Keywords
corneal tissue engineering, bibliometric analysis, patent analysis, clinical adoption, technology transfer
This article is included in the Eye Health gateway.
Tissue engineering research aims to address the global shortage of donated corneal tissue, yet challenges persist in clinical translation. This study assesses the pathway from basic research to clinical adoption in corneal tissue engineering.
Bibliometric and patent analyses were conducted using the Web of Science-Core Collection and Lens databases to identify top authors, countries, journals, publication trends, inventors, patent statuses, and affiliated companies. A quality-adjusted life year (QALY) analysis compared engineered corneal endothelium to full keratoplasty. A pilot study surveyed thirty ophthalmologist surgeons from eight Latin American countries.
A strong upward publication trend (R2 = 0.89, p = 1.53x10^-9) in corneal endothelium engineering was observed over the past decade, led by the USA, China, and Japan. Among 614 research papers, 26 patents and 10 companies were identified. Engineered corneal endothelium showed a QALY gain of 0.74 versus 0.07 of corneal transplants. Most survey respondents (97%) expressed interest in adopting engineered corneal endothelium for transplantation if affordability, biocompatibility, and functionality were assured.
While tissue engineering offers promise in alleviating corneal scarcity, a significant gap remains between scientific advancements and clinical adoption, presenting “death valleys.” Addressing this requires more efficient navigation of the interplay between scientific progress, technology adoption, and clinical practice.
corneal tissue engineering, bibliometric analysis, patent analysis, clinical adoption, technology transfer
Although corneal transplants are the most common type of transplant worldwide, a shortage of tissue donors hinders the need for necessary surgeries, leaving most patients without access to surgery that could restore their vision. Currently, only one cornea is available globally for every 70 individuals who are needed, and the majority of low- and middle-income countries lack access to eye banks.1 The International Agency for the Prevention of Blindness reports 12.7 million people on the waiting list for corneal tissue donors, making this a global need.2 Along with full cornea transplantation (penetrating keratoplasty), other surgical techniques involving only the transplantation of the corneal endothelium, such as Descemet membrane endothelial keratoplasty (DMEK) and Descemet stripping endothelial keratoplasty (DSEK), have been developed. However, all these surgical procedures have common limitations, such as rejection of the graft, graft detachment, and loss of cell viability in the middle term, which results in the need for an additional graft in most cases.3,4
In response to this issue, the field of tissue engineering applied to the corneal endothelium has emerged as a significant and potentially effective therapeutic intervention path. Several methods have emerged for isolating and harvesting corneal endothelial cells, as have approaches for producing scaffolds for the transplantation of these cells, such as decellularized stroma and other biomaterials.5 This is demonstrated by the growing trend in publications in the field in the last 20 years.6 Although these developments hope to alleviate the current scarcity of corneal tissue for transplants, the pathway for adopting emergent technologies in clinical practice faces two “death valleys”: the transition between academic research and technology transfer and then clinical practice adoption.7,8 Several factors influence this, including a shortage of multidisciplinary approaches, lack of funding, and practices in the technology transfer process, leading to a gap between scientific advances and practical implementation.
The goal of scientific progress is to benefit society. Quality-adjusted life years (QALYs), which enable us to estimate the potential impact of a novel treatment in contrast with the gold standard treatment in terms of quality of life beyond cost-effectiveness, serve as helpful tool to support the clinical adoption of emerging technologies.9 Prior research evaluated two surgical methods for transplanting corneal endothelium in terms of QALYs10 and the results of cost reduction between the use of donor corneal tissue obtained from eye banks and the transplantation of engineered corneal endothelium.11
We aimed to trace the trajectory from fundamental research to practical clinical application in the realm of corneal transplantation. We delve into the intricate interplay between scientific progress, technology adoption, and clinical practice to shed light on the barriers hindering the translation of innovative technologies into practical applications. This exploration is not merely a pursuit of academic curiosity but also a call for action, emphasizing the urgent need for sustained collaboration, financial support, and further in-depth studies. The aim of this study was to bridge the persistent gap between theoretical promise and practical implementation, ultimately revolutionizing the landscape of corneal transplantation for the improvement of global eye health.
2.1.1 Search strategy
We searched the Web of Science-Core Collection (WOS-SCC)12 database using topic as a field of search with the following keywords: tissue engineering, regenerative, reconstruction, corneal endothelium, and descement in the following query: TS=(“tissue* engineer*” OR regener* OR reconstruct*) AND TS=((cornea* AND endothel*) OR descement*). This search yielded a total of 1030 articles. The documents were then filtered by document type, including only original articles published in any language between 2003 and 2023. The data were searched and extracted on the same day (June 29, 2023).
The full records, including the cited references of all 614 selected articles in plain text format from the WOS-SCC, were exported. The raw file was uploaded to VOS-Viewer Software13 https://www.vosviewer.com/ to screen the author’s names and to create a thesaurus file. The ‘found and replace’ function was used in open-line software to update all the information in the database and to make the metadata consistent between the VOS-Viewer and Biblioshiny formats.
The bibliometric analysis was conducted using VOS-Viewer software 313 and the bibliometrix and biblioshiny R packages14 https://www.bibliometrix.org/home/index.php/layout/biblioshiny. VOS-Viewer Software was used to filter the top 25 most relevant authors and journals by creating a list according to their selection thresholds of the minimum number of documents and citations. Biblioshiny was used to perform a descriptive analysis of the bibliometric indicators, evaluate the citations of the top 25 authors and journals, and determine the impact factor and collaboration networks between the top authors and countries.
We searched the Patent Public Search (USPTO)15 database. We used the following query: “corneal endothelium” AND (“tissue engineering” OR “regenerative” OR “reconstruction”) AND (biomaterials OR cell therapy OR scaffold OR membrane) NOT (“organoids” OR “epithelium”). This search yielded a total of 126 patents. A search of the European Patent Office database16 using the following query: “corneal endothelium in the title AND tissue engineering OR scaffold OR biomaterial in the title or abstract” retrieved 6 results. Given the difference in the number of results retrieved from these two databases, we conducted a third search using the Lens database17 with the following query: (title:((cornea AND endothelium) AND (cell therapy OR scaffold OR biomaterial OR tissue engineering OR membrane OR reconstruction NOT epithelium)) OR abstract:((cornea AND endothelium) AND (cell therapy OR scaffold OR biomaterial OR tissue engineering OR membrane OR reconstruction NOT epithelium)) OR claim:((cornea AND endothelium) AND (cell therapy OR scaffold OR tissue engineering OR membrane OR reconstruction NOT epithelium))). This search retrieved 181 results. The data search was conducted on August 28, 2023. The “analysis” function of the Lens database was used to generate graphs about the number of patents over time, type of documents, legal status, top inventors, jurisdiction, and most cited documents from 1980 to present.
We used the results on Biblioshiny based on the publication affiliation from the bibliometric analysis search. We chose the affiliations that were companies, and then we selected those that had an active website. From those companies, we chose those that had products related to engineered corneal tissue. Data relating to the company’s country, type of product, year of foundation, clinical use and phase of production were analyzed.
For the QALY analysis, we created a Markov model18 that considered both the quality and quantity of life in the Mexican population using parameters such as life expectancy, cost of corneal transplant, and mean age of transplant. We considered three categories of vision health: blindness, sight, and mortality. Each category has several transition possibilities. Patients can transition from “sight” to “blind” and vice versa and then to death, referring to the end as the absorbing state, given that once an individual enters the state, they remain there.19 Only the transition from blindness to sight incurs expenses, including the cost of obtaining cells from one corneal donor and generating the necessary cells for up to ten engineered corneal endothelium constructs.20 We used a hypothetical cohort of 1000 blind patients aged 35 years and ran the program for 40 years to calculate life expectancy. A discount rate of 3.5% over a 10-year period was used in the computations. We projected that the intervention would reduce the waiting list from three to one year since more transplants would be accessible. We calculated the cost per QALY gained for each intervention and compared it to Mexico’s GDP per capita (8,346.7 USD) to determine whether the interventions were cost-effective, assuming the same cost per patient. The reference cost for a corneal transplant was $6000 USD,21 and the calculated cost of an engineered corneal endothelium tissue was $2400 USD (taken from a preliminary cost analysis from data not shown considering donor cornea acquisition, laboratory consumables, reagents, biocompatible scaffold production cost, QA testing, and a GMP-compliant laboratory, among others). The transplant survival rate/duration was 87% after one year, 72% after three years, 54% after six years, and 42% after ten years.22
We created an online 10-question formulary using Survey Monkey, an online software platform with free license options, to assess Latin American ophthalmology surgeons’ attitudes toward the use of engineered tissue for corneal endothelium restoration. The formulary was distributed during October 2022 by email throughout the community of Latin American ophthalmology surgeons, and information about the respondent’s location, willingness to use a synthetic biocompatible membrane to transplant corneal endothelial cells, preferred characteristics (thickness, insertion port size, presentation, rigidity, and transparency level), and price range of the bioengineered tissue was recorded to assess the potential of transplanting engineered corneal endothelium instead of donated corneal endothelium. The replies were analyzed using descriptive statistics.
A total of 416 of the 1030 screened articles were excluded (those published before 2003, review articles, meeting abstracts, proceedings papers, book chapters, editorial material, letters, early access, corrections, or retracted publications), and 614 were included in the bibliometric analysis. A flow diagram describing the flow information used for article screening and selection is shown in Figure 1.
The articles were published by 2,572 authors from 49 different countries in six different languages—English, French, German, Korean, Portuguese, and Russian—in 206 different journals and used a total of 17,194 references. In total, these articles had 12,042 citations, excluding self-citations. To analyze the trend in the number of publications and correlate it to the year, we performed a polynomial regression. Using a log transformation of the year as an independent variable, we observed a continuous annual growth rate that stabilized in 2021. We fitted a squared polynomial equation to predict the number of articles as a function of year, and we obtained a statistically significant R-squared value of 0.89 and a p value of 1.53×10-9, demonstrating a trend toward an increase in the number of articles related to corneal endothelium regeneration, as shown in Figure 2a.
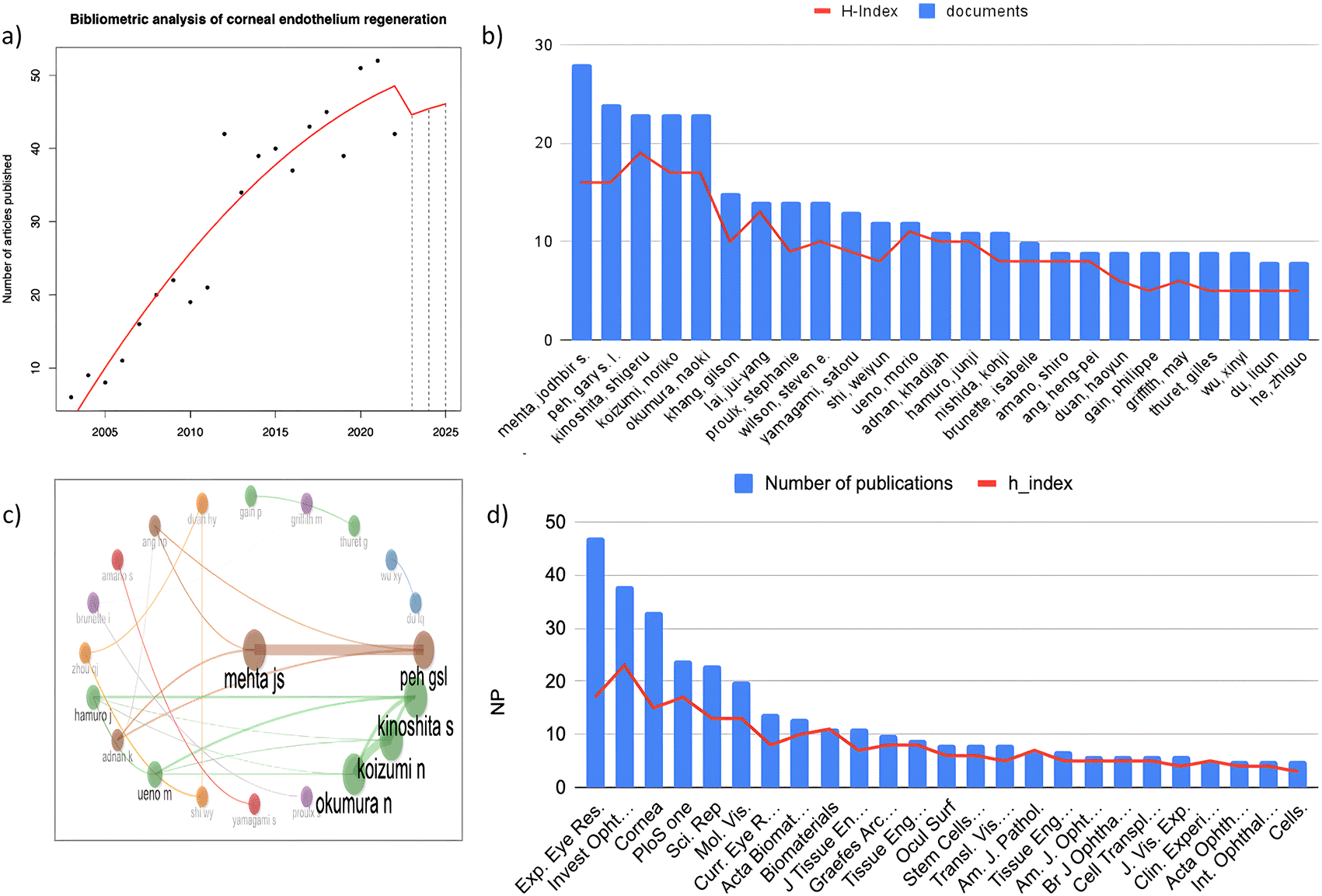
a) Annual growth rate of the number of publications on corneal endothelium regeneration. b) Top 25 authors. c) Top 25 Journals. d) Author collaboration.
The analysis included a total of 2,572 authors contributing to the 614 selected original articles, resulting in an average of 0.23 documents per author. Eight authors had single-authored documents. The average number of coauthors per document was 6.34, and the average number of international coauthorships was 25.57%. Figure 2b shows the top 25 authors according to their publication rate, and Figure 2c shows the relationships between the top authors in the field who had more than 8 publications and 159 citations. The most productive authors on this specific topic are Metha JS (28 articles), Peh GSL (24 articles), Kinoshita S (23 articles), Koizumi N (23 articles), and Okumura N (23 articles). There is a strong coauthor relationship between Peh GS and Metha JS, as well as between Kinoshita S, Koizumi N, and Okumura, all of whom currently form the most productive research groups in the field.
The study included 206 journals with 614 published papers, yielding a total of 0.33 documents per journal. The top 25 journals with at least 5 articles and 35 citations are shown in Figure 2d. According to JCR 2022, these 25 journals have an average impact factor of 4.238 and are in quartile 2; 11 (52%) are in the category of ophthalmology, 5 (20%) are in the category of cell & tissue engineering, 3 (12%) are in the category of multidisciplinary sciences, 2 (8%) are in the category of material science, and 2 (8%) are in other categories. These journal articles have been referenced more than 558 times and have a local H-index greater than 13. Exp. Eye Res. (47) is the most relevant journal.
A search of the Lens database returned 181 results. We applied the categorization filter to the following: A61f2/14 (eye parts, e.g. lenses, corneal implants; artificial eyes making thereof from organic plastic material), A61f2/142 (artificial or natural cornea replacement implant for repair of defective corneal tissue), A61p27/02 (Drugs for disorders of the senses, ophthalmic agents) A61l27/24 (materials for {grafts or} prostheses or for coating {grafts or} prostheses, collagen) A61l27/3604 (materials for {grafts or} prostheses or for coating {grafts or} prostheses, {characterized by the human or animal origin of the biological material, e.g. hair, fascia, fish scales, silk, shellac, pericardium, pleura, renal tissue, amniotic membrane, parenchymal tissue, fetal tissue, muscle tissue, fat tissue, enamel}), A61l27/38 (materials for {grafts or} prostheses or for coating {grafts or} prostheses, {containing added animal cells}), A61l27/3834 (materials for {grafts or} prostheses or for coating {grafts or} prostheses {Cells able to produce different cell types, e.g. hematopoietic stem cells, mesenchymal stem cells, marrow stromal cells, embryonic stem cells}, and C12n5/071 (Vertebrate cells or tissues, e.g. human cells or tissues). There were 26 patents who continued to be examined. The top inventors were Cong R., Fan T., Kinoshita S., Ueno M., Zhao J., Koizumi, Gutermuth A., Yang X., Duang H., and Hori J (Figure 3a). Among those, Kinoshita, Ueno, Koizumi, and Duan were also among the most cited authors in the bibliometric analysis. Four patents were granted, one was an additional patent, and twenty-one were patent applications between 1992 and 2024 (Figure 3b). The legal status of 10 of the patents was active, with only three being discontinued (Figure 3c). The jurisdiction with the largest number of patents is the United States, followed by the World Intellectual Property Organization (WIPO) and European patents (Figure 3d). The most cited patent, with 62 citations, was “Methods employed in replacement of the corneal endothelium”, of White T., granted in 1992; it was followed by “Human Corneal Endothelial Cell-Derived Precursor Cells, Cellular Aggregates, Methods for Manufacturing the Same, and Methods for Transplanting Precursor Cells and Cellular Aggregates”, of Amano, published in 2009, with 19 citations, currently discontinued; and “In Vitro Cornea Equivalent Model”, of Parenteau, published in 1994, with 6 citations.
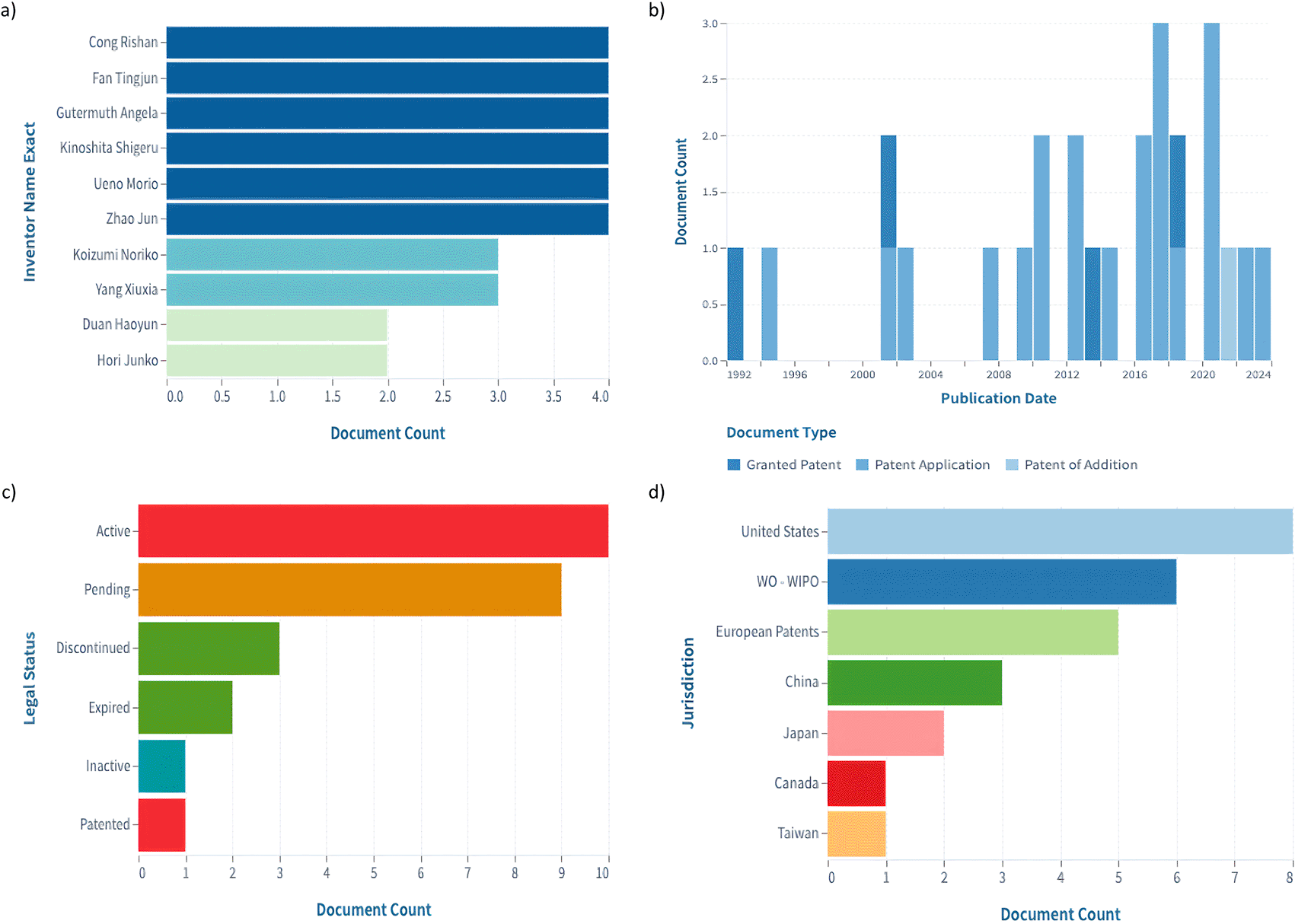
a) Number of documents per inventor. b) Document type versus publication date. c) Number of documents by legal status. d) Number of documents per jurisdiction (data and images retrieved from Lens.org).
The affiliations of the articles included in the bibliometric analysis yielded 53 companies. Twenty of those had an active webpage. Of those, 10 had products related to the original research paper from which the affiliation was taken (Table 1). These companies were founded from 2001 to 2022. These countries are in four different regions: five in the USA, two in Japan, two in China and one in Spain. Five of these companies have products on the market (Linkocare, CorneaGen, Takara Bio, BioTissue, and Cell Science & Technology Institute); two are developing phase II clinical trials (Trefoil Therapeutics and Emmecell); and one is in a phase I clinical trial (Body Organ Biomedical Corp); two of them are in the preclinical phase (Vissum Group and Cellusion). Three of these companies have products whose main use is corneal transplantation (Linkocare, CorneaGen, and Cellusion). One of the companies (Corneagen) appears as the affiliation of three documents with five of the top authors from the bibliometric analysis (Kinoshita, Hamuro, Ueno, Okumura, and Koizumi); two of them are also among the top patent inventors (Kinoshita and Koizumi).
| Company | Country | Product | Year of foundation | Use | Phase of production | Publication affiliation |
|---|---|---|---|---|---|---|
| Body Organ Biomedical Corp | China | Fish-scale collagen membrane | 2007 | Temporary corneal barrier for corneal perforation | Phase I | 23 |
| Cell sci & technol inst inc | China | Cell therapy vehicle (cell culture media) | 2001 | Regenerative medicine, cell culture | In the market | 24 |
| Cellusion inc | Japan | Corneal endothelium and injector | 2009 | Corneal transplants | Preclinical | 25 |
| Emmecell | Cell therapy technology for corneal edema | 2018 | Corneal restoration | Phase II | 26,27 | |
| Linkocare | USA | Bioengineered corneal implants and membranes | 2019 | Corneal transplants | In the market | 28 |
| Sightlife Surgical Inc (now CorneaGen) | USA | Ready-to-transplant corneal tissue Cross-linked cornea | 2006 | Corneal transplants | In the market | 29–33 |
| Takara Bio | Japan | Stem cell transplant | 2017 | Corneal restoration | In the market | 34 |
| TissueTech Inc (BioTissue) | USA | Prokera; Amniograft, Amnioguard | 2012 | Corneal bandage | In the market | 35–41 |
| Trefoil therapeutics | USA | TTHX1114 (engineered FGF1) | 2021 | Corneal endothelium and epithelium restoration | Phase II | 42 |
| Vissum Group | Spain | Stem cell transplant | 2022 | Corneal restoration | Preclinical | 43 |
The Markov model based QALY study showed that engineered corneal endothelium intervention is associated with greater QALY gains than full keratoplasty is. The analysis revealed that endothelial corneal transplant had a QALY increase of 0.07 compared to that of engineered corneal endothelium intervention (0.74 QALY gain on a scale of 0 to 1). Table 2 shows the comparison between the QALYs gained and the cost of the transplantation of engineered tissue and full keratoplasty.
A total of 30 ophthalmology surgeons with subspecialties in the cornea answered the questionnaire. The participants were from 8 Latin American countries: Colombia, Ecuador, Venezuela, Mexico, Spain, Costa Rica, Paraguay, and the Dominican Republic (Figure 4a). In a question with open answers, the key features for selecting an engineered corneal endothelium as the first choice for transplantation were biocompatibility, cost, manipulability, and transparency. More respondents mentioned efficacy, ease of insertion, orientation, resistance during manipulation, accessibility, thickness, adherence, and viability time (Figure 4b).
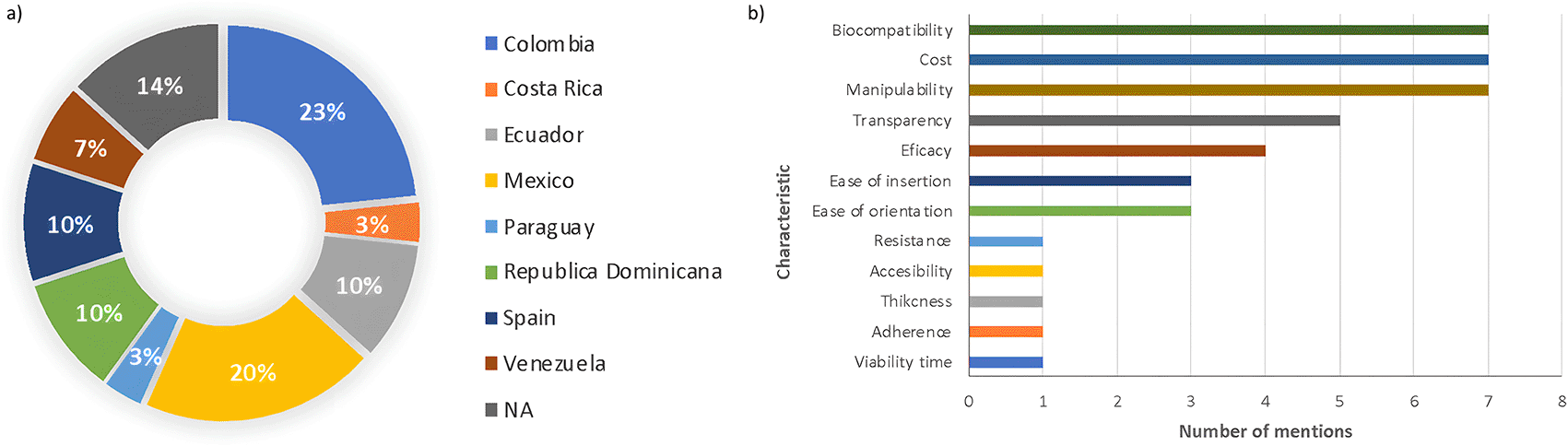
a) Nationality distribution of the survey respondents. b) Features favoring engineered corneal endothelium for transplantation over full keratoplasty.
The majority of participants (97%) expressed the belief that synthetic membrane-based engineered tissue would be beneficial for corneal endothelium transplantation. The participants’ preferences for key characteristics of an engineered corneal endothelium are outlined in Table 3.
The cornea is one of the most common transplanted tissues worldwide, primarily because corneal opacity is among the leading causes of blindness and vision impairment.44 As the global population continues to grow and life expectancy increases, the persistent scarcity of corneal tissue donors remains a primary obstacle to accessing corneal transplantation. Presently, there is a significant deficit in the availability of corneal grafts, with just one cornea accessible for every 70 individuals in need.1 Penetrating keratoplasty has traditionally served as the conventional and widely practiced surgical method for managing diverse corneal conditions, irrespective of the specific layer affected, including endothelial issues. However, recent advancements in technology and innovations in the field have given rise to endothelial keratoplasty techniques over the past two decades.4 These techniques entail the transplantation of specific corneal components and present an alternative and progressive approach to treating such conditions. Despite these surgical advances, the issue of donor tissue scarcity remains unresolved. This study aimed to trace the trajectory from fundamental research to practical clinical application.
Efforts to address the tissue donor shortage are now underway through comprehensive basic, translational, and clinical research initiatives. Tissue engineering has made significant strides, marked by notable developments in the isolation and culture of corneal endothelial cells, coupled with the production of biocompatible scaffolds, which have improved the prospects of tissue-engineered grafts.45 As demonstrated in the bibliometric analysis, there has been continuous growth in publications related to corneal endothelial engineering over the past two decades,6 which aligns with the data that indicate a decline in publications on penetrating keratoplasty.46 However, challenges persist in translating this scientific progress to clinical application due to diverse factors, such as limited research time and financial constraints. The median time to receive the first independent grant was 8 years only among ophthalmology clinician scientists. Nevertheless, the time taken to achieve independence as a researcher correlates with greater institutional support and earlier success in obtaining extramural grants.47 Furthermore, addressing the challenge of collaboration and integration between basic and clinical research is crucial for successful translational medicine.7 This is proven in our bibliometric analysis, where most of the documents were located in ophthalmology journals, with fewer contributions in fields such as cell and tissue engineering, multidisciplinary studies, and material sciences. This gap may signify a potential shortage of multidisciplinary approaches within the field, which is crucial for addressing the ongoing challenge of corneal tissue scarcity.
The first “Death Valley” in the path to clinical application occurs during the transitory period between technology transfer and academic research.7,8 This aligns with the findings of our patent study, which reduced the number of patents from 614 publications to 26 using a search strategy akin to the one utilized in the bibliometric analysis. Only four of the 25 most cited authors in the bibliometric analysis were among the top 10 inventors in the patents. This could be related to several factors, referred to as obstacles to the growth of technology transfer, such as the lack of engagement and cooperation between scientists and technologists.48 Several surgical techniques have been developed in the field of ophthalmology to improve visual health. Collaboration with experts in the fields of material sciences, nanotechnology, biotechnology, robotics, etc., was necessary for these advancements. This necessitates strengthening the training of research personnel doing eye surgery in transdisciplinary teams, providing access to financial resources for funding, and exchanging practices that enhance the work of Institutional Review Boards to improve the technology transfer pathway.49
Between the stage of technology transfer and clinical application, there is a second “Death Valley ”.7,8 Robust research and actual use of breakthrough technologies in the rapidly evolving medical technology landscape are significantly out of alignment, especially in the field of ophthalmology. Despite the abundance of research articles demonstrating advancements in disciplines such as artificial intelligence (AI), tissue engineering, and surgical improvements, the translation of these discoveries into practical applications has been remarkably poor. Industry 5.0 is anticipated to meet the demands of the ophthalmology sector by combining artificial intelligence and telemedicine.49,50 This difference is emphasized by the fact that, out of more than 600 research articles, only 26 patents and 10 companies are actively utilizing technology focused on engineering the corneal endothelium. This disparity demands a thorough examination of the barriers hindering the straightforward translation of test findings into therapeutic settings. This phase, which involves a lack of financing throughout the pre-seed and seed stages of the implementation of innovative ideas, is a well-discussed aspect that affects the translation of scientific breakthroughs.48 Five of the 10 companies analyzed are in the midst of ongoing research, while the other five already have products on the market. Particularly noteworthy is the presence of Corneagen, which was the affiliation for three documents authored by three of the most cited researchers in our bibliometric analysis. These companies are actively engaged in various aspects of the field, such as cell therapy, engineered tissue, devices designed for tissue transplantation, specialized media, and biomaterials used as bandages.
Considering the challenges that limit the practical application of novel technologies in ophthalmology, a thorough analysis of their potential impact on patient outcomes is crucial. As we investigate the field of engineered corneal endothelium, an evaluation assessing the benefits of this alternative strategy in comparison to traditional corneal transplantation is necessary. A prior study underscored the economic and accessibility advantages of the engineered corneal endothelium, positioning it as a more viable and cost-effective choice for patients.11 The application of QALY analysis, a metric gauging health outcome based on both length and quality of life,51 has been previously employed to assess the cost-effectiveness of ultrathin DSAEK (10). In the present study, the QALY analysis was tailored to the specific parameters of the Mexican population, given that it is the country with the most corneal transplants in Latin America according to the 2019 report of the Eye Bank Association of America,52 which established a foundational perspective. This approach not only accentuates the regional advantages of engineered corneal endothelium in terms of cost but also extends its benefits to life expectancy, graft survival rates, and the reduction of transplant waiting lists. These assumptions are likely applicable to other Latin American nations contending analogous challenges related to corneal blindness and donor scarcity.53–55
Effective collaboration among a variety of stakeholders, including patients, doctors, public health specialists, machine learning engineers, data scientists, statisticians, and field scientists, is required for the successful integration of cutting-edge technology into clinical practice.56 Notably, a clear framework for decision-making is often missing from the implementation of such technologies. In earlier research, the importance of “flexibility of usage” became a top priority criterion in “physician-specific” factors in the framework used to make decisions about the adoption of new surgical technologies.57 To bridge this conceptual understanding with tangible insights, we conducted a survey into the perspectives of Latin American ophthalmology surgeons. Despite limitations in terms of respondent numbers and survey questions, the data obtained offer initial insights into factors deemed crucial by these surgeons when contemplating the adoption of engineered corneal endothelium. Safety, effectiveness, cost, and procedural viability emerged as paramount criteria, aligning with the prioritization criteria for surgical technology adoption.57,58 This finding confirms the promise of this technology by bridging the gap between theoretical advantages and real-world attitudes among practitioners. Further in-depth studies with a wider group of respondents will confirm these preliminary data.
In summary, this study highlights the existing gap between the significant scientific advancements in corneal endothelium engineering and its practical application in clinical contexts. This study emphasizes the need to reconsider traditional research pathways and academic models, emphasizing a shift toward prioritizing healthcare impact and patient outcomes. Recognizing corneal transplantation as a crucial public health concern, the adoption of novel technologies should extend beyond research labs and actively target real-world implementation. While bibliometric analysis indicates a noticeable shift toward corneal endothelial engineering and emphasizes the importance of multidisciplinary collaboration, certain limitations exist. The database constraints hindered a comprehensive understanding of the types of studies analyzed, limiting our insight into the evolving evidence for engineered corneal tissue in clinical applications. Additionally, our company analysis was based solely on bibliometric affiliations, potentially overlooking other technologies. The examination of patents revealed challenges in translating innovations, emphasizing obstacles to practical applications. Industry trends underscore the misalignment between research production and real-world technology use, particularly in ophthalmology. The QALY analysis demonstrated the benefits of adopting engineered corneal endothelium in clinical practice. A Latin American survey among ophthalmologists offered valuable perspectives on the factors influencing technology adoption. Overall, these findings emphasize the complex interplay among scientific progress, technology adoption, and clinical practice, calling for sustained collaboration, financial support, and further in-depth studies to bridge the gap between theoretical promise and practical implementation in corneal transplantation.
Figshare: Corneal Endothelium Bibliometric and Patent Analysis. https://doi.org/10.6084/m9.figshare.25697556.v1 59
The project contains the following underlying data:
• Supplementary File 1.xlsx. (Web of Science Raw File Bibliometric Analysis)
• Supplementary File 2.xlsx. (Bibliometric Analysis Top 25 Journals)
• Supplementary File 3.xlsx. (List of 26 patents from Lens.org)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, et al.: Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) Statement: Updated Reporting Guidance for Health Economic Evaluations.Value Health. 2022; 25 (1): 3-9 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Health economics
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cornea, Refractive Surgery and External Diseases
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 10 Jun 24 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)