Keywords
Steroids, Treatment, antiviral drugs, COVID-19, Favipiravir, Maraviroc, severe disease
Coronavirus disease 2019 (COVID-19) has created the need to evaluate drugs such as favipiravir (FPV), an antiviral inhibitor of RNA-dependent RNA-polymerase (RdRp), and Maraviroc (MVC), an antiretroviral that antagonizes the chemokine receptor CCR5, which could affect the modulation of inflammation and viral replication in the treatment of COVID-19. We sought to evaluate the effect of MVC and/or FPV plus systemic steroid (SS) vs. SS alone on the viral load and progression to critical disease.
Sixteen patients with severe COVID-19 were evaluated in three treatment arms: 1) SS only (n=6), 2) SS plus one test drug MVC or FPV (n=5), and 3) SS plus both test drugs (MVC and FPV, n=5). The viral load was determined for N, E, and RdRp viral genes.
A significant decrease in viral load was observed in the three treatment groups, with a larger effect size in the group that combined SS with both test drugs. The E, N, and RdRp genes with Cohen’s d were 120%, 123%, and 50%, respectively.
The largest effect on viral load reduction, as measured by effect size, was observed in the combination treatment group; however, no statistical significance was found, and it did not prevent progression to critical illness.
Steroids, Treatment, antiviral drugs, COVID-19, Favipiravir, Maraviroc, severe disease
We have included many important references in the introduction section and discussion parts about new compounds/drugs, that are effective against the different variants of SARS-CoV2, as well as the last reports for COVID-19 disease treatment in general.
See the authors' detailed response to the review by Amgad M Rabie
The abrupt emergence of COVID-19 took the world's healthcare services by surprise like no other global public health event in the last century. Medicine had neither vaccines nor treatments that could help contain the exponential spread of infections and the increasing deaths observed in the first stages of the pandemic.1,2
Consequently, one of the first responses of the medical communities around the world was to propose a myriad of clinical trials in search of an already known drug that could be repurposed to target one of the pathogenic mechanisms of the novel infection that was being understood in a parallel manner to the advance of the pandemic.3–5
This elicited the emergence of multiple trials where the antiviral capacity of many previously existing drugs for different stages of the infection was evaluated with a wide variety of results such as remdesivir,6 ensitrelvir,7 nirmatrelvir,8 teriflunomide,9 and nucleoside/nucleotide analogs.10–12
In hospitalized patients with coronavirus disease 2019 (COVID-19), five percent of patients with severe disease can progress to critical condition; therefore, the WHO designed a 10-point progression and severity score.13 Most patients who progress to severe disease have at least one risk factor, such as old age, type 2 diabetes mellitus (T2DM), obesity, hypertension (HTN),14 and high viral load with a cycle threshold (Ct) <25.15 High viral load at admission and within the first 12 days, as well as old age and comorbidities, are independently associated with the risk of intubation and mortality.15,16 The severity of COVID-19 prompted both new therapeutic strategies; dexamethasone helped to reduce mortality17 and at the same time, in silico studies revealed an area of opportunity for Favipiravir (FPV), a prodrug developed in 2002 for the influenza virus as a ribonucleotide analog that inhibits the viral replication by inhibiting the viral enzyme RNA-dependent RNA-polymerase (RdRp).18–20 Moreover, its known adverse effects include thoracic pain, diarrhea, hyperuricemia, and liver damage.21–25 Another repositioned drug is Maraviroc (MVC), developed for human immunodeficiency virus (HIV), which antagonizes the chemokine receptor CCR5,26 additionally modulates inflammation,27–29 and could have a dual block of CCR5 and the NSP5 (3CLpro or Mpro) viral protease of the SARS-CoV-2 virus, which is responsible for the regulation of replication.28,30 It could also inhibit S-protein-mediated cell fusion and syncytia-forming capacity.29 The most frequent adverse effects were skin rash, infections, bronchitis, cough, and fever.31 Subsequently, the development of vaccines has reduced mortality,32 and the antivirals approved thus far are not fully effective, and their availability is limited.33,34 Notwithstanding, new infections continue to occur in vulnerable people, so there is still a need for effective drugs. Therefore, this study aimed to assess the effect of MVC and/or FPV plus systemic steroids (SS) vs. SS only on the viral load and progression to critical disease in COVID-19 patients.
This was a randomized, unicentric, parallel, single-blinded study (only the patient was blinded to the allocation). The participants were severe, non-critical COVID-19 patients admitted between July 2021 and June 2022 who met the following inclusion criteria: Age 18-80 years, within the first 12 days of onset of symptoms, oxygen saturation ≤90%, positive test for SARS-CoV-2, and at least one risk factor for progression to critical: T2DM, Body mass index (BMI) ≥30, HTN, and age ≥65 years. The women agreed to use contraceptives for up to 90 days. Exclusion criteria were breastfeeding or pregnant women, encephalopathy, renal function ≤ 30 ml/min/1.73 m2 estimated by CKD-EPI, acute coronary disease, any state of immunosuppression, and treatment with psychotropics. All the participants signed an informed consent form. We screened 237 patients, of which 19 met the inclusion criteria, 16 completed the assigned treatment, and were analyzed, as shown in Figure 1—CONSORT flow diagram.
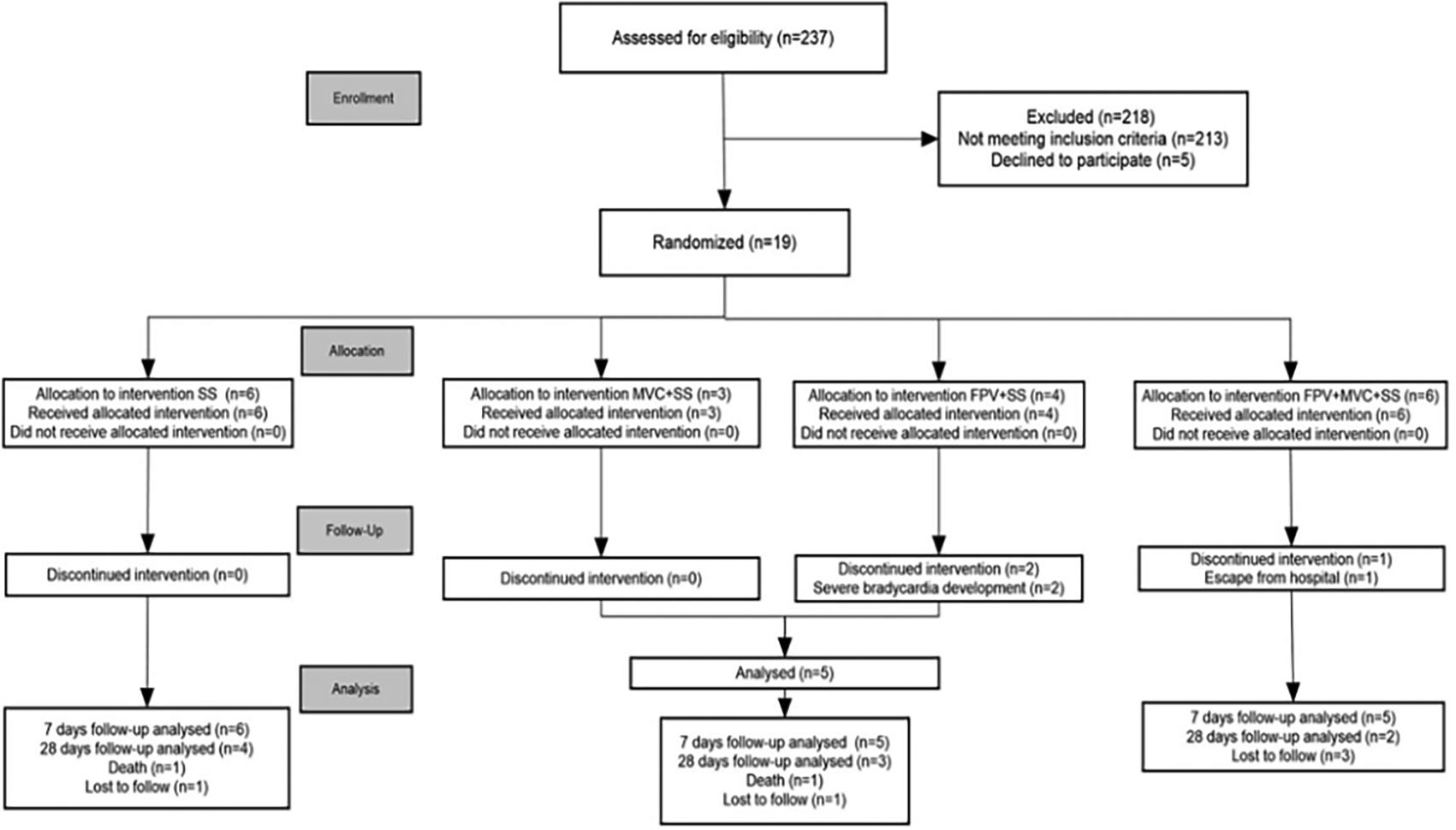
Due to the low recruitment rate, the treatment group with only MVC plus SS or FPV plus SS was grouped into a single treatment group named only one antiviral. Three patient was not included in the analysis because one patient of the FPV group developed severe sinus bradycardic (35 bpm) on the second day of treatment, so it was suspended for the patient's safety; this patient was on concomitant treatment with an antitussive (dropropizine), which has been associated with bradycardia due to its alpha-blocker effect, and therefore was also suspended; three days after suspension, the patient recovered heart rate without additional further treatment or procedure, another patient also development severe sinus bradycardia (<40 bpm) on the third day parallel to the intubation process, demanding the use of vasopressors, and dobutamine, recovered heart rate the same day; the remaining patient escaped from the hospital, then only analyzed 16 patients.
SS, Systemic Steroid only; MVC+SS, Maraviroc, and Systemic Steroid; FPV+SS, Favipiravir, and Systemic Steroid; FPV+MVC+SS; Favipiravir plus Maraviroc and Systemic Steroid; bpm, beats per minute.
Patients were recruited from the emergency room, infectology, or pneumology services. They were randomly assigned to one of four parallel groups in a 1:1:1:1 ratio; patients were allocated according to a chart of aleatory numbers stratified by sex; using your hospital record number, randomization was by a computer-generated random number list prepared by an investigator with no clinical involvement in the trial. After the researcher obtained the patient’s consent, the research nurse telephoned a contact independent of the recruitment process for allocation consignment. The nurses and investigators were trained in good clinical practice. Treatments were defined as follows: only (SS), MVC+SS, FPV+SS, and MVC+FPV+SS. Due to the low recruitment rate and adverse events of FPV, the clinical trial was closed, and the treatment groups with only MVC plus SS or FPV plus SS were gathered as a single group called treatment with only one antiviral for statistical analysis, as shown in Figure 1.
Outcomes
Primary outcomes: Decrease the viral load, and progression to critical disease.
Sample size calculation was performed using the statistical package GPower 3.1.9®, applying an F-family test for between-factors repeated measures ANOVA considering three treatment groups, three viral load measurements, and an effect size of 0.5, (odds ratio: 6) by Magleby et al.,16 and 0.8 power, obtaining a total of 24 patients: six per group, plus an additional 20% for losses, thus summing eight per group.
Dosing: MVC (300 mg Selzentry, GSK) was administered orally at 300 mg twice a day for ten days.35 FPV (200 mg Avigan, Fujifilm Pharma) was administered orally, 1600 mg twice daily on the first day, and 600 mg twice daily on days 2-7.36 SS: All patients received intravenous steroids: dexamethasone (6 mg daily) or its steroid equivalent,17 enoxaparin, or fractioned heparin. Patients who missed two dose administrations for any reason were excluded from the protocol. The patients’ participation could also end before term due to a complication or life-threatening adverse event, and clinical assessment was performed daily for seven days, and on day 28.
RNA was extracted from nasopharyngeal exudates using QIAamp® and the QIAGEN kit. Viral genes E, N, and RdRp were identified by qRT-PCR using the GeneFinder™ COVID-19 Plus RealAmp kit (OSANG Healthcare, South Korea), according to the manufacturer’s protocol. The results were analyzed using QuantStudioTM Design & Analysis version 1.5.1 (Thermo Fisher Scientific, USA). The Ct was determined in duplicate to identify the concordance between measurements, and the reported values are the means of measurements. The viral load was calculated from calibration curves and reported as the number of copies per microliter of RNA extracted per sample. Briefly, primer pairs were designed to amplify each of the three viral genes detected with the kit (E, N, and RdRp) using one of the positive samples with a Ct value of <25 for all three genes. The PCR amplification products were purified and quantified, and 1:10 dilutions with known copy numbers were generated for subsequent analysis by qRT-PCR. The Ct values obtained were plotted against log (number of copies/μl) to calculate a linear fitting equation (R2>0.97) for each viral gene. Finally, the Ct values for each gene in the samples were used to calculate the number of copies/μl of RNA, using the fitting equations of the calibration curve for each gene sequence. Libraries were prepared using the Illumina COVIDSeq test protocol, following the manufacturer’s instructions. First-strand synthesis was performed using the RNA samples. The synthesized cDNA was amplified using ARTIC primers v4 for multiplex PCR, generating 98 amplicons across the SARS-CoV-2 genome. The PCR-amplified product was tagged and adapted using IDT for Illumina Nextera UD Indices Set A, B, C, and D (384 indices) (Illumina, San Diego, CA, USA). Sequencing was performed on the NextSeq 2000 platform (Illumina, San Diego, CA, USA).
All adverse events, related or unrelated to the study drugs, were analyzed by the Pharmacovigilance Service of the Hospital and duly notified to the competing authorities.
Given that the patients were discharged before ten days, we analyzed the data up to day seven; chi-square and Kruskal-Wallis tests were used to compare clinical and laboratory characteristics in the three treatment groups. The effect of the three treatments on viral load was measured using effect size (Cohen’s d). T-tests for related samples were performed to compare the clinical and laboratory characteristics within the groups. SPSS version 26 (IBM, Chicago, IL, USA) was used for all the statistical analyses. The significance level was set at p < 0.05 for all statistical tests.
This clinical trial was approved by the Committee of Bioethics, Research, and Biosafety of Hospital General de Mexico “Dr. Eduardo Liceaga” (registration number DI/20/407/04/38) and by the Federal Commission for Protection Against Sanitary Risk (COFEPRIS). The procedures followed were in accordance with the ethical standards of the responsible institutional committee on human experimentation, World Medical Association, and Helsinki Declaration.
We included 16 patients with severe COVID-19 (9 women, seven men) with a mean age of 53±14 years. The clinical characteristics, viral loads, and adverse events of each treatment group are summarized in Table 1.
| Antiviral combination (MVC + FPV + SS) n=5 | Single antiviral (MVC+SS or FPV+SS) n=5 | Non-Antiviral treatment SS only n=6 | p<0.05* | ||
|---|---|---|---|---|---|
| Age | mean±sd | 58±8 | 52±14 | 48±18 | 0.49a |
| Female | n (%) | 3 (60) | 3 (60) | 3 (50) | 0.93b |
| Days between the start of symptoms and hospital admission | mean±sd | 7±2 | 7±3 | 7±3 | 0.97a |
| Number of risk factors for Critical disease | |||||
| One | n (%) | 0 (0) | 2 (40) | 5 (80) | 0.19 |
| Two | n (%) | 4 (80) | 1 (20) | 0 (0) | |
| Three | n (%) | 1 (20) | 1 (20) | 1 (20) | |
| Four | n (%) | 0 (0) | 1 (20) | 0 (0) | |
| CRP mg/L | mean±sd | 47±51 | 91±40 | 141±101 | 0.23a |
| D Dimer mg/L | mean±sd | 954±486 | 4237±8242 | 1196±944 | 0.28a |
| LDH U/L | mean±sd | 286±48 | 332±106 | 324±113 | 0.72a |
| Creatinine mg/dl | mean±sd | 0.77±0.21 | 0.79±0.32 | 0.79±0.14 | 0.98a |
| Primary Outcome | |||||
| No progress to Critical disease | n (%) | 4 (80) | 4 (80) | 5 (83.4) | 0.22 |
| Secondary Outcomes | |||||
| Decrease ≥ 2 points on the WHO score (Improvement on day 7) | n (%) | 1 (20) | 2 (40) | 1 (16.6) | 0.68b |
| Death (28 days) | n (%) | 0 (0) | 1 (33.3)d | 1 (33.3)e | 0.22b |
| Range of days of hospital stay | Min-max | 7 to 53 | 7 to 11 days | 6 to 54 days | 0.69a |
| Adverse Events | n (%) | 2 (40) | 2 (40) | 2 (40) | 0.22b |
| n (%) per patient | 1(20) Uncontrolled glycemia, atrial fibrillation, deep venous thrombosis SDRA, sacral ulcer 1(20) asymptomatic sinus bradycardia.f | 1(20)d SDRA pulmonary septic shock AKI stage 3 KDIGO upper gastrointestinal bleeding, dead 1(20) symptomatic sinus bradycardia. | 1(16.6)e Urinary sepsis SDRA, dead 1(16.6) Uncontrolled glycemia Empyema, Mild hyponatremia Abdominal pain. | ||
| CRP mg/L (Day 7) | mean±sd | 31±41 | 3.5±1.49c* | 38±43c* | 0.80a |
| D Dimer mg/L (Day 7) | mean±sd | 9136±18749 | 821±415 | 592±338 | 0.31a |
| LDH U/L (Day 7) | mean±sd | 199±6 | 324±113 | 275±178 | 0.83a |
| Creatinine mg/dl (Day 7) | mean±sd | 1.17±0.61 | 1.4±1.7 | 0.67±0.14 | 0.52a |
| Follow-up (28 days) | n (%) | 2 (40) | 3 (60) | 4 (83.3) | 0.33b |
| n (%) Per patient | 1 (20) Hospitalized and extubation. oxygen requirement, weakness, neuropathy, recovery from AKI Poor blood glucose control 1 (20) oxygen requirement wet cough, hyporexia, fatigue, depression, uncontrolled HTN. | 1 (20) Dyspnea, chest pain myalgia 1 (20) Hair loss, Blurred vision resumed his normal activities. 1 (20) Oxygen requirement hoarseness. | 1 (16.6) Dyspnea, headache, dysgeusia, anxiety, hair loss, insomnia 1 (16.6) Tachycardia, headache, dizziness, dysphagia 1 (16.6) Back pain, headache, oxygen requirement 1 (16.6) Hospitalized, poor blood glucose control, empyema, oxygen requirement. | ||
Four of 16 patients reached a decrease of ≥ 2 points in the WHO score on day seven; 50 percent of which were in the single antiviral group; however, no statistically significant difference was found.
The largest viral load reduction was observed in the MVC and FPV+MVC groups (days three, and seven compared with day 0) (Figure 2). The delta variant was identified in 10 of 16 patients, and the remaining six patients were infected with the Omicron variant.
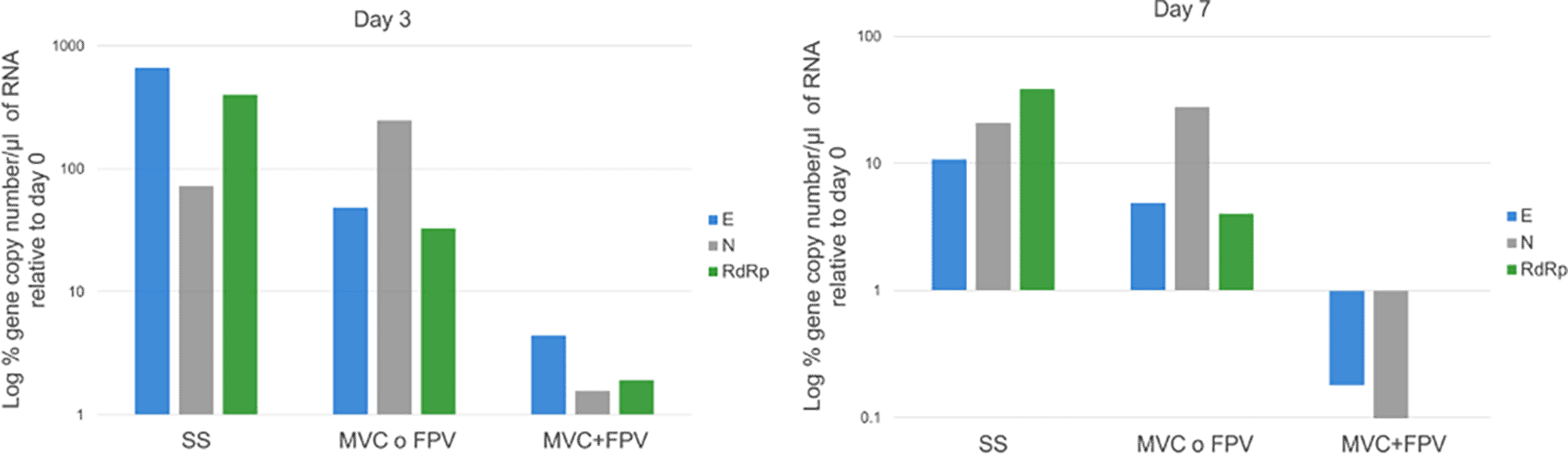
Percentage of change on day 3 vs day 0 in nasopharyngeal exudate in the three treatment groups. The most considerable percentage of reduction in the number of copies per microliter of RNA was observed in the MVC and FPV+MVC groups. Percentage of change on day 7 vs day 0 in nasopharyngeal exudate in the three treatment groups. The decrease in viral load measured in the percentage of the gene copies per microliter of RNA of nasopharyngeal exudate on day seven was larger in the MVC and FPV+MVC groups.
Three patients had bradycardia, two had FPV, and one had FPV+MVC. According to the Pharmacovigilance Service, these are related to FPV. All patients recovered; however, two patients were excluded from the study because of severe bradycardia and were not included in the analysis. Figure 1—CONSORT flow diagram.
This group included five patients (three women and two men). Of the five patients, one received two doses of vaccination, and in the remaining four patients, none received at least one dose. The mean patient age was 58±8 years.
The viral load was analyzed in five patients. This group had a larger effect size in reducing the viral load at day seven compared with the other treatment groups; genes E, N, and RdRp with Cohen’s d of 120%, 123%, and 50%, respectively (Table 2). One of the five patients maintained a persistently high viral load, and this patient survived. The Omicron variant was found in four out of five patients; the remaining patient had the delta variant.
| Primary outcome | Antiviral combination MVC + FPV + SS n=5 | Single antiviral (MVC+SS or FPV+SS) n=5 | Non-Antiviral treatment SS only n=6 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 (mean±ds) | Day 7 (mean±ds) | Cohen’s d (%) | 95% CI | Day 0 (mean±ds) | Day 7 (mean±ds) | Cohen’s d (%) | 95%CI | Day 0 (mean±ds) | Day 7 (mean±ds) | Cohen’s d (%) | 95%CI | |
| Gene E copies/μl of RNAa | 1738.6±1625 | 353.1±468 | 120 | -0.18 to -2.49 | 158107.9±298903 | 6781.8±13456 | 72 | -0.56 to -1.99 | 123811.8± 269967 | 1589.7±3143 | 64 | -0.52 to 1.8 |
| Gene N copies/μl of RNAa | 174.2±173 | 20.2±35.7 | 123 | -0.12 to -2.58 | 89877.2±143987 | 486.5±962 | 87 | -0.42 to -2.18 | 61942.2±137659 | 227.5±542 | 63 | -0.53 to -1.79 |
| Gene RdRp copies/μl of RNAa | 3882.4±3702 | 1974.4±3867 | 50 | -0.75 to -1.76 | 112028.8±175143 | 5806.9±11341 | 86 | -0.44 to-2.15 | 3882.4±3702 | 197.4±3867 | 97 | -0.22 to -2.17 |
In this study respect to the effect of three treatments on the viral load, and to avoid progress to critical disease in patients with severe COVID-19 found a reduction, and a quicker decrease in viral load in the combined treatment group (FPV+MVC) notwithstanding this did not prevent the progression to critical disease which could be attributed to the small size of the sample, notwithstanding several factors may affect the results; regarding Favipiravir, three significant factors that may affect the results: heterogeneity in doses, and duration of treatment (loading dose from 1600 to 4800 mg, maintenance dose of 1200 to 2000 mg, 2, 3, 4 doses for the next 4-13 days), older age, baseline disease severity.21,22,37–39 In one study, FPV slightly improved clinical and radiological manifestations or decreased viral load22 but in another study, it failed to improve viral elimination.38 Siripongboonsitti et al 2023 found in patients with mild to moderate COVID-19, the use of favipiravir prevented severe COVID-19 when personalized, early treatment was performed, and dose adjustment was made based on ethnicity and body weight.39
Currently, some antiviral drugs are approved by the FDA for emergency use,33,34 whose main objective is to avoid hospital admission and death in unvaccinated, with mild to moderate disease, and without a requirement of oxygen, within the first seven days of the onset of symptoms. Recent studies evaluating Molnupiravir, Nirmatrelvir-Ritonavir, and Sotrovimab have shown positive results in preventing hospitalization when administered within the first 5-7 days of the onset of symptoms.40,41 In contrast, our study focused primarily on patients with severe clinical symptoms, in which the objective was to prevent their evolution to be critical.
Analysis of the epidemic waves of COVID-19 suggests that the SARS-CoV-2 virus evolved into less lethal variants, and the start of vaccination modified the severity of the infection; the oxygen requirement decreased from 80% to 17%, as well as a decrease in admission to the ICU, average days of hospital stay, and mortality.42 Therefore, conditions changed dramatically for the benefit of our population since 42% of the subjects in this study were recruited between the third and fourth COVID-19 waves when Delta and Omicron were the predominant variants. The Omicron variant has been described to have a 44% reduction in hospital admissions and a 69% lower risk of death than confirmed cases of the delta variant.43 Delta and Omicron variants have shorter incubation periods, whereas higher infectious viral loads were detected in patients infected with Delta than in patients infected with Omicron.44,45
The clearance time of viral load is slower in non-vaccinated individuals.46 After one dose of vaccine, the number of Ct increased by 4.5 Ct, and with a booster, it increased by 2.4 Ct47; therefore, the start of vaccination could be a factor that influenced our results; nevertheless, the combined treatment reached the effect faster in Ct, and a larger effect size in reducing viral load at seven days; some antivirals found a significant reduction in viral load,33,34 remdesivir no provoke reduction, only positive clinical outcome.48
The most prevalent risk factors in the population were T2DM or HTN (63%), followed by obesity and age >65 years, which differs from other studies in which 79% had a BMI ≥ 30, followed by age >60 years, and T2DM.34
Regarding adverse events, we did not find hyperuricemia frequently reported in treatment with FPV;49 three of our patients developed bradycardia; and there are two case reports that associate the development of bradycardia with FPV as a possible rare adverse event.50,51
The limitations were that pharmacokinetics could not be performed, and the study could not be double-blinded. Finally, the sample size was small, which limited statistical analysis. The public health emergency mainly generated this, adding to the lack of finances, shortage of human resources, and obstacles of the regulatory system.
Regarding MVC in COVID-19, there are few registered clinical trials on ClinicalTrials.gov, including this, and none have evaluated its combination with favipiravir.
The development of drugs, whether approved or not, requires time and funding to determine the therapeutic target, the drug’s toxicity, and how to use it properly.52,53 It has been challenging to draw any consistently valid conclusions about the efficacy of drugs in the treatment of COVID-19 disease54,55; so far the cure has not been achieved with the use of a single particular drug.52,56 Therefore, it is very important to continue studying potential new therapeutic targets such as: drug repurposing strategies, pan-coronavirus drug targets, in vitro assays, mouse models against SARS-CoV-2 and next Strains.57,58
Register in Clinical Trials (NCT04475991). https://clinicaltrials.gov/study/NCT04475991?cond=NCT04475991&rank=1
For pre-registered analysis in clinical trials, we planned the analysis of data to be between the four treatment groups. However, because of the low recruitment rate and small sample size, it was necessary for statistical analysis to join the group of favipiravir plus systemic steroids with the group of Maraviroc plus systemic steroids in one antiviral treatment group.
Figshare: Medina, Elba; Sanchez, Laura; Barron, Valeria; Espinosa, Ana Maria; Villalobos, Alma; Leon, Mireya; et al. (2024). Repository COMVIVIR.xlsx. figshare. Dataset. https://doi.org/10.6084/m9.figshare.25017992.v3.59
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
CONAHCYT, CVU 785859. GlaxoSmithKline donated Maraviroc, and CCINSHAE (Comisión Coordinadora de Institutos Nacionales de Salud y Hospitales de Alta especialidad) donated Favipiravir. Hospital General de México “Dr. Eduardo Liceaga”.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Medicinal Chemistry & Drug Discovery
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
References
1. RABIE A: Future of the current anticoronaviral agents: A viewpoint on the validation for the next COVIDs and pandemics. BIOCELL. 2023; 47 (10): 2133-2139 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Medicinal Chemistry & Drug Discovery
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 2 (revision) 11 Jun 24 |
read |
|
Version 1 11 Mar 24 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (1)