Keywords
Squamous cell carcinoma, head and neck squamous cell carcinoma, OSCC, cancer stem cells, CD24, Oct4, immunohistochemistry
This article is included in the Oncology gateway.
Head and neck squamous cell carcinoma (HNSCC) encompasses a diverse range of tumors with varying degrees of differentiation. Within tumors, cancer stem cells (CSCs) are believed to play a crucial role in tumor initiation, progression, and resistance to treatment. This study aimed to explore the expression of two CSC markers i.e. CD24 and Oct4, in patients with HNSCC.
The study included 85 patients diagnosed with HNSCC. Immunohistochemistry was used to evaluate the expression of CD24 and Oct4 in tumor tissues obtained from patients with HNSCC. Staining intensity and the proportion of positive tumor cells were analyzed and compared across the various HNSCC grades and histological subtypes.
The findings revealed that the mean immunoreactivity score (IRS) for CD24 was significantly higher in non-conventional HNSCC subtypes compared to conventional types (p = 0.019). Additionally, a significant statistical association was observed between the IRS status of CD24 and histological subtypes (p = 0.043). Other demographic, clinical, and histological parameters showed non-significant associations with staining intensity, proportion score (PC), IRS status, and mean IRS (all p values > 0.05).
These findings indicate that CD24 could potentially serve as a CSC marker for distinguishing between conventional and non-conventional histological types of HNSCC. This suggests that CD24 holds promise as a valuable tool in identifying specific HNSCC subtypes characterized by CSCs.
Squamous cell carcinoma, head and neck squamous cell carcinoma, OSCC, cancer stem cells, CD24, Oct4, immunohistochemistry
In the past few years, extensive studies into the molecular processes that control HNSCC have significantly transformed how cancer patients, especially those with metastatic conditions, are treated. New approaches like targeted therapy and immune checkpoint inhibition have emerged and been used for HNSCC patients. Despite these breakthroughs, completely getting rid of or effectively managing HNSCC has remained elusive, as most patients eventually become resistant to treatment. The primary reason behind this resistance and the resulting treatment ineffectiveness is the presence of intratumoral heterogeneity, a crucial aspect of HNSCC. This refers to a mix of cells within the tumor that respond differently to cancer treatment due to varying levels of sensitivity.1
Two models have been proposed that may explain heterogeneity; the clonal evolution model, and the CSCs model. The term “CSC” refers to a malignant cancer cell having a stem cell characteristic. The CSCs theory claims that merely a small percentage of a tumor’s cells may renew the heterogeneity form, encourage spread, and cause recurrence, is increasing in popularity.2 CSCs typically compensate less than 1% of the total cells in many of these neoplasms, but they can compensate from 27% to 100% of the entire cancer cell population in extremely malignant tumors.3 In the context of HNSCC, it is suggested that CSCs play a role in initiating tumors, advancing their growth, causing metastasis, resisting drugs, and causing reoccurrence.1
A systematic review had reported 13 types of stem cell markers. The most commonly expressed CSC markers are CD44, aldehyde dehydrogenase, and CD133, which are responsible for tumorigenesis, self-renewal, and therapy resistance, whereas NANOG, SOX-2, and OCT-4 are involved in metastasis and invasion. Identification of an accurate panel of CSC markers is the need of the hour as non-specificity of the current markers poses a problem.4
In a number of cancer cells, the cytoplasmic protein CD24 is linked to the cellular membrane by glycosylphosphatidylinositol. Gynecologic, breast, prostate, bladder, renal, non-small cell carcinomas, and other human malignancies have high levels of CD24 expression.5 This suggests CD24 as an important marker in the diagnosis and prognosis of tumors. Further, it acts as a substitute ligand for platelet and vascular endothelial contact, that makes it easier for malignant cells to spread via circulation. It makes tumor cells prone to proliferating and sticking to fibronectin, collagen, and laminin. The correlations of CD24 with metastatic disease strengthen its significance as a predictive biomarker and a novel CSC marker.6
Okumura et al. (2021) stated that Octamer 3/4 (Oct 3/4) is an essential transcription factor and stem cell marker during human development, and it is also known as the Oct4 transcription factor gene or POU5F1. Studies have suggested presence of Oct 3/4 in both benign and malignant cells that have differentiated from human cells. Investigations have revealed that tumors with intensive Oct4 stem cell marker expression are associated with rapid disease progression, metastasis, and shorter cancer-related survival, as opposed to tumors with moderate to minimal Oct4 expression. Oct4 is confirmed as a target for CSCs and is critical in regulating stem cell self-renewal and maturation, as per Mohiuddin et al. (2020).7,8
Currently, there is no single biomarker to define the CSC population accurately for HNSCC. Therefore, assessing the expression of CSC markers in HNSCC appears to be the most rational approach for distinguishing high-risk cases of HNSCC from indolent. The aim of this study was to investigate the expression of two CSC markers (CD24 and Oct4), in tissue samples of HNSCC patients.
Each study participant gave their written informed consent. For this cross sectional study, formalin-fixed paraffin-embedded tissue samples from 85 patients diagnosed with HNSCC were collected. The samples were obtained from the Bakhtawar Amin Trust Teaching Hospital Multan, Pakistan. Included patients had different tumor grades: 67 cases classified as well to moderately differentiated SCC and 18 cases as poorly differentiated. Surgical resection was performed on all patients. Further details regarding the demographic, clinical, and histopathological characteristics of the HNSCC patients can be found in Table 1. In order to be eligible for inclusion criteria included confirmed diagnosis of HNSCC and available tissue samples for immunohistochemical analysis. Patients who had received neoadjuvant chemotherapy or radiotherapy were excluded.
| Baseline characteristics of HNSCC patients | Oct-4 SI | p-value | CD24 SI | p-value | ||
|---|---|---|---|---|---|---|
| N/WP (n = 30) | P/SP (n = 55) | N/LE (n = 13) | HE (n = 72) | |||
| Age | ||||||
| ≤40 years n (%) | 07 (43.8%) | 09 (54.2%) | 0.432 | 02 (12.5%) | 14 (87.5%) | 1.000 |
| >40 years n (%) | 23 (33.3%) | 46 (66.7%) | 11 (16%) | 58 (84%) | ||
| Gender | ||||||
| Females n (%) | 13 (42%) | 18 (58%) | 0.332 | 06 (19.4%) | 25 (79.6%) | 0.534 |
| Gender n (%) | 17 (31.5%) | 37 (68.5%) | 07 (13%) | 47 (87%) | ||
| HNSCC variant | ||||||
| OSCC n (%) | 15 (40.5%) | 22 (59.5%) | 0.374 | 07 (19%) | 30 (81%) | 0.415 |
| Others n (%) | 15 (32%) | 33 (68%) | 06 (12.5%) | 42 (87.5%) | ||
| HNSCC tumor size | ||||||
| T1+T2 n (%) | 28 (34.6%) | 53 (65.4%) | 0.611 | 13 (16%) | 68 (84%) | 1.000 |
| T3+T4 n (%) | 02 (50%) | 02 (50%) | 00 (00%) | 04 (100%) | ||
| HNSCC histological grade | ||||||
| Well/moderately differentiated n (%) | 25 (37%) | 42 (63%) | 0.452 | 08 (12%) | 59 (88%) | 0.137 |
| Poorly differentiated n (%) | 05 (28%) | 13 (72%) | 05 (28%) | 13 (72%) | ||
| HNSCC histological type | ||||||
| Conventional n (%) | 28 (35.4%) | 51 (64.6%) | 1.000 | 10 (45.6%) | 69 (54.4%) | 0.043* |
| Non-conventional n (%) | 02 (34%) | 04 (66%) | 03 (50%) | 03 (50%) | ||
Immunohistochemical analysis was conducted on 4 μm-thick sections of the formalin-fixed paraffin-embedded tissue samples. The avidin-biotin-peroxidase complex method was employed for this purpose. Initially, the tissue sections were deparaffinized and rehydrated. Antigen retrieval was performed by boiling the sections in citrate buffer (pH 6.0) for 15 minutes. To minimize nonspecific binding, the sections were then blocked with 10% normal goat serum for 30 minutes at room temperature.
Next, the sections were incubated overnight at 4°C with primary antibodies targeting CD24 (1:100, Abcam) and Oct4 (1:100, Abcam). Following this, the sections were washed with phosphate-buffered saline (PBS) and incubated with a biotinylated secondary antibody (1:200, Vector Laboratories) for 1 hour at room temperature. After another round of PBS washing, the sections were exposed to streptavidin-peroxidase complex (Vector Laboratories) for 30 minutes at room temperature. Subsequently, the sections were washed with PBS and developed using 3,3’-diaminobenzidine (DAB) (Vector Laboratories). Finally, the sections were counterstained with hematoxylin, dehydrated, and mounted using mounting medium.
The immunohistochemical staining of CD24 and Oct4 was independently assessed by two pathologists who were unaware of the clinical data. The staining intensity and the percentage of positively stained cells were evaluated. To measure the immunoreactivity score (IRS) for HNSCC tissue sections stained with the aforementioned antibodies, a semi-quantitative scale was utilized. The IRS was calculated by multiplying the staining intensity (SI) of tumor cells (0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining) by a proportion score (PC) based on the percentage of positive tumor cells (0 = negative, 1 < 25%, 2 = 26%-50%, 3 = 51%-80%, 4 = 81%-100%). The resulting PC and SI scores were multiplied, yielding an immunoreactivity score (IRS) ranging from 0 to 12. An IRS score of 9-12 was considered high, while a score of 0-8 was categorized as low.9
Statistical analysis was carried out utilizing SPSS 25.0 (SPPS Inc., Chicago, USA). Mean with standard deviation (SD) or median within the interquartile range was used to express continuous variables, whereas frequency and percentage were employed for categorical variables. To assess the disparities in means among groups, the One-way ANOVA test was employed. Results were regarded as statistically significant if the p-value was equal to or less than 0.05.
The baseline characteristics of patients with HNSCC were assessed, and their comparison was made with the SI, IRS positive or negative status and mean IRS of CD24 and Oct-4 antibodies (Table 1). The study included 85 patients, with 30 patients in the negative/weakly positive (N/WP) SI group and 55 patients in the positive/strongly positive (P/SP) SI group. The analysis yielded that in patients with conventional HNSCC, 35.4% in the N/WP group and 64.6% in the P/SP group had Oct-4 SI (p-value = 1.000). For CD24 SI, 45.6% of patients with conventional HNSCC in the N/WP group and 54.4% in the P/SP group showed staining intensity, yielding a significant p-value of 0.043. Among patients with non-conventional HNSCC, 34% in the N/WP group and 66% in the P/SP group had Oct-4 SI, while for CD24 SI, the percentages were 50% and 50%, respectively. It may be inferred from this finding that SI of CD24 is significantly associated with histological subtyping of HNSCC (Figure 1). While there existed no statistical association of CD24 and Oct-4 staining intensity with that of any other clinical or histological parameters (all p values >0.05).
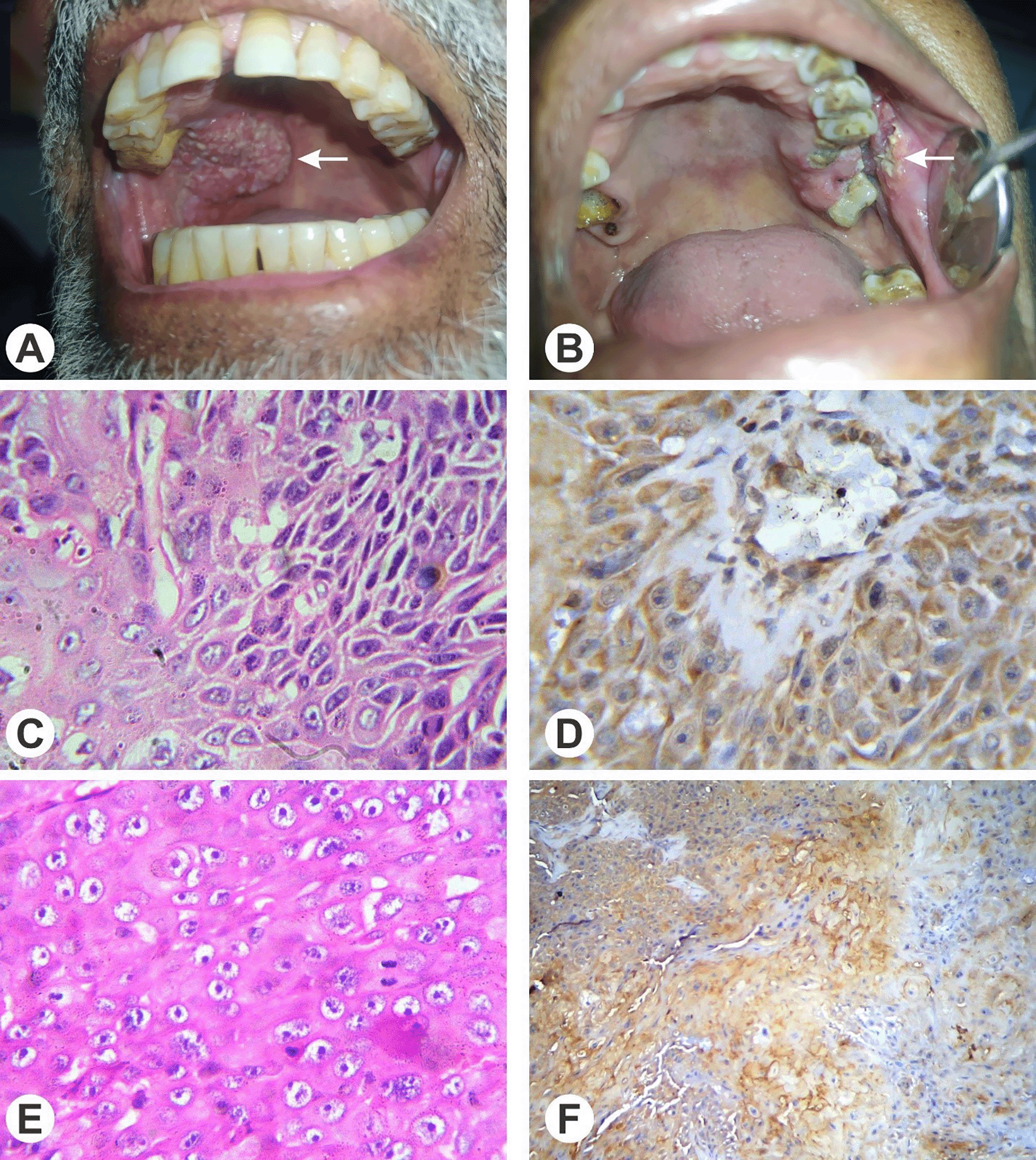
Table 2 shows a total of 85 patients, with 31 patients in the low (L) IRS group and 54 patients in the high (H) IRS group. In patients with conventional HNSCC, 62% in the low IRS group and 38% in the high IRS group had Oct-4 IRS. For CD24 IRS, 45.6% of patients with conventional HNSCC in the low IRS group and 54.4% in the high IRS group had immunoreactivity (p = 0.064). Among patients with non-conventional HNSCC, 83.3% in the low IRS group and 16.7% in the high IRS group had Oct-4 IRS, while for CD24 IRS, the percentages were 83.3% and 16.7%, respectively.
Table 3 provides a comparison of the mean IRS of Oct-4 and CD24 antibodies with the histological types and grades of HNSCC. The analysis included a total of 79 patients with conventional histological type and 6 patients with non-conventional histological type. In terms of CD24 IRS, the mean score for conventional histological type was 4.50 ± 3.49, with a 95% CI of 6.88-8.45. For non-conventional histological type, the mean IRS of CD24 was 7.67 ± 3.49, with a 95% CI of 1.09-7.23 (p = 0.019): indicating a statistically significant difference in CD24 IRS between the two histological types.
| Variables | n (%) | Antibody | Mean IRS ± S.D | 95% CI Mean | p value | |
|---|---|---|---|---|---|---|
| Histological type | Conventional | 79 | Oct4 | 5.34 ± 4.17 | 4.406-6.277 | 0.631 |
| Non-conventional | 06 | 4.50 ± 3.14 | 1.198-7.802 | |||
| Conventional | 79 | CD24 | 4.50 ± 3.49 | 6.88-8.45 | 0.019* | |
| Non-conventional | 06 | 7.67 ± 3.49 | 1.09-7.23 | |||
| Histological grade | WD/MD | 67 | Oct-4 | 5.35 ± 4.25 | 4.321-6.395 | 0.744 |
| PD | 18 | 5.00 ± 3.58 | 3.219-6.780 | |||
| WD/MD | 67 | CD24 | 7.50 ± 3.56 | 6.639-8.376 | 0.677 | |
| PD | 18 | 7.11 ± 3.62 | 5.306-8.915 | |||
In summary, the comparison of SI, IRS status and mean IRS of Oct-4 and CD24 antibodies with demographic, clinical and histological parameters of HNSCC did not reveal any statistically significant differences, except for CD24 SI, IRS status and mean IRS in relation to histological type (conventional vs. non-conventional), where a significant difference was observed.
Although, early stage cases of HNSCC have high cure rates, up to 50% of patients presents with advanced stage and full spectrum of complications of the disease.10 The category of late diagnosed group necessitates reliable diagnostic tool which can help in early diagnosis and treatment of disease. Presently there is a lack of clinically employable predictive indicator of disease. Hence, to look for potential predictors is paramount. The aim of this study was to investigate the expression of two CSC markers i.e. CD24 and Oct4, in different grades of HNSCC. CSCs are thought to be responsible for tumor initiation, progression, metastasis, drug resistance, and recurrence, making them a crucial focus in cancer research and treatment development.2 The findings of this current study shed light on the potential role of CD24 and Oct4 in distinguishing conventional and non-conventional histological types of HNSCC and offer insights into their significance in CSC biology.
Studying the expression profiling of these two CSCs markers is pivotal in understanding the disease nature. In the present study, a statistically significant difference in staining intensity of CD24 was observed in different histological subtypes (conventional and non-conventional types) HNSCC. In this study, the average immunoreactivity score (IRS) for CD24 was significantly high in non-conventional HNSCC subtypes compared to conventional types (p = 0.019). This suggests that CD24 may serve as a potential CSC marker for distinguishing different histological types of HNSCC. The implications of this finding are significant, as it may aid in identifying highly aggressive HNSCC cases characterized by CSCs, enabling more targeted and personalized treatment approaches.
These findings align with earlier research that has identified CD24 as a potential marker for cancer stem cells (CSCs) in different types of cancer. For instance, in ovarian cancer, CD24 expression correlated with chemoresistance and poor prognosis.11 These findings suggested the role of CD24’s as a CSC marker for different malignancies, warranting further investigation in HNSCC. It has been reported that expression of CD24 in cases of HNSCC with lymph nodal metastasis was high as compared to non-nodal involvement. Further, HNSCC cases with high expression of CD24 had poor overall survival when compared to low expression cases.12 Likewise, Han and colleagues indicated that the CD24 gene and its corresponding protein could potentially function as a diagnostic indicator or a target for treatment in HNSCC. Their study also revealed that CD24 expression was limited to a small subset of cells, underscoring the heterogeneous nature of tumors, and identified the presence of tumor-initiating cell subtypes (CD24+/CD44+) within HNSCC.13 A meta-analysis revealed that increased expression of CD24 has been observed across multiple types of tumors. The stimulation of the CD24/Siglec-10 pathway has been proven to enhance immune evasion by inhibiting the functionality of cytotoxic T cells and impeding macrophage-driven phagocytosis within tumors. The researchers also noted the absence of a uniform approach to determine CD24 positivity, as certain studies use flow cytometry while others rely on immunohistochemical assessment, leading to considerable variability.14 Zimmerer et al. conducted an in vitro study on OSCC cell lines and reported that almost 30% of tumor cells showed positive expression for CD24. They further reported that in terms of growing therapies based on personalized medicine, CD24 might be a potential target for modulating tumor angiogenesis or eliminating CSCs and thus tumor growth, progression, and metastasis.15
On the other hand, Oct4, a critical transcription factor and stem cell marker, has been implicated in tumor progression, metastasis, and shorter cancer-related survival. However, in the current study, no statistically significant associations was observed between Oct4 SI or IRS and the clinical and histological parameters of HNSCC (p > 0.05). This might indicate that the role of Oct4 in HNSCC progression and prognosis could be more complex, involving various molecular pathways and interactions. Regarding the relevancy of Oct4 in HNSCC, a study in 2020 was conducted on large cohort of 348 HNSCC patients to investigate its expression potential and impact on patient prognosis that revealed non satisfactory expression in any case of HNSCC which supports findings of the present study.16 Although multiple researchers reported Oct4 association with tumorigenicity, invasion and metastasis but in HNSCC the data is still lacking.17 Different properties of Oct4 isoforms could be the potential cause of confusion and controversies in its behavior in multiple cancer types.
The findings on Oct4 are consistent with some previous studies, while conflicting results have been reported in others. Mohiuddin et al. (2020) suggested high Oct4 expression in colorectal cancer association with advanced tumor stage and poor survival, suggesting a potential prognostic role. Conversely, in hepatocellular carcinoma.8 Oct4 expression was not significantly correlated with clinicopathological features or prognosis.18 These discrepancies may be attributed to tumor-specific differences in Oct4’s functional role and cellular context. Therefore, further research is warranted to elucidate Oct4’s precise role in the context of HNSCC.
Comparing the results of this study with previously reported data on CD24 and Oct4 expression in HNSCC may provide further insights into their relevance as CSC markers and potential prognostic indicators. A systematic review by Singh et al. (2021) identified CD44, aldehyde dehydrogenase, and CD133 as the most commonly expressed CSC markers in HNSCC, responsible for tumorigenesis, self-renewal, and therapy resistance. Meanwhile, NANOG, SOX-2, and OCT-4 were involved in metastasis and invasion.4 The identification of an accurate panel of CSC markers is essential to address the non-specificity of currently used markers and to improve understanding of CSC biology in HNSCC.
It is important to note that CSC markers are often expressed heterogeneously within tumors, and their expression levels may vary based on tumor stage, location, and patient demographics. Therefore, comprehensive meta-analyses and larger-scale studies involving diverse patient populations would be beneficial to establish a clearer understanding of CD24 and Oct4’s clinical significance in HNSCC.
In summary, this study provides valuable insights into the potential role of CD24 as a CSC marker for distinguishing conventional and non-conventional histological types of HNSCC. The findings suggested that CD24 could serve as a valuable tool in identifying specific HNSCC subtypes characterized by CSCs. However, further research is needed to validate these findings and explore the complex interactions between CSC markers and tumor biology in HNSCC. Understanding the roles of both CD24 and Oct4 in HNSCC may contribute to the development of targeted therapies and improved prognostic strategies for this aggressive malignancy. Future studies should aim to investigate larger patient cohorts and include a broader panel of CSC markers to elucidate the underlying molecular mechanisms and clinical implications of CSCs in HNSCC.
The ethical and technical approval to perform this study was granted by the Ethical Review Committee and the Advanced Studies & Research Board (No.UHS/Edu/126-16/1226, Dated: 17-06-2016) University of Health Sciences, Lahore, Pakistan. This study also strictly adheres to the guidelines given in Helsinki Declaration of World Medical Association. Each study participant gave their written informed consent. Consent to publish the details has been obtained from all individuals and all efforts were made to anonymize individual’s data.
Harvard Dataverse: CD24 Oct4 Immunohistochemical Expression in HNSCC. https://doi.org/10.7910/DVN/QGX2E3. 19
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
This work is supported by the University of Health Sciences (UHS) Lahore and Higher Education Commission (HEC) of Pakistan.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: I could understand every aspect of this study because i have done research on Oral cancer stem cells in OSCC
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 23 Apr 24 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)