Keywords
Colonoscopy, remimazolam, midazolam, sedation, anesthesia.
Remimazolam is an ester-based ultra-short-acting benzodiazepine that efficiently achieves sedation within a short period and is now being assessed as a suitable alternative to midazolam. This meta-analysis aims to pool the available data assessing and focusing on the safety aspect of remimazolam compared with midazolam.
A multi-center randomized control trial for patients undergoing endoscopic procedures like colonoscopy was conducted, comparing remimazolam to placebo for the midazolam group as the intervention group. The safety of remimazolam was the primary endpoint of this meta-analysis.
A total of 3 studies were included. The total study population was 697, including the placebo, remimazolam, and midazolam groups. The types of studies included are i. randomized, double-blind, parallel-group, active-controlled clinical trial ii. prospective, randomized, parallel-group study comparing remimazolam to placebo (blindly), RCT, and iii. prospective, double-blind, randomized, parallel-group study RCT.; Treatment-emergent adverse effects included vascular disorders (P=0.42), cardiac disorders (p=0.06), respiratory, thoracic, and mediastinal disorders (p=0.26), infections and infestations (0.88), hematologic abnormalities such as anemia (p=0.63), and derangements in Blood pressure (systolic p=0.47 and diastolic p=0.68 and respiratory parameters (p=0.34). Analysis of the reported data suggests that the remimazolam group had a significantly higher incidence of treatment-emergent adverse effects compared to the midazolam group (RR: 0.84; 95% CI [0.78, 0.91]; P <0.00001; I2 = 5%).
In conclusion, this meta-analysis of three randomized controlled trials showed outcomes favoring both remimazolam and midazolam as successful sedatives, yet the higher requirement of top-up dosage and rescue sedatives in the midazolam group indicates that remimazolam can be used as its replacement, especially in colonoscopy procedures.
Colonoscopy, remimazolam, midazolam, sedation, anesthesia.
Colonoscopy is one of the most widely performed diagnostic procedures. It has advanced surgical oncology due to its role in earlier cancer detection. Although relatively less time-intensive and invasive, patients often require the administration of opiates, sedatives, and hypnotics to help ameliorate post-procedural pain.1 Benzodiazepines (a class of sedatives approved by the FDA) have resulted in their ubiquitous use due to their multiple therapeutic benefits as anti-anxiolytic and antiseizure and their established sedating and analgesic properties.2 Moreover, various drugs can be employed for different populations and procedures due to their diverse potency and efficacy. Midazolam is currently employed for colonoscopy due to its high safety profile and ultra-short-acting properties. It is classically preferred over agents with a narrow therapeutic index, such as diazepam.3,4 However, midazolam, with a relatively longer mean half-life (three hrs; 1.8–6.4 hr), is associated with a slow return of cognition and carries a risk of systemic accumulation; therefore, limiting its use in patients with chronic kidney disease.2,5 Hence, alternative agents such as remimazolam with a similar biochemical structure and efficacy have been recently explored for their safety profile owing to their shorter recovery time.6,7 Unlike midazolam, this has been attributed to its earlier inactivation, hence decreasing the systemic effects.5 As an ultra-short-acting, ester-based drug, remimazolam can be administered intravenously to achieve sedation within 3–3.5 minutes.8 Rendering its quicker induction and recovery, as seen across trials, including bronchoscopy and colonoscopy, remimazolam was approved for use in the US as an anesthetic.7,9
This systematic review aims to evaluate the adequacy of remimazolam in replacing midazolam in colonoscopic procedures. Our review specifically looks at the randomized control trials comparing the safety of remimazolam and midazolam in patients undergoing colonoscopies to determine if the clinical effectiveness of remimazolam can be safely determined and whether this emerging drug could successfully replace midazolam in the future or not.
The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA 2020) standards and PRISMA checklist were used to conduct this meta-analysis.10,11 The literature search, quality assessment, data extraction, and statistical analysis were all accomplished by two independent reviewers (MA Khalid, M Sadiq). A third reviewer (MJ Siddiqui) was consulted in case of conflict for screening procedures. The report did not need ethics committee approval because raw data of human beings were not involved. The ultimate selection of studies for inclusion was determined through unanimous agreement among all authors. Additionally, we examined the reference lists of original papers, published reviews, systematic reviews, and meta-analyses to identify any trials that were not initially part of the database. Our search strategy was designed to encompass every randomized controlled trial (RCT) examining the efficacy of remimazolam in procedural sedation during colonoscopy.
Studies that fulfilled our PICO criteria were included. Studies that were meta-analyses or not released as published reports or case reports and studies in which the PICO criteria were not matched were all excluded.12,13 We also excluded clinical trials in which remimazolam was compared to any drugs other than midazolam. Non-human studies and publications in languages other than English were also excluded. We lastly excluded the latest meta-analysis, which focused on efficacy as it was outside the scope of our topic.12
PubMed/MEDLINE and the Cochrane Library databases were searched for this meta-analysis up to July 17th, 2023. MedRxiv and BioRxiv were also searched for preprints and other related articles. All significant studies were included using the relevant medical subject headings (Mesh terms). The used search strategy was: ((Remimazolam) OR (ONO 2745)) OR (ONO-2745)) OR ((CNS7056)) OR ((methyl 3-(8-bromo-1-methyl-6-(2-pyridinyl)-4H-imidazo(1,2-a)(1,4)benzodiazepin-4-yl)propanoate)) AND ((Midazolam) OR (Midazolam Maleate)) OR ((Maleate, Midazolam)) OR (Dormicum)) OR (Versed)) OR (Midazolam Hydrochloride)) OR (Hydrochloride, Midazolam)) OR (Ro 21-3981)) AND ((Colonoscopy) OR (Colonoscopic Surgical Procedures)) (Extended data: File 112).
Studies were included based on the following PICO selection criteria: (a) the population of interest was colonoscopy patients; (b) the outcome was safety/side effects with an intervention group of remimazolam with midazolam being the control group.
To enhance our search, we investigated the titles and abstracts for all the articles. Subsequently, all the selected articles were imported into EndNote Reference Library version X4 (Clarivate Analytics, Thomson Reuters Corporation, Philadelphia, Pennsylvania). One duplicate study was identified, which was eliminated. The articles were then screened on three levels: title, abstract, and full text. A quality assessment test was performed using Cochrane Collaboration’s tool for assessing the risk of bias; for reference, see the Extended data: File 2.12,13
The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) standards were used to conduct this meta-analysis.10,11 The literature search, quality assessment, data extraction, and statistical analysis were all accomplished by two independent reviewers. A third reviewer was consulted in case of conflict for screening procedures.
All statistical analyses were performed using Review Manager (version 5.4; Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014).14 The first two outcomes, completion of colonoscopy and need for rescue sedation, were subjected to sensitivity analysis to assess the specific impact of each study. However, the rest of the outcomes were reported only in two of the included studies, so sensitivity analysis could not be applied. To visually check the pooled data, forest plots were established. The outcomes of the reports were merged using a random-effects model and calculated as risk ratios (RR) with 95 % confidence intervals (CIs). In all cases, a p-value of less than 0.05 was considered significant. Higgins’s I2 statistic was used to quantify heterogeneity across studies, with I2 values between 25 and 50% representing mild heterogeneity, 50 to 75% representing moderate heterogeneity, and greater than 75% signifying severe heterogeneity.15
A preliminary search of the three electronic databases yielded thirteen potential studies. Four articles were selected based on the comparison of remimazolam and midazolam. After exclusions were done based on the similarity of the intervention and control group with no combination agents, three studies were left for analysis. The PRISMA flowchart (Figure 1, Extended data: File 2) summarizes the results of our literature search.12
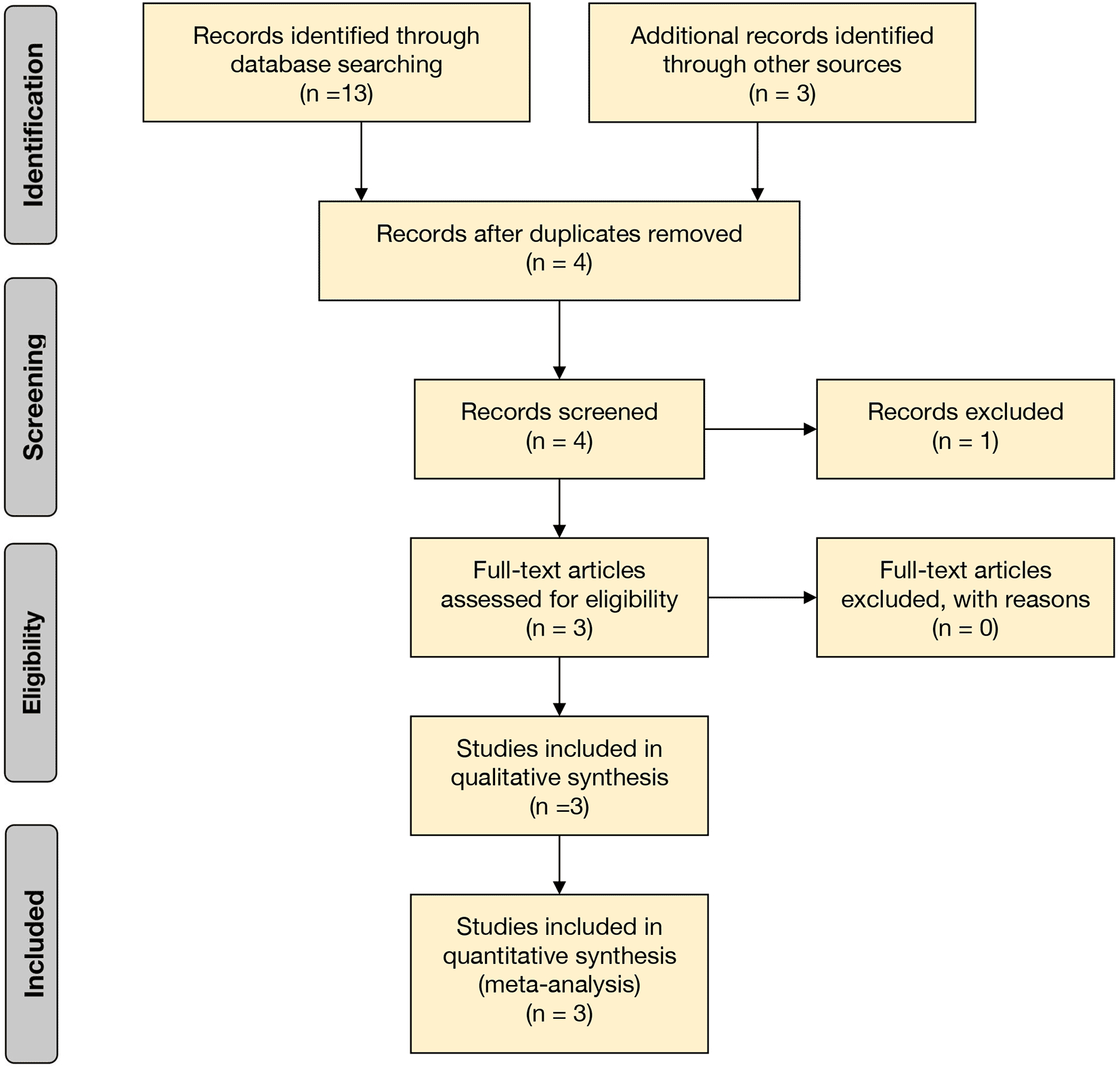
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi: 10.1371/journal.pmed1000097.
The PRISMA flowchart was used in our systematic review and meta-analysis to shortlist articles. The articles were included based on similar PICO and underwent specific exclusion and inclusion criteria to form the final list.
Three randomized controlled trials (RCTs) were used to compare remimazolam and midazolam.16–18 The Pambianco et al. study was a randomized, double-blind clinical trial. Patients received either remimazolam or midazolam for sedation during colonoscopy, with supplemental oxygen and fentanyl. Sedation adequacy was assessed by the Modified Observer’s Assessment of Alertness/Sedation score (≤3), and additional doses were given as needed. Success meant achieving sufficient sedation without rescue medication or ventilation.16
The Rex et al. phase III study was a randomized, double-blind study comparing remimazolam to placebo for outpatient colonoscopy, with an additional group receiving open-label midazolam. Study medications were administered by the endoscopist, and fentanyl was given before the drugs. The primary endpoint required successful colonoscopy completion, no rescue medication, and limited medication doses within specific time intervals.17
Another Rex et al. study was a randomized, double-blind trial comparing remimazolam to placebo, with an open-label arm for midazolam, in sedating 79 ASA III/IV patients during colonoscopy. It was the third Phase III trial for remimazolam in procedural sedation, focusing on evaluating remimazolam’s safety as the primary endpoint.18
All three studies included patients aged 18 to 70, scheduled to undergo a routine diagnostic or therapeutic colonoscopy. The patients had an ASA Score of I, II, or III, were within a weight range of 55–130 kg, and their BMI was 18 to 33 kg/m2 or less.12
According to the quality assessment table, all studies carried a rigorous methodological approach and were linked with a low risk of bias; Table 1.12
Results of the meta-analysis
Of the three included studies, the total study population was found to be 697, including the placebo, remimazolam, and midazolam groups.16–18 The analyzed outcome was the safety of the two drugs as assessed by the incidence of treatment-emergent adverse effects (TEAEs) such as vascular disorders, cardiac disorders, respiratory, thoracic, and mediastinal disorders, infections and infestations, hematologic abnormalities such as anemia, and derangement in hemodynamic and respiratory parameters. The characteristics of the three studies are presented in the Extended data: File 3 and Extended data: File 4. The results are represented as forest plots in the Extended data: File 5.12
Treatment-emergent adverse effects
A multitude of possible adverse effects were observed and reported for the placebo, remimazolam, and midazolam groups, including vascular disorders, cardiac disorders, respiratory, thoracic, and mediastinal disorders, decreased respiratory rate, infections, and infestations, increased diastolic blood pressure, increased systolic blood pressure, and anemia. Analysis of the reported data suggests that the TEAEs have a significantly higher incidence in remimazolam (RR: 0.84; 95%CI [0.78, 0.91]; P <0.00001; I2 = 5%) compared to the midazolam group.
Our meta-analysis comprising three RCTs with a total study population of 697 aims to evaluate the safety profile of remimazolam as an alternative sedative in patients undergoing colonoscopy. In accordance with the results, it is indicated that remimazolam is an overall safe sedative that can be considered for use as a replacement for midazolam in patients undergoing colonoscopy. Unlike the previous meta-analysis “Efficacy of Remimazolam for Procedural Sedation in American Society of Anesthesiologists (ASA) I to IV Patients Undergoing Colonoscopy”, this systematic review and meta-analysis maintain a strict focus on the safety aspect of remimazolam. The primary outcome highlights all the treatment-emergent adverse effects, not the procedural success predictors such as completion of endoscopy, need for rescue sedative, and additional need for top-up dosages.19
In the three RCTs to draw a comparison between remimazolam and midazolam, remimazolam was found to have a similar safety profile when compared to midazolam. However, pooled effects revealed remimazolam to be associated with increased treatment-emergent adverse effects. The mean terminal elimination half-life of remimazolam was estimated at 0.75 hours, and that of midazolam was 4.3 hours. Remimazolam was found to have a shorter half-life, early bioavailability, and early return of the patient to conscious levels.18 The early recovery is also explained by the ultra-short-acting nature of the drug, as discussed earlier.20 Another possible explanation for this early recovery with remimazolam compared with midazolam is the difference in nature of the metabolites of the two drugs. Midazolam has an active metabolite, a-hydroxy-midazolam, which has the potential risk of repeat sedation when the active metabolite becomes bioavailable, whereas no metabolites of remimazolam have been reported to cause repeat sedation.21 Additionally, remimazolam has a lesser degree of drug interaction in comparison to midazolam and uneventful use in a patient with myotonic dystrophy endorses its safety profile.22,23
These findings are in contrast to a previous meta-analysis evaluating the safety and efficacy of remimazolam in various endoscopic procedures.24 Remimazolam was linked with fewer cases of hypotension in comparison to midazolam. Additionally, both groups carried an equal risk of undergoing postoperative hemodynamic and respiratory derangements. The discrepancy could be attributed to the absence of subgroup analysis on patients undergoing colonoscopy, a larger sample size (1996 vs. 697), and a more diverse patient population than ours. Moreover, in order to achieve a similar anesthesia effect, often larger doses of remimazolam need to be administered, which could explain the increased incidence of TEAEs.25 Isolated incidences of anaphylaxis and failure of flumazenil to fully reverse the sedative effects of the drug have raised suspicions regarding the reported exceedingly safe nature of the drug.26–28 Meanwhile, bradycardia, hypotension, and injection site pain occurred less often in the remimazolam group.29,30 Furthermore, the drug may become less popular with patients due to its inability to decrease postoperative nausea and vomiting.31
Although many important characteristics highlighting the effectiveness and potential of remimazolam as an alternative sedative are available, the high failure rate of the drug in inducing sedation in 11/44 patients in one clinical trial cannot be ignored. This is coupled with the fact that one patient was seen to have an adverse event (hypotension and oxygen desaturation).32 Thus, it is evident that the use of remimazolam is subject to a higher risk of TEAEs.
The review had some limitations, including statistical heterogeneity in some meta-analyses, reliance on observational data, and potential bias in included studies. Fentanyl dose may have contributed to inconsistent results in the Pambianco and Rex Phase III study. Future reviews may consider pooling results from studies with the same dose for the same procedure. This review focused on randomized controlled trials. This meta-analysis incorporated only three trials, which implies that the conclusion may lack decisiveness. However, the results indicated that several outcomes had a sufficient sample size and statistical significance.
The studies included in the meta-analysis showed that both the drugs under observation had multiple TEAEs, with remimazolam having a higher frequency of adverse effects compared to midazolam. Nonetheless, patients administered with midazolam were at a higher risk of repeat sedation.
In conclusion, despite a significant difference in the safety profile, the data indicates that more research and, specifically, more RCTs must be performed to find the most effective sedative agent with the least possible treatment-emergent side effects. Furthermore, other aspects of safety, such as depth of sedation and further intra-procedural and post-procedural outcomes, need to be assessed in future studies.
All data underlying the results are available as part of the article and no additional source data are required.
Figshare: Extended data for ‘Safety of remimazolam in comparison with midazolam for colonoscopy: A systematic review and meta-analysis’, https://doi.org/10.6084/m9.figshare.24105291.v2. 12
This project contains the following extended data:
• Supplementary file 1: This includes the relevant search string(s) and the databases used.
• Supplementary file 2: This includes the PRISMA flowchart.
• Supplementary file 3: This includes the quality assessment tool and articles included.
• Supplementary file 4: This includes the 3 Randomized Control Trials and a table showing their characteristics and findings.
• Supplementary file 5: This includes the relevant forest plot comparing Remimazolam and Midazolam with specific p-values. Treatment emergent adverse effects are focused in this study, with further subcategories of adverse effects being discussed.
Figshare: PRISMA checklist for ‘Safety of remimazolam in comparison with midazolam for colonoscopy: A systematic review and meta-analysis’, https://doi.org/10.6084/m9.figshare.24105321.v2. 11
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: my expertice is endoscopy including sedation for endoscopy
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Gastroenterology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 22 Mar 24 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)