Keywords
aflatoxin, ochratoxin, antibody titer, broiler, toxin binders, food production
In broiler farming, vaccination against Newcastle disease (ND) is essential. Nonetheless, during the post-vaccination phase, production may be negatively impacted by mycotoxin contamination in feed. This study aimed to evaluate the effect of mycotoxin binders on immune and intestinal histopathology ameliorations against ND in vaccinated broilers with aflatoxin B1 (AFB1) and ochratoxin A (OTA) toxication.
A total of 20 broilers were randomly assigned into 4 groups with 5 replications i.e. (C-) broiler groups with basal feed, (C+) broiler groups with AFB1 and OTA feed contamination, (T1) and (T2) broiler groups with exposed AFB1, OTA, and toxin binders as feed additives with dose 1.1 g/kg and 1.6 g/kg feed, respectively. ND vaccination was carried out on day 7 and 21. Antibody titers were evaluated from serum samples of broiler on days 14, 28, and 35 for further hemagglutination inhibition (HI) test. Histopathology of the cecum and colon organs was evaluated using HE staining on day 36. HI test and histological scoring were analyzed using the One-Way ANOVA, followed by Duncan’s test with a p < 0.05 in SPSS v.26 software (IBM Corp., Armonk, NY).
As a result, histopathological improvement of the cecum and colon was reported based on mucosal rupture, hemorrhage and necrosis on day 35. A significant increase in antibody titer was observed on day 35 in the C− and T2 groups, which differed significantly (p < 0.05) from the C+ and T1 groups.
This study revealed that a 1.6 g/kg toxin binder dose in feed can increase antibody titer and histopathology of cecum and colon in broiler chickens after ND vaccination fed with mycotoxin-contaminated feed.
aflatoxin, ochratoxin, antibody titer, broiler, toxin binders, food production
In the revised version of the manuscript, several improvements were implemented to enhance its clarity and scientific rigor. The abstract was refined to better emphasize the key findings, while the introduction was expanded to include the study's contributions and address existing research gaps. The methodology section was strengthened by incorporating details on ethical approval, experimental design, animal treatments, mycotoxin preparation, hematoxylin-eosin (HE) staining procedures, and statistical analyses. Additionally, figure and table legends were supplemented with explanatory captions, and relevant references were added as recommended to support and enrich the discussion.
See the authors' detailed response to the review by Essam S Soliman
See the authors' detailed response to the review by Agung Irawan
See the authors' detailed response to the review by Rifat Ullah Khan
See the authors' detailed response to the review by Mohammad Miftakhus Sholikin
See the authors' detailed response to the review by Ammar M. Al-Aalim
The presence of immunosuppressant substances, such as mycotoxins including aflatoxins B1 (AFB1) and ochratoxins A (OTA),1 and in tandem are the mycotoxin combination that has been shown to have high toxicity.2 Which can decrease the body’s immune response to vaccination, may be the reason for antibody titer findings that indicate no protection against vaccination.3 Immunosuppressive disorders result from T cell proliferation failure carried on by immunotoxic processes, which prevents the body from responding to the incoming vaccination.4,5 In the short term, OTA and AFB1 interacting to contaminate chicken feed will provide a synergistic negative effect.6 The digestive system has the highest risk of exposure since animal feed is the first point in the food chain where mix mycotoxin can enter directly through plant goods like grains.7 The process of digesting and absorbing dietary fibre in chickens is significantly aided by the large intestine.8,9
Ochratoxin A profoundly alters the microbiota’s composition and metabolism in the large intestine, which has an impact on the process of fermentative digestion that occurs there.10 Aflatoxin B1 plays a significant role in the production of reactive oxygen species (ROS), which damage DNA and cause lesions in the mitochondria.11,12 Damage to the membranes and nuclear structures of cells can result in necrosis or death of the cells.13 Mycotoxicosis is a poisoning that happens when an animal eats feed that is too toxic for their body to tolerate. When mix mycotoxin is exposed to the large intestine, it first disrupts the synthesis and breakdown of proteins, which alters the integrity of the intestine and damages the intestinal mucosa. Mix mycotoxin exposure will eventually cause oxidative damage. Excessive production of ROS can result in tissue damage and impede the healing process, ultimately contributing to cellular necrosis.14
In the gastrointestinal system, mycotoxin binders generally act as adsorbents. One form of adsorbent that has been extensively researched in vivo and in vitro that is effective at removing mycotoxins from the body is bentonite (Dioctahedral montmorillonite). Because the fluid in the gastrointestinal system has a negative charge on its surface, bentonite will be distributed there and utilise cation exchange processes to absorb different biomolecules, including aflatoxin.15 Mycotoxin binders are generally composed of natural or synthetic adsorbent materials rather than single molecular entities. Their primary mechanism involves binding mycotoxins within the gastrointestinal tract, thereby promoting their elimination via fecal excretion. Mycotoxin binders have the ability to reduce the amount of mix mycotoxin in feed, which in turn lessens the amount of mix mycotoxin that penetrates the bloodstream and other bodily organs and causes mycotoxicosis.16 Mycotoxin binders in this research, also known as biotransforming agents, with different function in that they alter the chemical makeup of mycotoxins to be non-toxic molecules. This biotransforming agent was classified as yeast (Trichosporon mycotoxinivorans). As a biotransforming agent, the aforementioned yeast can hydrolise amide bonds in the chemical structure of OTA to break it down into new chemical and non-toxic molecules.17 Vaccination against Newcastle disease (ND) in broiler farming is essential. Nonetheless, during the post-vaccination phase, production may be negatively impacted by mycotoxin contamination in feed.
This study aimed to evaluate the effect of mycotoxin binders on immune and intestinal histopathology ameliorations against ND in vaccinated broilers with AFB1 and OTA toxication.
This study was approved by the Ethical Committee of Animal Care and Use, Faculty of Veterinary Medicine, Universitas Airlangga (No. 1.KEH.033.02.2023) on February 10, 2023. This study was also reported according to the Animal Research: Reporting of in vivo Experiments (ARRIVE) guidelines 2.0: author checklist.44 All efforts were made to ameliorate any suffering of animals. Animal handling was carried out in accordance with institutional guidelines and the American Veterinary Medical Association standards, 2020 (AVMA, 2020).
All experiments in this study were conducted from February 27 to June 10, 2023 in an animal laboratory. Antibody titer evaluation was conducted in the microbiology laboratory, while histopathology evaluation was conducted in the pathology laboratory, Faculty of Veterinary Medicine, Universitas Airlangga.
This study employed a true experimental design to assess the effects of a dietary mycotoxin binder (Mycofix®, Cat No. 20026289-FTS, PT. Biomin Indonesia) on antibody titers and intestinal histopathology (cecum and colon) in broiler chickens exposed to AFB1 and OTA following ND vaccination. A completely randomized design (CRD) was utilized. Sample size determination was based on the Federer formula: t(n − 1) ≥ 15, where t represents the number of treatment groups and n the number of replicates per treatment. Accordingly, twenty Cobb-strain broiler chickens were randomly assigned to four treatment groups, with five birds per group (n = 5).
Day-old male Cobb broiler chicks (sourced from PT. Charoen Pokphand Indonesia, Jakarta, Indonesia) were sexed based on feather morphology. The chicks were housed in sanitized battery cages (30.5 × 35 cm) under controlled temperature and photoperiod conditions, with unrestricted access to feed and water. A 7-day acclimatization period was provided prior to the initiation of experimental treatments. All birds received vaccinations against ND and avian influenza (AI) on days 7 and 21.
The five steps of preparation before acclimatization of day-old chicks (DOC) are as follows: 1. Clean and remove all physical materials from a chicken cage; 2. Wash and clean all equipment and the cage with soap and water, using scraping; 3. Spray liquid disinfectant on all equipment and the cage and close the cage for a week; 4. Spray solid disinfectant (lime) and mix it with water in the cage and close the cage for a week; 5. Fumigate with permanganate and formalin, close the cage, as this can irritate; and finally, wait a week before DOC arrives.
A total of 20 DOC broiler chickens Cobb strain were reared for 35 days as final stock following broiler rearing standards. Rearing standards for broiler experiments include: basic ventilation system (open house), rodent and flyproof, cleaning and disinfection protocol (five steps before DOC arrived), ambient temperature (18 to 24°C), relative humidity (50 to 70%), manual feeding system, watering system (manual, ad libitum, and groundwater), lighting program (light color at 20 lux intensity for the first 7 days and 17-20 hours duration), and lighting program. From day 8 to day 35, the chicks were subjected to one of four dietary treatment regimens. They were randomly allocated into four treatment groups, each comprising five replicates. The negative control group (C−) received only the commercial basal diet (CP511 starter and CP512 finisher; PT. Charoen Pokphand Indonesia). The positive control group (C+) was fed the same basal diet contaminated with AFB1 at 0.16 mg/kg (Fermentek Ltd., Jerusalem, Israel) and OTA at 0.16 mg/kg (GLP BIO, Shanghai, China). Treatment group 1 (T1) was provided the contaminated basal diet supplemented with 1.1 g/kg of a commercial mycotoxin binder comprising bentonite, Bacillus subtilis, T. mycotoxinivorans, and phytogenic compounds (Mycofix®, Cat No. 20026289-FTS, PT. Biomin Indonesia). Treatment group 2 (T2) received the same contaminated diet supplemented with 1.6 g/kg of the mycotoxin binder. All feed formulations and mixing procedures were conducted at the Feed Laboratory, Faculty of Veterinary Medicine, Universitas Airlangga. Feed was administered twice daily, while water was provided ad libitum throughout the experimental period.
The mycotoxin binder used in this study not only adsorbs mycotoxins but also biotransforms them into non-toxic metabolites. Bentonite effectively binds the polar AFB1, whereas OTA, which is non-polar, requires biotransformation into less toxic forms, a process facilitated by Trichoderma reesei.
Newcastle Disease (ND) vaccination was administered to seven-day-old broiler chickens using the La Sota inactivated ND vaccine (Medivac®, Cat No. 3526004001, PT. Medion Farma, Indonesia) via ocular instillation at a dose of one drop per bird. A booster vaccination was given on day 21 using the same inactivated vaccine at a dose of 0.5 mL per bird via intramuscular injection. Antibody titers were assessed through the hemagglutination inhibition (HI) test using serum samples collected on days 14, 28, and 35. Additionally, cecum and colon tissues were harvested on day 36 for histopathological examination using hematoxylin-eosin (HE) staining.
Erythrocytes suspension was collected from ND virus antibody-negative donor chickens. Blood was drawn from the brachial vein and collected in tubes containing EDTA solution (BD Vacutainer® EDTA Tubes, Cat No. 366643, USA). The blood was washed thrice using PBS by centrifuging at 1.500 g for 10 minutes. The buffy coat and plasma fluid were discarded at each washing. The erythrocyte residue was then diluted with PBS to a concentration of 0.5%.
A total of 1 mL blood samples without anti-coagulant were collected through the brachial vein in 20 broiler chickens aged 14, 28, and 35 days. Blood was then allowed until collected serum and transferred into an Ependorf tube. The HA test was performed to determine the antigen titer that will be used for the HI test. This test begins with filling the microplate wells (A1–A12) with 25 μl of PBS. Furthermore, 25 μl of antigen was inserted into the A1 and A12 wells (antigen control). The antigen and PBS in the A1 well were then homogenized with the suction-blow technique using a 25 μl micropipette, then 25 μl of liquid was transferred from one well to the next well, and so on until it reached the A11 well. The remaining 25 μl in the micropipette was discarded. Furthermore, all A1–A12 wells were filled with 50 μl of 0.5% chicken erythrocyte suspension. The microplate was then shaken and incubated at room temperature for 30 minutes, and the titer was ready to evaluate.
The microplate was filled with 25 μl of PBS solution into number 1–5 wells in rows A and B (duplicate titration) using a micropipette. Then, the first well in rows A and B was filled with 25 μl of ND antigen. Antigen and PBS in 1 well were then homogenized by the suction-blowing technique using a 25 μl micropipette, then 25 μl of liquid was transferred from one well to the next well, and so on until reaching the 4 wells. Furthermore, all wells were filled with 50 μl of 0.5% chicken erythrocyte suspension. The microplate was then shaken and incubated at room temperature for 30 minutes, and the titer was ready to interpret.
The first until the twelfth microplate wells were filled with 25 μl of PBS using a micropipette. Then, the first and twelfth wells are filled with 25 μl of serum to be checked for titer. Serum and PBS were mixed in the first well by the suction-blowing technique using a micropipette, then 25 μl of liquid was transferred from the first well to the next well, and so on until the tenth well. Next, wells 1–10 were filled with 25 μl of ND antigen. The microplate was then shaken and incubated for 30 minutes at room temperature. Next, all wells were filled with 50 μl of 0.5% chicken erythrocyte suspension and incubated for 30 minutes for further evaluation.
On the 36th day, the cervical dislocation procedure was used to perform euthanasia.18 All efforts were made to ameliorate any suffering of animals. In the event that the study’s chickens displayed signs of illness prior to the termination time, they received vitamin supplements to enhance their health and were treated in accordance with the disease’s symptoms. Nevertheless, the deceased chicken was taken out and insinerated if it died before the termination time.
The procedure of manual cervical dislocation (CD) involves twisting and stretching the neck with the hands. After being picked up, the chickens were fed to help them relax. As we gradually repositioned our hold, we waited for the chickens to calm down. The non-dominant hand was used to grasp the chicken legs. We slowly turned the chicken so that its feet were pointing up at our chest. To keep them motionless, the chicken feet were securely held close to the base of their rear end. Grasping the upper part of the chicken’s neck, where the soft, spongy vertebrae meet the brain stem, fingers were wrapped around them. Gently, the thumb and index finger were encircling this section of the chicken’s neck. After that, the neck of the fowl was angled 90 degrees downward. The chicken’s body was gradually moved to position its head away from the dominant side. Their feet were securely grasped while the head and neck of the chicken were arranged in an L configuration, the beak pointing downward. The twisting motion dislocates the vertebral column from the skull and ruptures or stretches the blood vessels, while the stretching action tears the neck muscles, ligaments, connective tissues, and blood vessels. The cessation of the pupillary light reflex and nictitating membrane reflex were death indicators in broiler chickens.18,19 The process of incineration involves burning carcasses or residues at temperatures as high as 850 °C to create an inorganic ash. All infectious agents are anticipated to be destroyed by the treatment. Ash usually makes about 1–5% of the initial volume of the carcass, though this will vary depending on the type of incinerator, the method, the fuel, and the species of animal.20
Tissue sampling was conducted via abdominal dissection following euthanasia. The cecum and colon from a total of 20 broiler chickens were excised and immediately fixed in 10% formalin solution within labeled containers to preserve the tissues for histopathological analysis using hematoxylin-eosin (HE) staining. The fixed tissues were embedded in paraffin, sectioned at a thickness of 5 μm, and subsequently stained with HE. Histopathological evaluation was performed using a light microscope (Nikon Eclipse E200, Japan), with inflammatory cell infiltration assessed at 100× magnification and morphological alterations in the cecum and colon examined at 400× magnification. For each sample, five randomly selected fields were analyzed and scored by blinded observers to minimize bias. The extent of pathological alterations was quantified using a standardized scoring system based on the proportion of affected tissue area: score 0 indicated no observable changes, score 1 corresponded to pathological findings in less than 25% of the field, score 2 represented involvement of 26–50%, score 3 indicated 51–75% affected, and score 4 denoted more than 76% pathological changes within the examined tissue. This method allowed for a systematic and semi-quantitative assessment of histopathological severity.21
The hemagglutination inhibition (HI) test data were organized and assessed for normality using the Shapiro-Wilk test. Data conforming to a normal distribution were subsequently analyzed via one-way analysis of variance (ANOVA), followed by Duncan’s multiple range post hoc test for pairwise comparisons. Histopathological scores, which were non-parametric, were analyzed using the Kruskal–Wallis test, with pairwise comparisons conducted using the Mann–Whitney U test. All statistical analyses were performed using SPSS version 26.0 for Windows (IBM Corp., Armonk, NY, USA), with significance determined at a 95% confidence interval.
Results in this research have increased in the mean antibody titer observed on day 35 compared to days 14 and 28, with significant changes observed in serum samples based on the C+ group, which was significantly different from the C- and T2 groups (p < 0.05).
Based on hemagglutination inhibition (HI) test, as a result, erythrocyte sediment at the bottom of the microplate without a hemagglutination process results in a positive HI test; conversely, hemagglutination in the form of a diffused erythrocyte layer at the bottom of the microplate without an erythrocyte sediment results in a negative outcome. Based on Figure 1, presents the HI test, (A) depicts the absence of hemagglutination, which is defined as erythrocyte sedimentation when the microplate is tilted, and (B) illustrates the occurrence of hemagglutination, which is defined as erythrocyte absence at the microplate well’s bottom. The maximum serum dilution that could prevent hemagglutination against antigens was also used to calculate the HI titer.
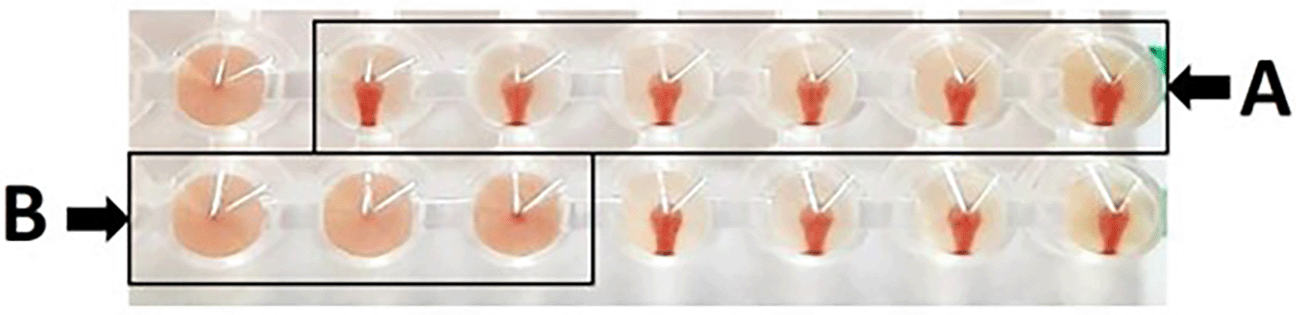
Panel (A) demonstrates the absence of hemagglutination, characterized by the sedimentation of erythrocytes upon tilting the microplate. In contrast, panel (B) illustrates the presence of hemagglutination, indicated by the lack of erythrocyte sedimentation at the bottom of the microplate well.
There were no discernible changes between groups C-, C+, T1, and T2 when the antibody titer of broiler chickens was examined at 14 days of age, or 7 days after the initial ND vaccination. Analysis by average revealed that all treatment groups’ antibody titers seemed to be moderate and did not yet exceed ND-protective titers (≥7 HI log 2).
On day 35, broiler antibody titers varied (p = 0.010) between groups, indicating that mycotoxin binder may have an impact on the development of ND antibody titers. According to the results of the Duncan test, there were no significant differences between the treatment groups C- with T1, C- with T2, C+ with T1, and T1 with T2, but the C+ with C- and C+ with T2 groups were significantly different, as shown by different letter superscripts in the same column ( Table 1). Conversely, a significant increase in antibody titer was observed in broilers at 35 days of age compared to the mean titers recorded at 14 and 28 days. The relatively low antibody titer at 14 days of age—seven days following the initial ND vaccination—can be attributed to the early phase of the immune response, during which the immune system is still in the process of recognizing and responding to the initial antigen exposure. This stage, known as the primary immune response, represents the body’s initial immunological reaction to a novel antigen.22,23
Histopathological improvement of the cecum and colon was reported based on reduced mucosal rupture, hemorrhage and necrosis on day 35.
The findings of the scoring analysis of the broiler chickens’ mucosal rupture, necrosis, and hemorrhage in the cecum ( Table 2) and colon ( Table 3). The control group (C-), had the lowest mean rank. The C+ treatment group, had the highest mean rank. The T1 treatment group and Treatment group T2 as indicated in Table 2. Furthermore, the T2 group demonstrated a progressive improvement in the histological differences related to mucosal rupture, hemorrhage, and necrosis in the cecum organ ( Figures 2–4).
| Treatment group | Parameters (Mean rank) | ||
|---|---|---|---|
| Mucosal rupture | Hemorrhage | Necrosis | |
| C- | 3.8c | 4.7c | 5.0c |
| C+ | 17.9a | 17.6a | 17.5a |
| T1 | 13.1b | 13.1b | 12.9b |
| T2 | 7.2c | 6.6c | 6.6c |
| Treatment group | Parameters (Mean rank) | ||
|---|---|---|---|
| Mucosal rupture | Hemorrhage | Necrosis | |
| C- | 4.5c | 5.0c | 4.2c |
| C+ | 17.9a | 18.0a | 17.3a |
| T1 | 12.8b | 12.8b | 13.2b |
| T2 | 6.8c | 6.2c | 7.3c |

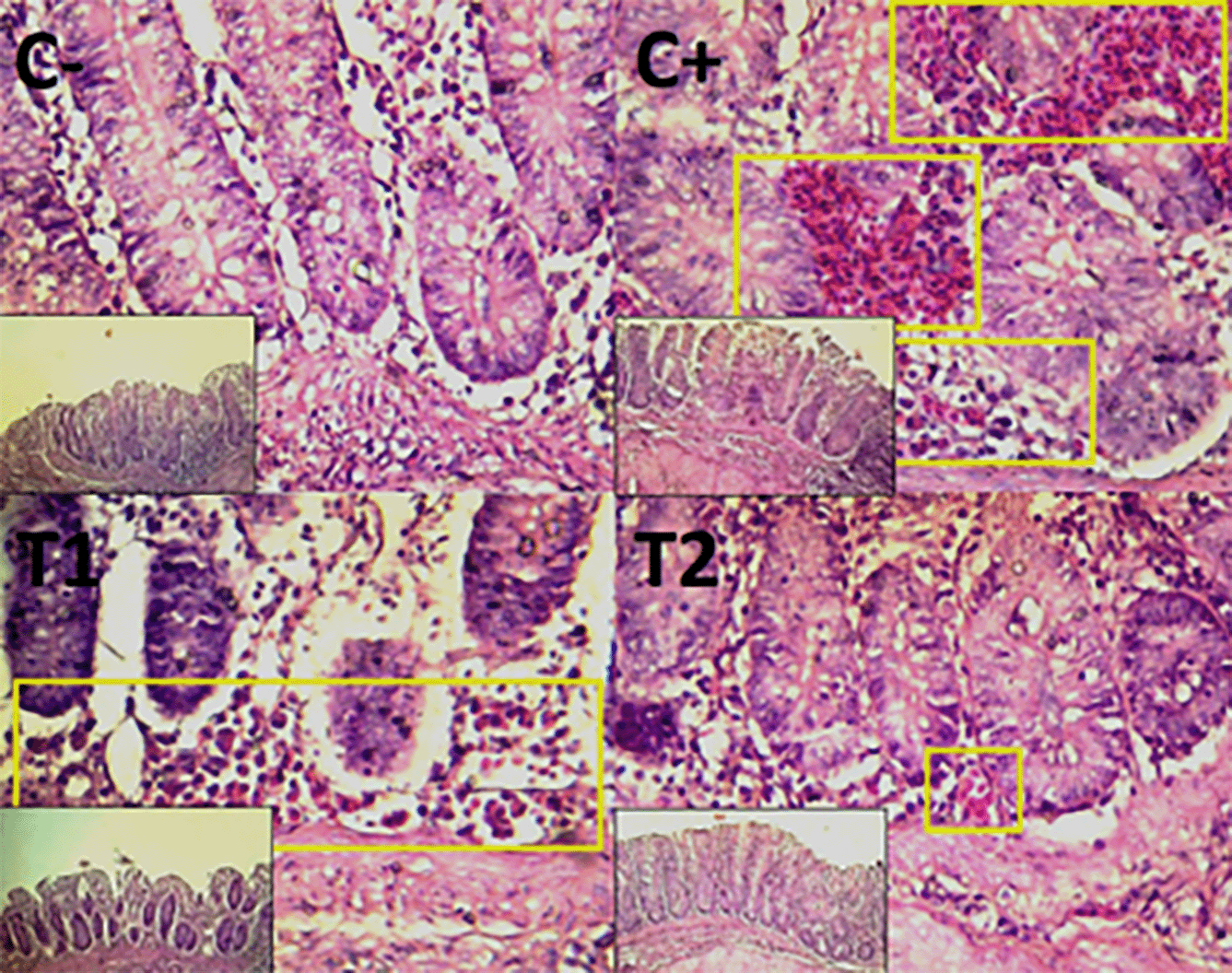

The control group, treatment group C-, had the lowest mean rank. Treatment group C+, had the highest mean rank. The T1 treatment group. Table 3 shows that the T2 treatment group demonstrated a progressive improvement in the histological differences of mucosal rupture, haemorrhage, and necrosis in the colon organ ( Figures 5–7).
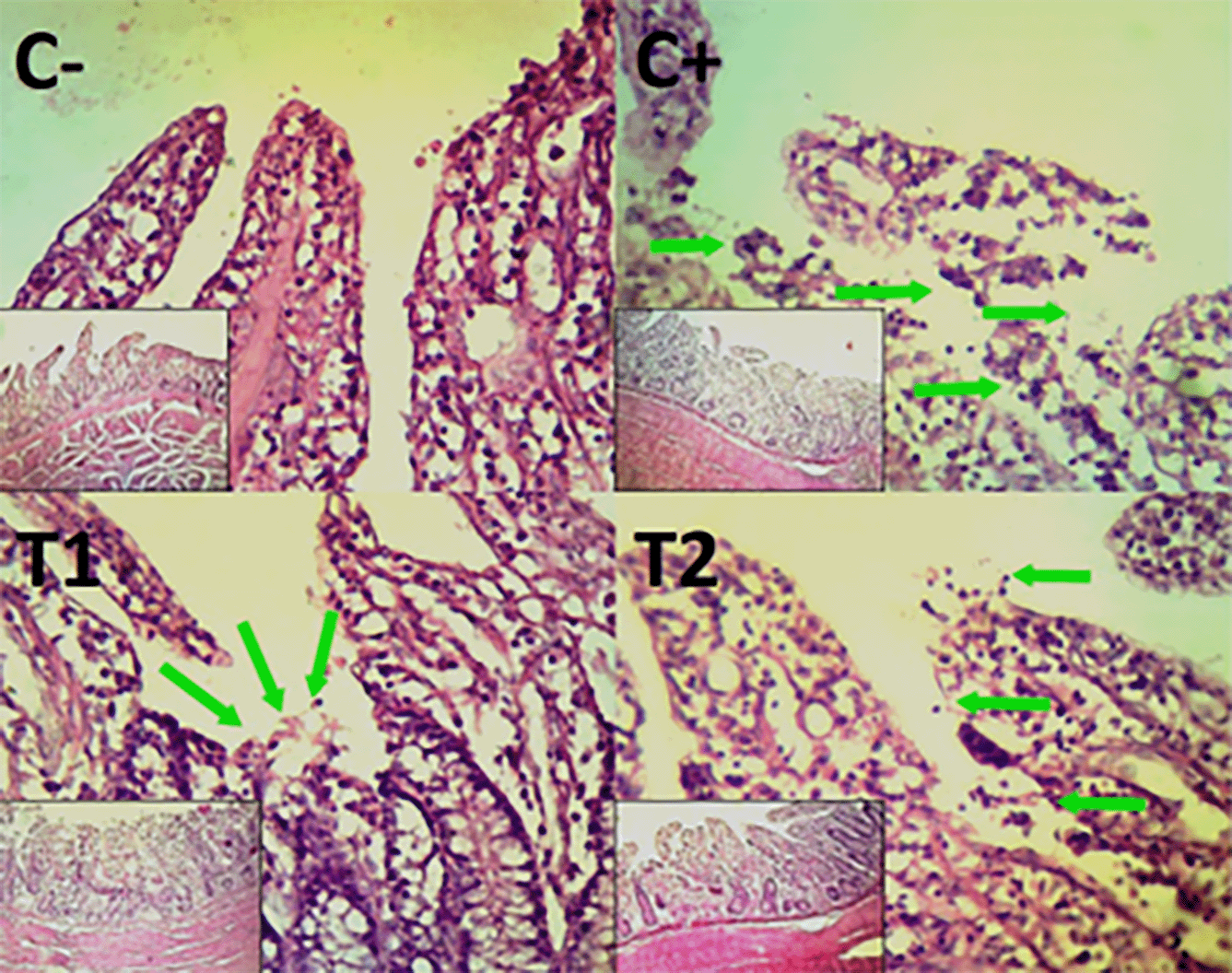

The primary immune responses that initiated by cell-mediated immunity (CMI) often follow a more gradual course than secondary immunological responses. T cells that serve the role of CMI will develop into effector T cells, or T helper (CD4+) and T cytotoxic (CD8+). Both CD8+ and CD4+ go through three phases in their response to antigens: the expansion phase, the death phase, and the memory phase.24 Approximately seven days after antigen entry, the organism enters the expansion phase, characterized by the proliferation of immune cells. This is subsequently followed by the contraction phase, during which homeostatic mechanisms lead to the apoptosis of more than 90% of activated T cells.25,26
The investigation of antibody titer evaluation in this research performed seven days after booster vaccination in 28-day-old broilers following ND vaccination revealed no significant difference between the C-, C+, T1, and T2 groups. In contrast to the antibody titer at 14 days of age, the average antibody titer in the C-, C+, T1, and T2 groups increased. Immunoglobulin or antibody levels tend to be low seven days after the first vaccination when the HI test is conducted because of this mechanism, which prevents IL-2 produced by CD4+ and CD8+ from signaling B cells to perform maximum cell differentiation. In order to remember and identify antigens to contribute to the secondary immune response during booster immunization, the remaining active T cells will go into the memory phase.27
The development of a secondary immune response could be the cause of the increase in antibody titer at 28 days of age. Booster vaccinations containing the same antigen can accelerate the proliferation and differentiation of B cells by activating memory cells produced from the main immune response.28
In all treatment groups, the average antibody titer appears to have increased, but it is still below protective levels (≥ 7 HI log 2). Because the body’s production of antibodies against the inactive vaccine has not yet reached its maximal titer, this is conceivable. According to a prior study, because inactive vaccines contain an oil adjuvant, vaccination with them will result in the highest antibody titer occurring 14–21 days after the vaccination.24 In order to improve the immune response, adjuvants are substances that are added to vaccines. By slowing down the body’s process of destroying antigens, adjuvants prolong the time that the vaccination comes into touch with macrophages and lymphocytes, which delays the vaccine’s ability to induce the development of antibodies.29
The broiler group with the lowest average antibody titer among the other treatment groups is C+, which was exposed to AFB1 and OTA without the use of any additional mycotoxin binder agents. According to a prior study, immunotoxic diseases might arise in the body as a result of mycotoxin exposure.30 The primary immune cell apoptotic pathways, autophagy, and oxidative stress will all play a role in the immunotoxic mechanism. These cells include B lymphocytes, dendritic cells, macrophages, neutrophils, and T cells. Moreover, the body experiences immunosuppression, autoimmunity, immunological dysfunction, and hypersensitivity reactions as a result of mycotoxin exposure.31
The impact of chronic mycotoxicosis conditions linked to weight loss, cancer, immunosuppression, and pathological conditions with other slow onset times is known as immunosuppression. In this study, the effect of immunosuppression resulting from mycotoxin exposure on post-vaccination antibody titers has not been observed significantly at 14 and 28 days of age examination. The type, duration, dose, age, and species of the mycotoxin all affect the intensity and symptoms of exposure. If mycotoxins are given to chickens for longer than three to four weeks, the result is chronic mycotoxicosis.32,33
The analysis of antibody titers at 35 days of age revealed significantly different findings in group C+ with T2 (p < 0.05) in addition to group C+ with C-. T2 is a group of broilers given commercial feed that has been exposed to AFB1 and OTA as well as a mycotoxin binder agent at a dose of 1.6 g/kg feed. In order to prevent the toxicity of mycotoxins from spreading throughout the body, mycotoxin binder agents as feed additives typically function by lowering the bioavailability of mycotoxins while they are still in the gastrointestinal tract. Mycotoxin binders, such as bentonite clay, and mycotoxin modifiers, such as T. mycotoxinivorans yeast, are two key components found in mycotoxin binder agent products. When it comes to removing mycotoxin exposure from the body, the two of them collaborate well.34
As an enterosorbent, bentonite can bind polar mycotoxins like AFB1 in a certain way. It disperses into the gastrointestinal tract’s fluid, becoming negatively charged. This negative charge can then absorb aflatoxins through cation exchange reactions or positive charges from AFB1 dicarbonyl bonds.35 The pH, binder content, mycotoxin kind and dosage, and incubation duration are just a few of the numerous variables that impact an adsorbent’s capacity to interact with mycotoxins.36,37 Unlike bentonite, the yeast T. mycotoxinivorans has the capacity to decrease the bioavailability of OTA by the hydrolysis of the amide bond structure that links the hydro-isocoumarin ring and the phenylalanine group. Two less hazardous chemicals, L-β-phenylalanine and α-ochratoxin (OTα), will be produced if the amide link is effectively hydrolysed.38,39
Using mycotoxin binder chemicals as feed additives to bind mix mycotoxin can help prevent uncontrollably contaminated mycotoxin mixes. Using mycotoxin binders, the mix of mycotoxin is detoxified according to its specific features. In this work, the polar toxin known as AFB1 is detoxified using bentonite, an adsorbent that will boost enzyme activity and prevent exposure to the toxin in the digestive system. Mycotoxin binders function by biotransformation, which is the process of converting mixed mycotoxin into non-toxic metabolites, as opposed to OTA, which is non-polar.39,40 The absorption mechanism of mycotoxin binders in the present study is presumed to be consistent with findings from previous research, which demonstrated that visible light absorption facilitates the efficient degradation of target materials.41
Mycotoxin binder is an option that can be utilised as a feed additive in the large intestine, with an ideal dosage of 1.6 g/kg feed. The integrity of the intestinal mucosa will be impacted if the toxicity of mix mycotoxin declines. It will result in fewer goblet cells in the large intestine, which will make the intestinal mucosa less susceptible to injury and mucosal rupture.10
In an attempt to detoxify the toxins created by mixed mycotoxin and lessen their toxicity, a mycotoxin binder is utilized, with an ideal dosage of 1.6 g/kg feed. Aflatoxin B1 will be adsorbed by the mycotoxin binder, but OTA will be biotransformed. By detoxifying the poison created by mixing mycotoxin, a mycotoxin binder will lower the amount of toxin generated. Lower toxin levels will undoubtedly have an impact on endothelial damage brought on by exposure to mixed mycotoxin; as endothelial damage declines, the endothelium’s role as a mediator of fluid or plasma release that aids in hemostasis will rise.10,12
As a mycotoxin deactivator for AFB1 and OTA, the use of a mycotoxin binder at an optimal inclusion rate of 1.6 g/kg of feed represents an effective intervention strategy. The mode of action of the binder depends on the chemical properties of the target mycotoxin. Aflatoxin B1, due to its polar molecular structure and capacity to form strong, stable bonds, is primarily neutralized through adsorption. However, conventional adsorbents such as aluminum silicates, which expand and disintegrate upon hydration, are less effective under such conditions. Similarly, zeolite loses its adsorptive properties at acidic pH levels, such as pH 3. In contrast, the detoxification of OTA involves enzymatic biotransformation, wherein the mycotoxin is degraded into non-toxic metabolites. The mechanisms targeted by the mycotoxin binder in this study are consistent with findings from previous research, which have demonstrated that certain bioactive compounds—such as quercetin—can mitigate organ damage by inhibiting disease progression.42 Furthermore, the combined action of mycotoxins does not induce oxidative stress, necrosis, or cell death, provided the toxins have been effectively neutralized or eliminated.12,21
The findings of this study indicate that supplementation with a mycotoxin binder at a dosage of 1.6 g/kg feed in broilers exposed to AFB1 and OTA positively influences the development of antibody titers following ND vaccination and provides protective effects against tissue rupture, hemorrhage, and necrosis in the cecum and colon.
Erma Safitri, Siti Darodjah Rasad, Goo Jang, and Mitsuhiro Takagi: Supervision, Research Conceptualization, Methodology and Research Observation, Writing—Original Draft Preparation; Hery Purnobasuki, Tita Damayanti Lestari, Suzanita Utama, Eka Pramyrtha Hestianah, Mohammad Anam Al Arif, Chairul Anwar Nidom, Sri Mulyati, Sri Hidanah, and Jola Rahmahani: Curation for Data, Analysis and Formal Investigation, Resources of References, Visualization, Writing—Review and Editing; Merisa Wahyu Erdhina and Maulida Ilma Sadida: Curation for Data, Software and Validation; Martia Rani Tacharina and Muhammad Thohawi Elziyad Purnama: Curation for Data, Proof Read, Review and Editing.
Figshare: Database of hemagglutination inhibition test data, histopathology scoring of cecum and colon, and original histopathology figures. https://doi.org/10.6084/m9.figshare.27248592.v2.43
The extended data set contains the following data:
• Antibody titer
• Cecum histopathology scoring
• Colon histopathology scoring
• Histopathology figure of cecum
• Histopathology figure of colon
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Figshare: ARRIVE Guidelines 2.0 for Mycotoxin binders study. https://doi.org/10.6084/m9.figshare.27900534.v1.44
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
To analyse the quantitative data, SPSS version 26 (IBM Corp., Armonk, NY) was used. If readers are looking for an open-source substitute, JASP (https://jasp-stats.org/) can be used to conduct comparable analyses.
The authors express their profound gratitude to Dr. Gadis Meinar Sari, dr., M. Kes, Chairman of the Institute for Research and Community Service, Universitas Airlangga, and Prof. Dr. Mirni Lamid, drh., M.P., the Dean of the Faculty of Veterinary Medicine, Universitas Airlangga Surabaya for providing the research facilities.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Animal Nutrition
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Animal Nutrition
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Veterinary Immunology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Zhu Z, Zhou S, Tian D, Li GZ, et al.: Fabrication of Polydopamine/hemin/TiO2 Composites with Enhanced Visible Light Absorption for Efficient Photocatalytic Degradation of Methylene Blue.Polymers (Basel). 2025; 17 (3). PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Poultry science
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Putra RP, Astuti D, Respati AN, Ningsih N, et al.: Protective effects of feed additives on broiler chickens exposed to aflatoxins-contaminated feed: a systematic review and meta-analysis.Vet Res Commun. 2024; 48 (1): 225-244 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Nutritional biochemistry of poultry and ruminants related to feed, feed technology, and feeding.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: 1. Preventive medicine and prophylactics of animals (Large and small), poultry, and fish. 2. Biosafety and biosecurity measures, plans, and strategies in animal and poultry farms. 3. Green environment and waste management and reuse. 4. Combating means of infectious and contagious diseases. 5. Air and water quality, and environmental sanitation. 6. Epidemiology of infectious and contagious disease and measurement of disease occurrence.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
|
Version 3 (revision) 25 Jul 25 |
read | ||||
|
Version 2 (revision) 27 Jan 25 |
read | read | read | ||
|
Version 1 13 Dec 24 |
read | read | |||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)