Keywords
branch retinal vein occlusion, COVID-19, SARS-CoV-2 infection, macular edema, oral contraceptive
Oral contraceptive use, vaccination for Coronavirus disease 2019 (COVID-19), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are risk factors for venous thromboembolism. Branch retinal vein occlusion (BRVO) generally develops mid-60s patients. Herein, we present a case of superotemporal BRVO caused by the above mentioned risk factors in a young woman. To the best of our knowledge, this is the first report about superotemporal BRVO associated with oral contraceptives, COVID-19 vaccination, and SARS-CoV-2.
A 21-year-old woman presented with loss of visual acuity in her right eye for 10 days. She had been receiving oral contraceptives for 2 years for oligomenorrhea before noticing ophthalmological symptoms. Despite having received two doses of an mRNA COVID-19 vaccine, she contracted COVID-19 and developed fever, sore throat, cough, low back pain, and general malaise about 40 days before the initial visit. However, only cough persisted for more than a month. The right eye showed superotemporal BRVO with macular edema (ME). She did not smoke nor had diabetes or hypertension. Blood test results, including cardiolipin antibody IgG, were normal. She was treated with an intravitreal aflibercept injection. ME in the fundus showed rapid improvement and resolution. Although more than 20 months have passed since the first injection, there has been no relapse of ME.
The combination of oral contraceptive use, COVID-19 vaccination, and subsequent SARS-CoV-2 infection could induce the development of venous thromboembolism, thereby leading to superotemporal BRVO. Given that cases of COVID-19 have increased globally, patients with retinal vein occlusion who use oral contraceptives are likely to be encountered more frequently.
branch retinal vein occlusion, COVID-19, SARS-CoV-2 infection, macular edema, oral contraceptive
We revised the manuscript following the reviewer’s comment.
See the authors' detailed response to the review by Pradeep Kumar Panigrahi
The Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is the latest pandemic and has lasted approximately 3 years in Japan. A special characteristic of COVID-19 is its propensity to cause venous thromboembolism (VTE),1 which could result in fatal complications. In ophthalmology, COVID-19 reportedly causes retinal vein occlusion (RVO).2–4
Branch RVO (BRVO) is a major retinal vascular disease that occurs following venous thrombosis at arteriovenous crossing points. Arteriosclerosis accompanied by hypertension is a risk factor for BRVO; therefore, it primarily affects older people.
BRVO causes blood–retinal barrier dysfunction, resulting in macular edema (ME). Photoreceptor cell apoptosis caused by chronic ME is responsible for the reduced vision in patients with BRVO. Therefore, a missed treatment window may lead to irreversible loss of vision.5,6 Anti-vascular endothelial growth factor agents are the first-line treatment for ME secondary to BRVO.7
Over 100 million women worldwide practice contraception and use intrauterine devices, combined estrogen and progestin oral contraceptives, and progestin -only preparations (oral contraceptives, implants, or injections).8 Oral contraceptives contain estrogen, which increase the risk of VTE due to activation the coagulation cascade. The incidence of VTE in women receiving oral contraceptives is approximately twice as high as that in the normal population.9 Generally, the age group susceptible to RVO associated with oral contraceptive use is younger than that for typical RVO.
Herein, we present a case of a 21-year-old woman using oral contraceptives who developed superotemporal BRVO with ME following COVID-19. To the best of our knowledge, this is the first report suggesting that oral contraceptive intake combined with COVID-19 may be a risk factor for the development of superotemporal BRVO.
A 21-year-old woman was diagnosed with polycystic ovary syndrome accompanied by oligomenorrhea at 19 years old. Consequently, she had been taking oral contraceptives (norethisterone and ethinylestradiol mix tablet) for the improvement of oligomenorrhea. She did not have diabetes or systemic hypertension, and she did not smoke. She had no family history of VTE. Her height, body weight, and body mass index were 1.62 m, 52 kg, and 19.8, respectively. Although she had received an mRNA COVID-19 vaccine twice approximately 5 months prior to presentation, she experienced fever, sore throat, cough, low back pain, and general malaise, prompting her to visit a clinic. Polymerase chain reaction of her saliva sample showed a positive reaction to SARS-CoV-2. Her cough persisted for a month, but other symptoms improved without post-COVID-19 sequelae.
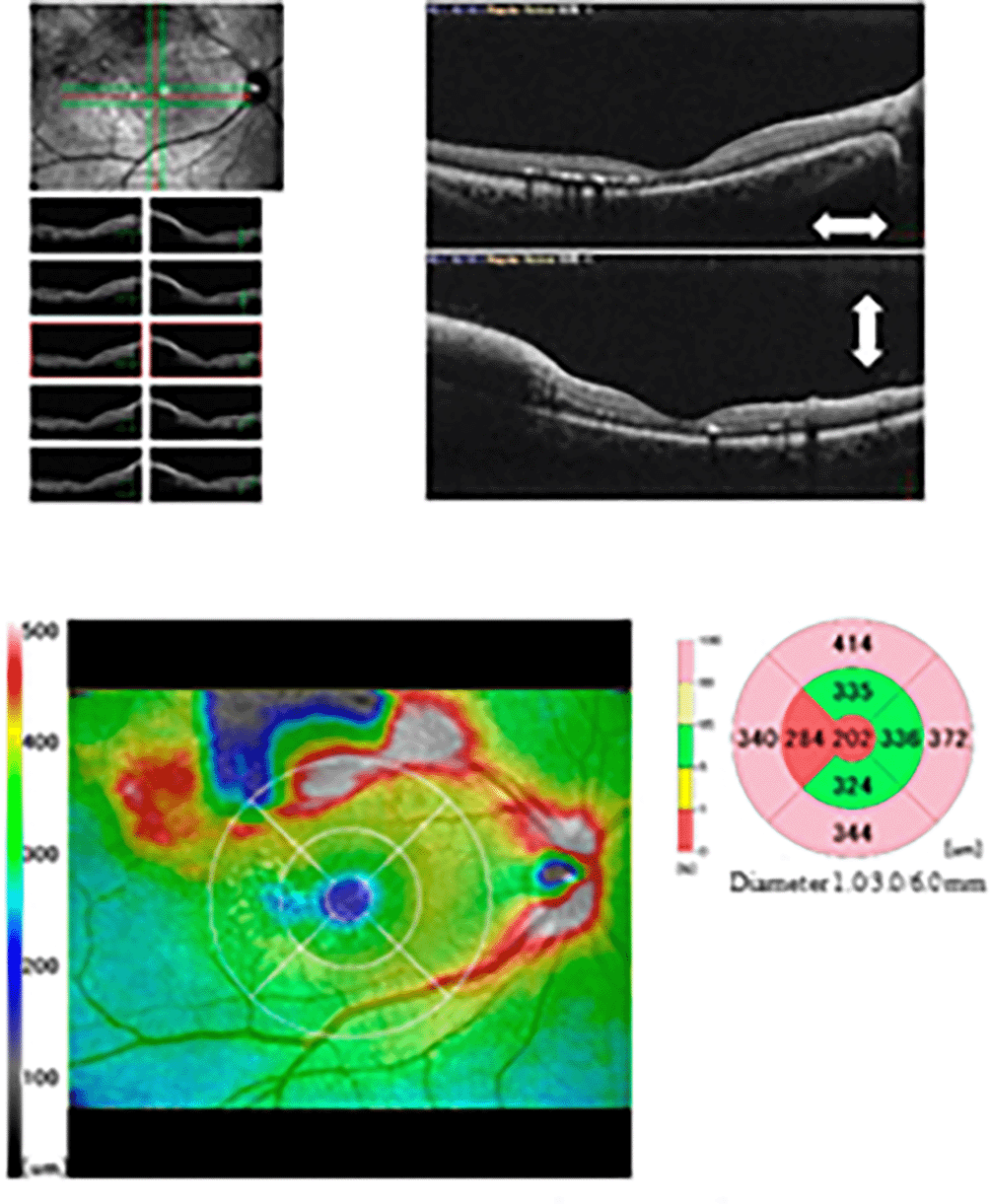
Approximately 40 days after being diagnosed with COVID-19, she presented with decreased vision in the right eye and was diagnosed with ME secondary to superotemporal BRVO. On her initial visit to our hospital, the decimal best-corrected visual acuity (BCVA) values were 0.4 in the right eye and 1.2 in the left eye. In both eyes, intraocular pressure was normal, and slit-lamp biomicroscopy did not detect inflammation. Fundus examination revealed retinal hemorrhage in the superior-temporal quadrant of the retina in the right eye ( Figure 1). Optical coherence tomography (RS-3000 Advance, Nidek Corporation, Japan) demonstrated cystoid ME and intraretinal fluid in the right eye ( Figure 2). Blood test results, including cardiolipin antibody IgG, were within normal limits ( Tables 1 and 2).
The clinical diagnosis was ME secondary to superotemporal BRVO following COVID-19. We contacted her gynecologist about her eye condition, and oral contraceptives for oligomenorrhea were changed to a progestational hormone agent (dydrogesterone). Additionally, the patient was treated with intravitreal aflibercept (Eylea®; Regeneron, Tarrytown, NY, USA). ME resolved after 1 month ( Figure 3), and the decimal BCVA improved to 1.2 in the right eye. After more than 20 months since the first administration, no additional intravitreal aflibercept has been administered. No ME was noted after 24 months ( Figure 4), and the decimal BCVA remained at 1.2.
The estimated incidence of combined oral contraceptive-related ocular complications is 1 in 230,000 persons and includes dry eyes, corneal edema, lens opacities and retinal neuro–ophthalmologic, or vascular complications.8 Sinawat et al. analyzed patients with RVO aged <50 years and reported that 3 of 70 patients with central RVO had taken oral contraceptives for 5–6 years and 1 of 30 patients with BRVO had taken oral contraceptives for 10 years.10 As persons aged mid-60s are the most susceptible to RVO, our case is extremely rare. According to a 2013 survey regarding VTE, the risk of VTE in women receiving oral contraceptives is twice as high as that in women not receiving oral contraceptives.9 Lidegaard et al. reported that the VTE risk related to oral contraceptive use is 1.0 for women aged 15–19 years, 1.32 for 20–24 years, 1.99 for 25–29 years, 2.91 for 30–34 years, 4.01 for 35–39 years, 5.29 for 40–44 years, and 6.58 for 45–49 years.11 Therefore, the VTE risk increases with increasing age.11 As our patient was 21 years old, the risk for RVO appeared to be low.
Coagulation disorder in COVID-19 is thought to occur through vascular damage caused by virus infection.12 Various factors, including reduced antithrombogenicity of the vascular endothelium, release of von Willebrand factor and coagulation factor VIII, complement activation, increased fibrinogen, and cytokine storm, are intricately interwoven.12 Thrombus can then form in any vessel, such as arteries, veins, and capillaries.12
Battaglia et al. reported that among 144 patients with BRVO, 128 (88.9%) had temporal BRVO, while 16 (11.1%) had nasal BRVO.13 The two groups showed no differences in systemic hypertension, diabetes mellitus, glaucoma, or ischemic heart disease.13 Nasal BRVO cases exhibited better visual acuity but higher levels of capillary non-perfusion, retinal neovascularization, and vitreous hemorrhage.13 Kumral et al. found that among 64 BRVO patients, 38 had superotemporal BRVO, and 26 had inferior temporal BRVO.14 Superotemporal BRVO required significantly more intravitreal ranibizumab injections.14 In our case of superotemporal BRVO, ME resolved with a single intravitreal aflibercept injection.
Several reports have described BRVO development following SARS-CoV-2 infection.2,4 SARS-CoV-2 infection is a high-risk factor of VTE.1 Pur et al. reported a case of BRVO after mRNA COVID-19 vaccination.15 They postulated that the vaccine evoked an immunological response that induced VTE in a healthy patient.15 A thrombotic cause was unclear from the patient’s blood results. The literature shows that D-dimer is commonly elevated in patients with COVID-1916 and oral contraceptive users.17 However, no special blood factors have been reported linking RVO with oral contraceptive use, SARS-CoV-2 infection, and COVID-19 vaccination. Identifying the cause was challenging. Thus, the combination of oral contraceptive use, SARS-CoV-2 infection, and COVID-19 vaccination could be a risk factor for the development of RVO. To the best of our knowledge, this is the first reported case of superotemporal BRVO involving all three risk factors.
Written informed consent for publication of the clinical details and clinical images was obtained from the patient.
We have uploaded our report to Research Square in the form of a preprint (DOI: https://doi.org/10.21203/rs.3.rs-2067517/v1).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
No
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Vitreo-retina, medical retina, surgical retina
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 2 (revision) 17 Feb 25 |
|
|
Version 1 08 May 24 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)