistar rats. Significant differences were observed between groups in the laboratory analysis, such as: segmented cells, monocytes, creatinine, aspartate aminotransferase, potassium, and bicarbonate levels, demonstrating the varied impact of BD on these parameters. The study shows that early detection is important to avoid progressive somatic deterioration and ensure better organ function. Although the experimental model and sample size in this work were not ideal, it has the potential to contribute to the understanding that individual responses to BD oscillate, complicating its diagnosis and emphasizing the need for further investigation to improve clinical diagnostic precision.
We confirm that all efforts were made to ameliorate suffering of animals applying the rules settled to animal pain management and suffering in the “Brazilian Guide to Good Animal Euthanasia Practices” (available online in: https://www.cfmv.gov.br/guia-brasileiro-de-boas-praticas-para-a-eutanasia-em-animais/comunicacao/publicacoes/2020/08/03/#1) dictated by the Brazilian Federal Council of Veterinary Medicine (CFMV). Ethical Principles in Animal Research adopted by the Brazilian College of Animal Experimentation (COBEA) and were approved by the Ethics Committee on the Use of Animals (CEUA) of the Faculty of Medicine (FMB) of UNESP, Botucatu Campus, protocol No. 0259/2018 on 16th January 2019.
Figshare: Parâmetros hematológicos, gasométricos e bioquímicos na indução da morte encefálica em ratos Wistar, DOI: https://doi.org/10.6084/m9.figshare.27586602.v633
This project contains the following underlying data:
• PARÂMETROS HEMATOLÓGICOS, GASOMÉTRICOS E.docx
• Underlying data spreadsheet.xlsx
• IACUC
• mazzante_nmg_dr_bot_sub-english.docx
• Underlyingdataspreadsheet-english.xlsx
• ARRIVE Checklist
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Figshare: Arrive checklist for ‘ Parâmetros hematológicos, gasométricos e bioquímicos na indução da morte encefálica em ratos Wistar’, DOI: https://doi.org/10.6084/m9.figshare.27586602.v6
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We thank the School of Veterinary Medicine and Animal Science of São Paulo State University for their support and encouragement during this study.
The authors declare that the Chat GPT – 4 in the response writing process to improve readability and language.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Brain death, disorders of consciousness, neuroimaging, general neurology
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: organ procurement and transplantation, cardiac arrest, ECMO, respiratory failure, critical care echocardiography
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Renal transplantation, donor preconditioning, molecular aspects of marginal kidneys
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Renal transplantation, donor preconditioning, molecular aspects of marginal kidneys
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: organ procurement and transplantation, cardiac arrest, ECMO, respiratory failure, critical care echocardiography
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 06 May 25 |
read | read | read |
|
Version 1 06 Dec 24 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)